Multi-institutional retrospective analysis of FOLFlRl in patients with advanced biliary tract cancers
2020-01-16JonathanMizrahiValerieGunchickKabirModyLianchunXiaoPhanikeerthiSurapaneniRachnaShroffVaibhavSahai
Jonathan D Mizrahi, Valerie Gunchick, Kabir Mody, Lianchun Xiao, Phanikeerthi Surapaneni, Rachna T Shroff,Vaibhav Sahai
Jonathan D Mizrahi, Lianchun Xiao, Division of Cancer Medicine, The University of Texas MD Anderson Cancer Center, Houston, TX 77005, United States
Valerie Gunchick, Vaibhav Sahai, Department of Internal Medicine, University of Michigan,Ann Arbor, MI 48109, United States
Kabir Mody, Phanikeerthi Surapaneni, Division of Medical Oncology, Mayo Clinic Cancer Center, Jacksonville, FL 32224, United States
Rachna T Shroff, University of Arizona Cancer Center, University of Arizona, Tucson, AZ 85724, United States
Abstract
Key words: Biliary tract neoplasms; Fluorouracil; Irinotecan; Cholangiocarcinoma;Retrospective studies
INTRODUCTION
Biliary tract cancers (BTCs) are rare but aggressive malignancies that arise from epithelial cells in the bile ducts or gallbladder. BTCs are anatomically classified as intrahepatic and extrahepatic (perihilar and distal) cholangiocarcinoma (CCA), and gallbladder carcinoma (GBCA)[1-3].
In the United States alone, more than 12000 people are estimated to be diagnosed with BTC in 2019[4]. Advanced BTCs are considered aggressive cancers with a reported median overall survival (OS) of approximately 12 mo. Over 85000 people lost their lives to BTC between 1999 and 2014[5], and mortality rates continue to rise[5,6].It is clear that more effective management strategies are needed to reverse these rising rates, particularly for patients with advanced BTCs. Standard of care first-line therapy for these patients involves multi-agent chemotherapy with gemcitabine and cisplatin[7-9]. In the phase 3 ABC-02 trial published in 2010, gemcitabine and cisplatin was demonstrated to improve median OS to 11.7 mo from 8.1 mo with gemcitabine alone. However, durable response rates are infrequent, and a substantial number of patients progress quickly. Additional strategies and subsequent lines of therapy remain largely investigational with no clear standard at present, although FOLinic acid and Fluorouracil in combination with either OXaliplatin (FOLFOX) or IRInotecan(FOLFIRI) are often used[10-12].
The efficacy of FOLFIRI as a first or second-line treatment has been previously assessed in small retrospective studies. In a single institution review of 17 patients with advanced BTC treated with FOLFIRI as first-line therapy, the authors noted a median progression free survival (PFS) and OS of 2.6 and 6.5 mo, respectively[13]. In another retrospective analysis of 64 patients with advanced BTC treated with either FOLFIRI or XELIRI as second-line therapy, Brieauet al[10]observed a similar median PFS and OS of 2.6 and 6.2 mo, respectively. Additionally, a smaller retrospective analysis of five BTC patients treated with either FOLFIRI or FOLFOX as second-line therapy reported a median PFS and OS of 4.4 and 6.1 mo, respectively[14].
The primary objective of this retrospective analysis was to identify the efficacy of irinotecan-based regimens in the management of patients with advanced BTC in a larger multi-institutional cohort.
MATERIALS AND METHODS
Study cohort
The study was individually approved by the institutional review boards at University of Michigan, University of Texas MD Anderson Cancer Center and Mayo Clinic Cancer Center at Jacksonville. The informed consent was waived for this HIPAA compliant retrospective study. The eligibility criteria included patients aged 18 years or older with pathologic confirmation of BTC and advanced unresectable or metastatic disease on imaging. Eligible subjects must have received irinotecan-based systemic chemotherapy. Patients with ICD9 and ICD10 diagnosis codes for BTC were retrospectively identified at each institution with at least one encounter between January 2007 and October 2017. Data were collected on patient demographics,subtype of BTC, response per RECIST v1.1, progression and survival. In addition,genomic analysis data were also collected, when available.
Statistical analysis
Descriptive statistics such as mean, standard deviation, median and range, frequency and percentage were used to summarize patient characteristics. The Kaplan-Meier method was applied to estimate survival outcomes (Figure 1),i.e., OS and PFS, and the log rank test was used for comparison of these outcomes between subgroups of patients. The OS time was calculated as the time period from the date of the treatment start to the date of death or to the date of the last follow-up for patients alive, and patients alive were censored for the analysis of OS. The PFS time was calculated as the time period from the date of start of treatment to the date of progression or death,whichever occurred first; and patients alive and without progression were censored to the date of the last follow-up. SAS software v9.4 (SAS institute Inc., Cary, NC, United States) and Splus software v8.2 (TIBCO Software Inc., Palo Alto, CA, Unites States).
RESULTS
A total of 98 consecutive patients who met the eligibility criteria were included in the analysis. The median age was 60 years (range, 22-86 years), and 46 (46.9%) subjects were women. Sixty-one patients were identified at MD Anderson, 26 at University of Michigan, and 11 at Mayo Clinic Cancer Center in Jacksonville. Seventy-four (75%)patients had distant metastases at the time of treatment with FOLFIRI, while 24 (25%)had locally advanced disease. The majority of patients had intrahepatic CCA (n= 71),compared with 17 with GBCA and 10 with extrahepatic CCA. The patient baseline characteristics are detailed in Table 1.
The median duration on FOLFIRI, or FOLFIRI-containing regimens, was 2.2 mo(range, 0.5 to 8.4), and the median PFS was 2.4 mo (95% confidence interval (CI): 1.7-3.1) for the entire cohort. The median PFS for patients treated with FOLFIRI as 1st, 2nd,3rdor 4th- Nthline therapy was 3.1 (95%CI: 1.4-4.8), 2.4 (95%CI: 1.8-3.7), 2.3 (95%CI:1.5-3.1) and 1.5 (95%CI: 0.9-2.0) mo, respectively. The median OS for patients treated with FOLFIRI as 1st, 2nd, 3rdor 4th- Nthline therapy was 12.3 (95%CI: 5.6-23.4), 7.7(95%CI: 4.9-10.5), 5.0 (95%CI: 3.6-7.3) and 7.5 (95%CI: 5.2-9.8) mo, respectively. The median OS for the cohort was 6.6 mo (95%CI: 4.7-8.4) from start of therapy. The best overall response rate was 9.8% per RECIST v1.1 with a disease control rate of 45.1%.
Thirteen patients received vascular endothelial growth factor targeted therapy with bevacizumab, and five patients received anti-epidermal growth factor receptor therapy with erlotinib (n =4) and panitumumab (n =1) concurrently with FOLFIRI.Patients in both of these groups of patients exhibited a median PFS of 2.7 mo.
There was no statistically significant difference in median PFS for patients with locally advanced disease when compared to those with distant metastases (3.2vs2.1 mo,P =0.16) at the time of FOLFIRI treatment. There was a trend towards prolonged median OS for patients with locally advanced cancer compared to those with distant metastases (9.3vs5.6 mo,P =0.08) (Table 2).
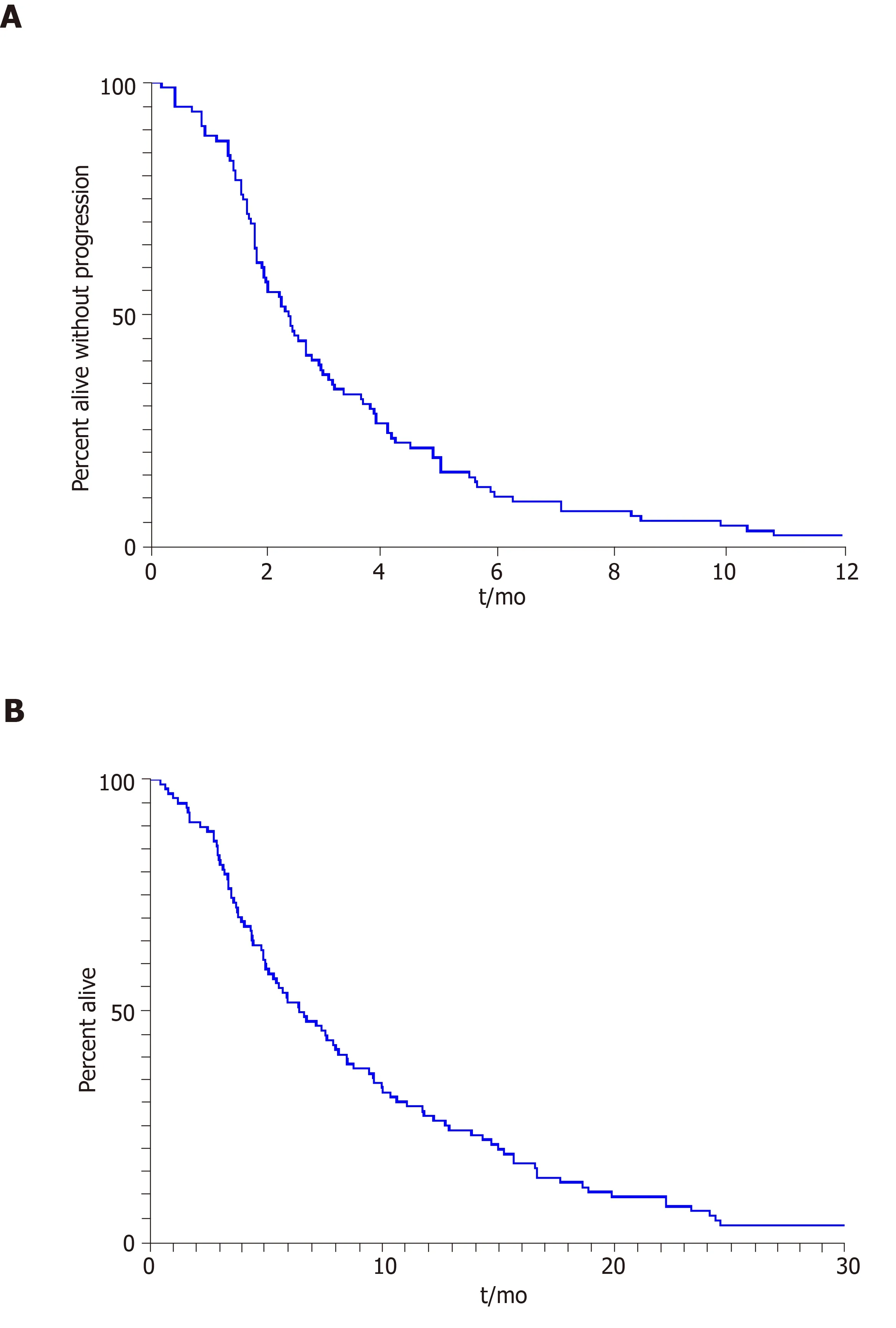
Figure 1 The Kaplan-Meier method was applied to estimate survival outcomes.
Thirty-four (35%) of the patients included in the study had genomic profiling of their BTC completed, including five with extrahepatic CCA, 27 with intrahepatic CCA and two with GBCA. The genomic profiling results are summarized in Table 3. The most frequent alterations identified included mutations inTP53(35.3%),IDH1andIDH2(29.4%) andKRAS(20.6%) genes.FGFR2 fusions were identified in four patients with intrahepatic CCA and two patients with extrahepatic CCA, however, theIDH1andIDH2mutations were restricted to the intrahepatic subtype.
DISCUSSION
In this multi-institution retrospective study, FOLFIRI, or FOLFIRI-containing regimens had modest efficacy with a median PFS of 2.4 mo and OS of 6.6 mo in patients with advanced unresectable or metastatic BTC. To our knowledge, this is the largest analysis of outcomes with FOLFIRI in BTCs. Expectedly, patients treated with FOLFIRI earlier in the course of their therapy tended to have longer PFS (P =0.53),likely due to use of other 5-fluorouracil (5-FU) containing regimens prior to FOLFIRI and development of multi-drug resistance. Additionally, patients with locally advanced stage may have longer PFS (3.2vs2.1 mo;P =0.16) compared to those with distant metastasis.
The majority of the patients included in our analysis had intrahepatic CCA (72%).This subtype of BTC may be associated with better outcomes compared to extrahepatic CCA and GBCA[15], which could potentially bias our results. However, a difference in survival was not seen in our patients based on subtype of BTC, though the sample size of patients with extrahepatic CCA and GBCA was small.
The survival outcomes we describe are comparable to those reported by Brieauetal[10]and Morettoet al[13]utilizing FOLFIRI and similar to published data regarding other chemotherapy regimens, such as FOLFOX in this patient population[16,17].FOLFOX has been evaluated in multiple prospective studies as second-line therapy with a reported time to progression of 3.1 mo in a 37 patient phase II trial from China[18]and a PFS of 3.9 mo in a 66 patient observational study from Japan[19]. A retrospective analysis of 144 patients with BTCs treated with second-line chemotherapy (70% regimens 5-FU based) at a single institution in Germany found an overall response rate of 9.7% with a disease control rate of 33.6% and median OS of 9.9 mo[20]. An additional retrospective study of 18 patients from an institution in Chile showed a median PFS and OS of 3.2 and 4.6 mo, respectively[17]. There are several other clinical trials accruing patients for second-line therapy, including the phase Ib/II trial evaluating the combination of 5-FU, folinic acid and nanoliposomal irinotecan in conjunction with an anti-PD1 antibody nivolumab (BilT-03)[21].
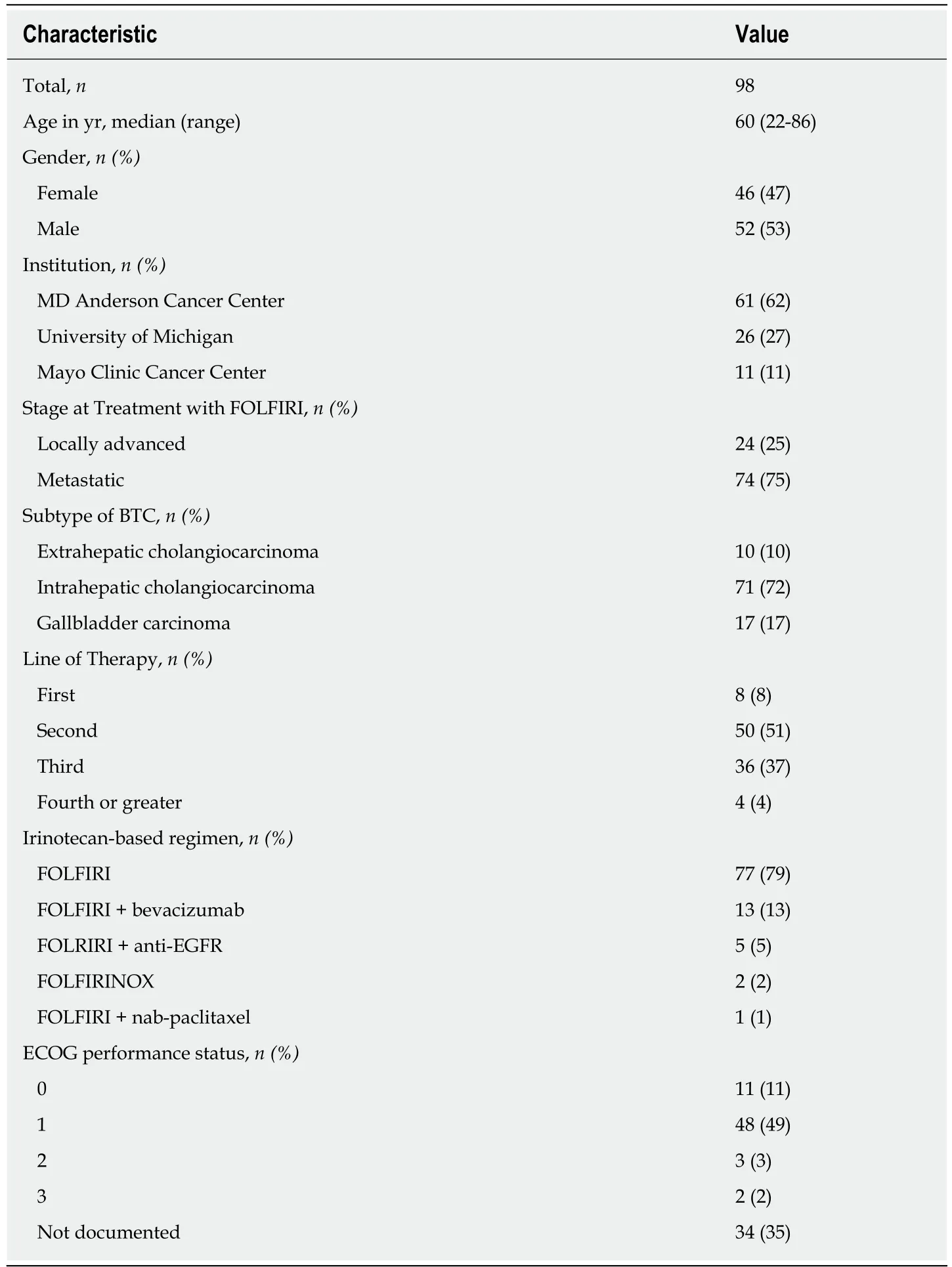
Table 1 Patient baseline characteristics
In the subgroup of 18 patients who were treated concurrently with either anti-EGFR or anti-vascular endothelial growth factor targeted therapy, there did not appear to be a clinical benefit of the additional drug, though this was a small cohort.This is in contrast to a single institution analysis from France of 13 patients with metastatic intrahepatic CCA treated with FOLFIRI with bevacizumab as second-linetherapy that reported a best overall response rate of 38.4%, median PFS of 8 mo and OS of 20 mo[22].
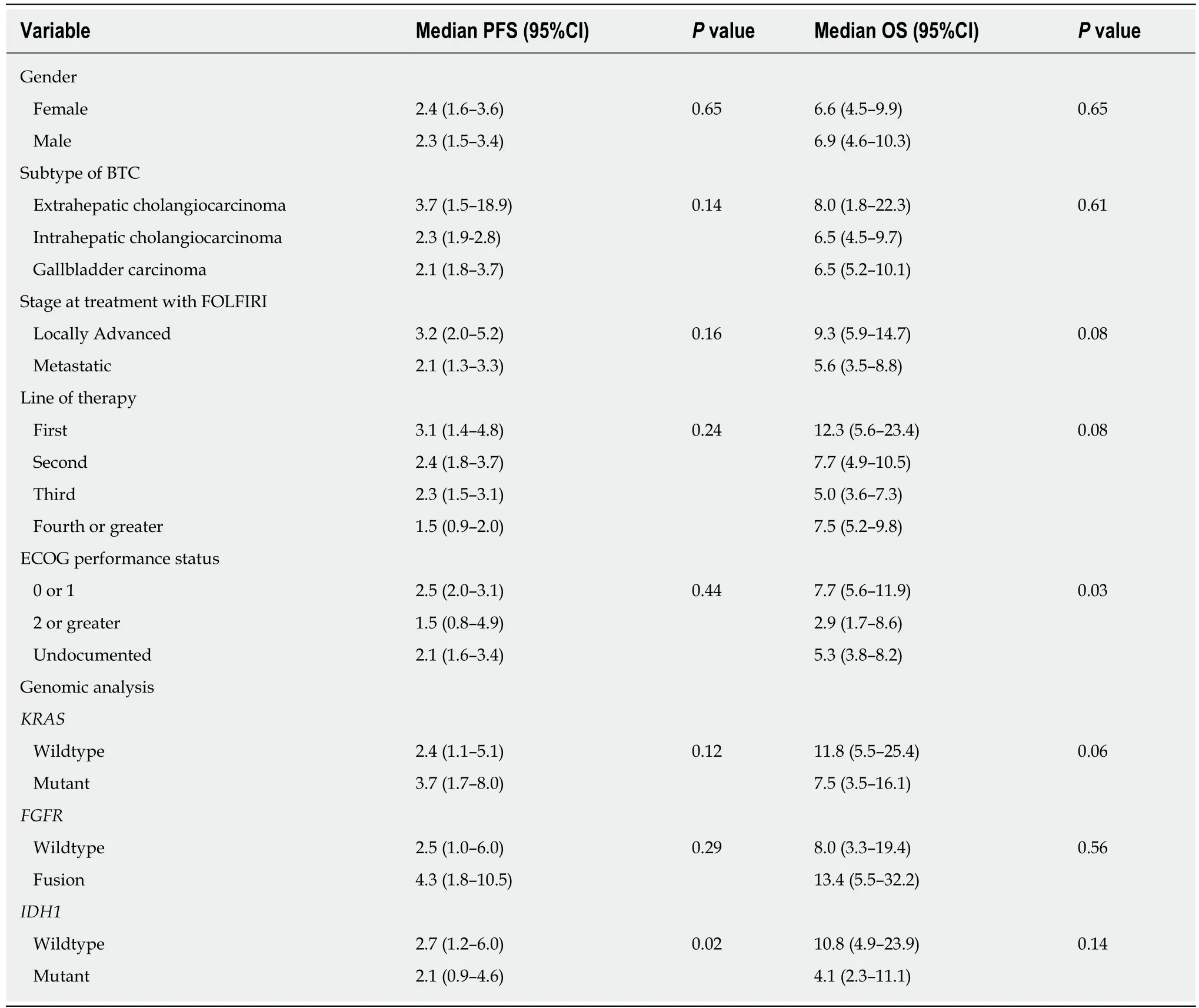
Table 2 Subgroup analysis of median progression-free survival
We identified no significant correlation between specific somatic mutations and patient outcomes or response to FOLFIRI, though this conclusion is limited by the small number of patients in our study. Recently, the most promising therapeutic advances in BTC have resulted from the identification and targeting of actionable driver somatic mutations. Multiple phase II clinical trials have yielded encouraging results by taking advantage of driver mutations such asFGFR,IDH1,IDH2andBRAF[23-26]. However, most patients with BTCs do not harbor mutations that are currently targetable, limiting the benefits of these recent advances to only a select cohort.
Given the lack of other standard therapies for patients with BTCs who have progressed on first-line therapy, our results indicate that FOLFIRI may indeed have a role in these patients. The results of our study further emphasize the need for more effective treatment options for patients with advanced unresectable or metastatic BTCs after failure of first-line systemic chemotherapy, especially in absence of actionable driver mutations.
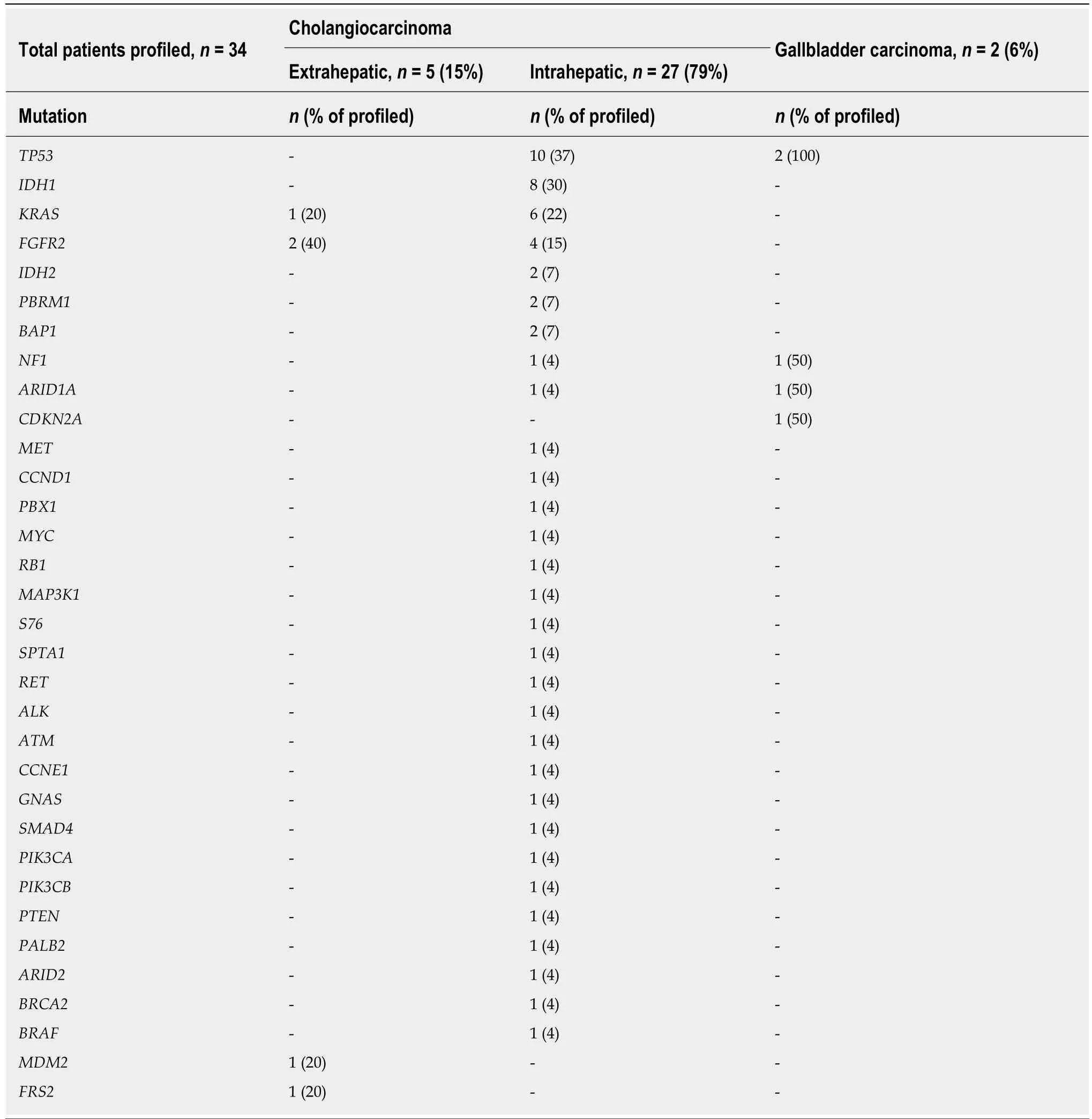
Table 3 Genomic profiling by tumor type
ARTICLE HIGHLIGHTS
Research background
Advanced biliary tract cancers (BTC) are aggressive malignancies without an established standard of care after progression on first-line combination chemotherapy with gemcitabine plus cisplatin. Fluoropyrimidine-based therapies, such as 5-fluorouracil plus either oxaliplatin(FOLFOX) or irinotecan (FOLFIRI) are commonly used in this setting. There is limited data on the efficacy of such regimens in patients with BTCs, particularly in the patients who have progressed on first-line therapy.
Research motivation
There is a significant need for evidence-based treatment of patients with advanced BTCs who have previously progressed of first-line systemic chemotherapy. Only small, primarily singleinstitution analyses have been published about the role of FOLFIRI in this population. We sought to combine the experiences of multiple institutions to provide the largest dataset with this regimen.
Research objectives
Our study assessed the efficacy of FOLFIRI in patients with BTC by measuring progression-free survival and overall survival.
Research methods
We retrospectively identified patients with advanced, unresectable BTC who were treated with FOLFIRI at three institutions: MD Anderson, University of Michigan and Mayo Clinic in Jacksonville. We collected data on survival, response per RECIST v1.1, patient demographics and tumor characteristics.
Research results
Ninety-eight patients were included in our analysis, most of whom were treated in the second and third-line setting. Median duration on FOLFIRI was 2.2 mo. Median progression-free survival was 2.4 mo (95%CI: 1.7-3.1), and median overall survival was 6.6 mo (95%CI: 4.7-8.4).
Research conclusions
The efficacy of FOLFIRI for patients with BTCs appears to be modest with survival outcomes that are similar to historical controls of other retrospectively examined second-line cytotoxic therapy options.
Research perspectives
Based on this multi-institutional analysis, FOLFIRI seems to have a limited role in the treatment of patients with BTCs, though there are no prospective studies that have assessed this regimen in this patient population. The recently reported results of the randomized phase III ABC-06 trial demonstrating an increase in OS with modified FOLFOX plus active symptom control compared to active symptom control alone likely makes this a more appealing treatment option for most patients who have progressed on gemcitabine plus cisplatin.
杂志排行
World Journal of Gastrointestinal Oncology的其它文章
- Precision medicine for gastrointestinal cancer: Recent progress and future perspective
- Digestive tract reconstruction options after laparoscopic gastrectomy for gastric cancer
- lnterpretation of the development of neoadjuvant therapy for gastric cancer based on the vicissitudes of the NCCN guidelines
- ldentification of candidate biomarkers correlated with pathogenesis of postoperative peritoneal adhesion by using microarray analysis
- Abnormal CD44 activation of hepatocytes with nonalcoholic fatty accumulation in rat hepatocarcinogenesis
- Laparoscopic dissection of the hepatic node: The trans lesser omentum approach
