Effect of particle gradation of aluminum on the explosion field pressure and temperature of RDX-based explosives in vacuum and air atmosphere
2020-01-07FanJiangXiaofengWangYafengHuangBoFengXuanTianYuleiNiuKunZhang
Fan Jiang,Xiao-feng Wang,Ya-feng Huang,Bo Feng,Xuan Tian,Yu-lei Niu,Kun Zhang
Xi'an Modern Chemistry Research Institute,Xi'an Zhangbadong Road 165,Xi'an,710065,Shanxi,PR China
ABSTRACT To optimize the energy output and improve the energy utilization efficiency of an aluminized explosive,an explosion device was developed and used to investigate the detonation pressure and temperature of R1 (Al6) aluminum powder and the aluminum powder particle gradation of R2 (Al6+Al13), R3(Al6+Al24)and R4(Al6+Al flake)in a confined space.By using gas chromatography,quantitative analysis and calculations were carried out to analyze the gaseous detonation products.Finally,the reaction ratios of the aluminum powder and the explosion reaction equations were calculated.The results show that in a confined space,the quasi-static pressures and equilibrium temperature of the aluminum powder in air are higher than in vacuum.In vacuum,the quasi-static pressures and equilibrium temperatures of the samples in descending order are R1>R3>R4>R2 and R3>R4>R1>R2,respectively.In air,the quasi-static pressures and equilibrium temperatures of the samples in descending order are R1>R2>R4>R3 and R1>R4>R2>R3,respectively.R4(Al6+Al flake)and R3(Al6+Al24)have relatively higher temperatures after detonation,which shows that the particle gradation method can enhance the reaction energy output of aluminum during the initial reaction stage of the explosion and increase the reaction ratio by 10.6%and 8.0%,respectively.In air,the reaction ratio of Al6 aluminum powder can reach as high as 78.16%,and the reaction ratio is slightly reduced after particle gradation.Finally,the reaction equations of the explosives in vacuum and in air were calculated by quantitative analysis of the explosion products,which provides a powerful basis for the study of RDX-based explosive reactions.
Keywords:Particle gradation Quasi-static pressure Equilibrium temperature Reaction ratio
1. Introduction
Aluminum, a metal material that has a high combustion enthalpy and a large energy density,is commonly used as an ingredient in energetic materials to enhance the air blast,increase the bubble energies of underwater weapons,raise the reaction temperatures and facilitate after-burning effects [1]. Although aluminum does not react quickly enough to take part in the reactions in the detonation zone,it may react with either the gaseous products resulting from detonation or the oxygen from the surrounding air, both of which can significantly promote the strength of the blast wave[2].Consequently,aluminum powder is often used to improve the performance of energetic materials[3-6].
Many studies have been carried out to investigate the influence of the particle size of aluminum powder on the performance of explosives[7-11].The former Soviet Union used particle gradation of micron sized aluminum and flake aluminum powder in the MC bomb model,which significantly improved the performance of explosives.Zhou et al.[12]studied four types of TNT/Al explosives with particle size of 50 nm,100 nm,1.50 μm and 9.79 μm.The results showed that the Chapman Jouguet(CJ)pressure of nano-sized aluminum is higher than that of micron-sized aluminum powder,and a smaller aluminum particle size used in TNT/Al explosives is associated with a slower detonation pressure attenuation.Waldemar[13]investigated the effect of particle size and the aluminum content on the detonation characteristics of RDX-based explosivescontaining 15%-60%aluminum and found that flaked aluminum additives result in more porous explosives than aluminum containing powder,thus causing changes in the detonation velocity.Brousseau[14]adopted a micro-nano aluminum powder particle grading method to increase the reaction ratio and detonation properties in his research.Muravyev et al.[15]showed that ultrafine aluminum powder can increase the burning speed and the combustion completeness by 2.5 times and 4 times,respectively,compared with micron-sized aluminum powder.Chinese scientists also researched the effects of particle size,particle shape and atmosphere on the blast characteristics[16-19].For example,Huang et al.[17]studied the effect of the particle size and particle shape of aluminum powder on the explosion field pressure and temperature in vacuum. After investigating four different particle sizes of aluminum powder,he found that the effects of the aluminum powder on the pressure are not consistent with temperature.Feng et al.[18]showed that when the mass fraction in an aluminized explosive is 35%,the explosive energy of the 13 μm aluminum powder is reduced by nearly 40%compared with that of the flake aluminum powder.Essentially,the relationship between the particle size of aluminum powder and the blast characteristics is still ambiguous[20]and research on the effect of the particle gradation of aluminum on the energy output of explosives is still insufficient.
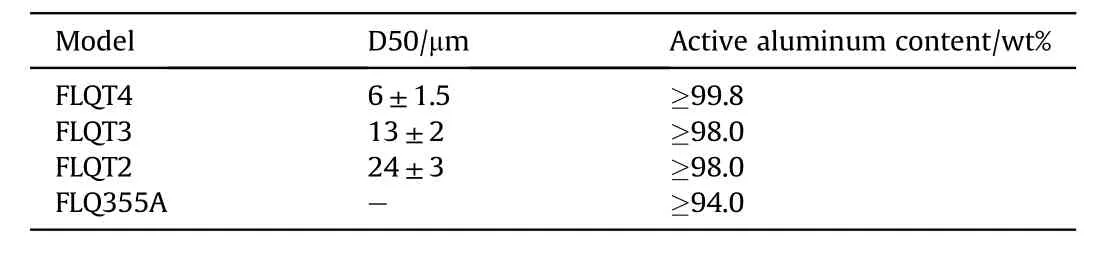
Table 1D50 and active aluminum content of aluminum powder.
To optimize the energy output and improve the energy utilization efficiency of the aluminized explosive,four aluminized explosives with different particle size distributions are designed in this paper to study the quasi-static temperature and pressure after explosion in confined space under vacuum and air condition.The gaseous products were quantitatively analysed using gas chromatography,and furthermore,the reaction ratio of the aluminum powder and the explosion reaction equations were calculated.
2. Experimental section
2.1. Reagents and materials
To prepare the explosive samples for testing,RDX was bought from Gansu Yinguang Chemical Industry Co.Ltd.of China.FLQT4,FLQT3 and FLQT2 aluminum powder were provided by Angang Group Aluminum Powder Co.Ltd.FLQ355A aluminum flake was bought from Harbin Dongqing Metal Powder Industry Co.Ltd.All aluminum powders comply with the GJB1738-1993 ultrafine aluminum powder specifciation.The D50 and the active aluminum content of the selected aluminum powder are shown in Table 1.The particle size of the flake aluminum powder is ≥355 μm(wt%≤0.1%)& ≥160 μm (wt%≤5.0%) and the average thickness of flake aluminum is 2.1 μm.
In this study,four different particle gradation of aluminum powder were considered.After gradating the FLQT4(marked as Al6) aluminum powder with FLQT3 (Al13), FLQT2 (Al24) and FLQ355A(Al flake),four different particle gradations of the samples were obtained.The mass fraction of Al6 in each sample accounts for 14%of the total mass of the aluminum powder.The particle size distribution of the aluminum powder was tested using Mastersizer 2000 laser particle size analyzer from British Malvern Corporation.The optical microscope image and the normal distribution for each of the gradated aluminum samples are shown in Fig.1.Each sample was tested at least three times.
Due to the addition of Al6 with a mass fraction of 14%,the particle size distribution is asymmetrical but appears to be elevated or bimodal for small particle sizes.The medium particle sizes of aluminum for R1, R2, R3, and R4 are 6.348 μm, 11.449 μm,22.287 μm,and 74.052 μm,respectively.The formulations of the test samples were 60RDX/35Al/5binder and the aluminum-oxygen ratio of the sample in vacuum and air environment is 0.800.The explosives were pressed to a theoretical density of about 96%.The charge density of the columns were measured using solvent extraction method. The formulations and charge densities are shown in Table 2.
The formulations were made as follows.Firstly,the binder was dissolved in ethyl acetate and placed in a water bath to raise the temperature to 60°C.Then,RDX and aluminum were added to the dissolved binder. The solution was stirred until the solvent evaporated and the material turned to brushed state.Afterwards,the material was screened,granulated and dried.Finally,the materials were pressed into columns(φ=25 mm),reserving a no.8 detonator hole.

Fig.1.Normal distribution of the particles after gradation.

Table 2Formulations and charge densities of particle gradated aluminized explosives.
2.2. Experimental facility
The experiment used a self-developed closed explosive device,as shown in Fig.2.
The device had a cylindrical body with a height of 400 mm,an inner diameter of 188 mm and a volume of 5.8 L.The pressure sensor used in this experimental device was an ultra-high temperature silicon piezoresistive sensor produced by Kulite Company of the United States.The explosive device has a maximum pressure resistance of 140 bar and can operate at temperatures ranging from -55°C to 538°C; the temperature sensor was a selfrecovering and fast-reacting tungsten-rhodium thermocouple developed by the American NANMAC Company.The temperature sensor had a response time of 10-5s and was able to withstand a maximum pressure of 135 MPa.The sensor was placed 40 mm away from the center of the top cap,and its lower end was placed 120 mm from the bottom of the end cap.The temperature range was-200°C-1300°C,and the error was less than 1%.
2.3. Experimental method
First,the ignition device was short-circuited.The sample was suspended 20 cm away from the top cap.Then,the detonator was connected to the ignition device,and the upper cap of the experimental device was closed.Next,the device was evacuated with a vacuum pump to the air.Then,the explosion device was slowly filled with nitrogen;this step was repeated,and finally,the nitrogen was pumped out,making the absolute pressure inside the device less than 100 Pa.For the air experimental group,the original air was used, and the atmospheric pressure was recorded(96.45 kPa).Finally,the sample was detonated,and the signal data were recorded within 20 s by the pressure and temperature sensors.Gas samples were collected with a gas sampling bag.
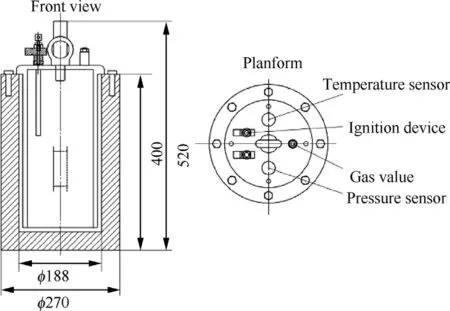
Fig.2.Schematic diagram of the explosive device.
3. Results and discussion
The detonation products produce a pressure that is much smaller than the peak value of the shock wave,but the gas pressure lasts longer.The process is relatively slow and can be approximated as a quasi-static process.In a confined space,irreversible processes attenuate the shock and convert their energy to quasi-static pressure,which is an important parameter used to characterize the energy released from the explosion reaction and the after-burning process[21].Usually,after detonation,an average pressure of several tens of milliseconds is used as the quasi-static pressure[22].Because the quasi-static pressure is maintained for a long period of time in the confined space,the impulse is very large and the damage effect on the target is sound.
Consequently,by studying the formulation of aluminized explosives,changes in the quasi-static pressure and temperature in vacuum and in air were investigated to study the completeness of the reaction of the aluminum powder by anaerobic combustion and aerobic combustion.In addition,to investigate the completeness of the reaction of the aluminum powder by anaerobic combustion and aerobic combustion,gaseous species produced by the explosion were analysed.
3.1. Pressure analysis of explosion field
From the explosion tank sealing experiment,a pressure versus time curve was obtained for the aluminized explosive with different aluminum powder particle size gradations;the time scale of the graph is reduced to 100 ms as shown in Fig.3.
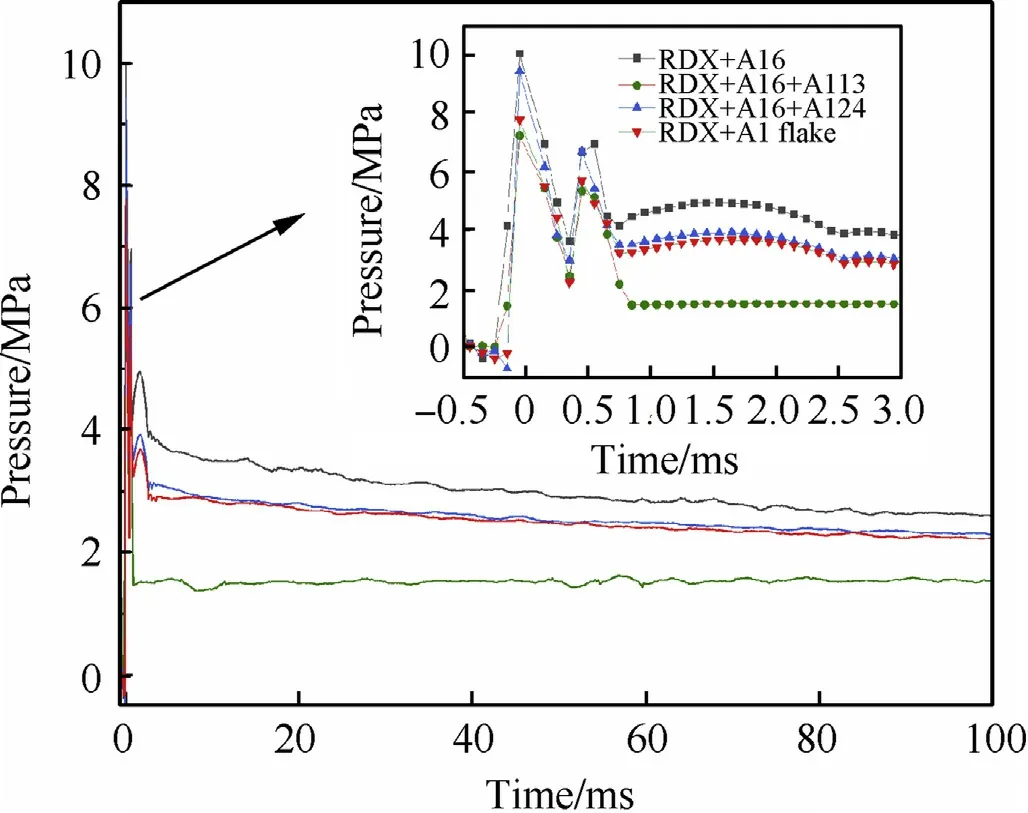
Fig.3.Pressure profile of different particle gradation of aluminum powder within 100 ms of the explosion in vacuum.
Under the shock effect, the explosive reacts violently and releases a large amount of heat and gas products during the reaction,which causes a sudden jump in the temperature and pressure in the closed chamber.After a relatively long period of heat exchange and material exchange,the system is balanced.As shown in the figure,in vacuum,the quasi-static pressure of the four particle size gradation of aluminum powder in descending order are R1(Al6)>R3(Al6+Al24)>R4(Al6+Al flake)>R2(Al6+Al13).
To restore the actual environment of the explosion,the samples are detonated in air.The pressure versus time curves resulting from the air detonation experiment are shown in Fig.4.
The results show that the shock wave overpressure and quasistatic pressure in air is greater than that of the explosion that occurred in vacuum,which is consistent with Feng's[18]experimental results. The quasi-static pressures of the samples in descending order are R1(Al6)>R2(Al6+Al13)>R4(Al6+Al flake)>R3(Al6+Al24). As shown in the experimental data, the Al6 aluminum powder has the highest quasi-static pressure both in vacuum and in air, which means that the gradation of the aluminum can slightly reduce the detonation quasi-static pressure.This result can be explained by the effects of inert thermal dilution.The aluminum powder does not participate in the reaction in the detonation reaction zone but instead acts as an inert substance.This occurs during the time when the aluminized explosive absorbs and consumes part of the energy,thereby reducing the total energy of the detonation wave.Therefore,the explosion pressure of the explosive is reduced.Numerous studies have shown that better thermal conductivity,smaller particle sizes and larger specific surface areas can contribute to greater thermal dilution effects.However,the detonation pressure is also affected by the charge density.An aluminum particle with a smaller particle size and a larger specific surface area can increase the charge density and increase the detonation pressure to a certain extent.As shown in previous results,R1 sample with Al6 aluminum powder has the highest charge density,and can compensate for the effect of thermal dilution on the decrease of quasi-static pressure.Consequently,the Al6 aluminum powder exerts such influence on the quasi-static pressure of the explosive due to the combined effects of dilution and the increased charge density.
3.2. Temperature analysis of explosion field
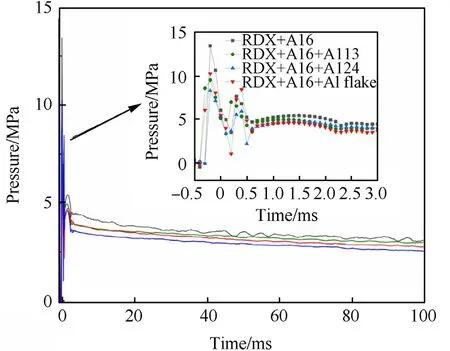
Fig.4.Pressure profile of different particle gradation of aluminum powder within 100 ms of the explosion in air.
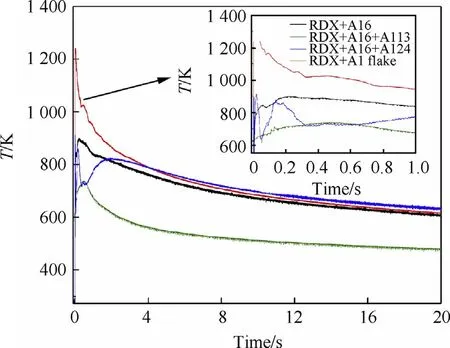
Fig.5.Temperature profile of different particle gradation of aluminum powder in vacuum within 20.0 s of the explosion.
Tests were performed to measure the temperature of the explosion field corresponding to the voltage and time.The equilibrium temperature was determined by calculating the average temperature of the last second of the explosion temperature field curve.The curves are shown in the Fig.5.
As shown in the Fig.5.The equilibrium temperature of the samples in descending order are R3(Al6+Al24)>R4(Al6+Al flake)>R1(Al6)>R2(Al6+Al13).After the explosives were detonated,the detonation products diffused rapidly.During that time,the temperature sensor responded quickly and generated peak voltage signals.Then the rapid dispersion of the gas caused a decrease in the temperature.The results show that the quasi-static pressure and equilibrium temperature of the explosion field are not correlated.It can be seen that the R4(Al6+Al flake)and R3(Al6+Al24)samples have relatively higher temperatures after detonation,which shows that the particle gradation method can enhance the reaction energy output of aluminum during the initial reaction stage of the explosion.The ambient temperature increased rapidly and then dropped after the detonation.However,the temperature went back up again resulting in a short plateau region.The main reason for this phenomenon is that R4(Al6+Al flake)and R3(Al6+Al24)have a good spatial distribution in a confined space,which makes them react better with explosive the gaseous explosion products and emit more heat.According to the secondary reaction theory[23]of aluminized explosives,the reaction of the aluminized explosive can be divided into two stages depending on the particle size of the aluminum powder and the environment.The first stage is the explosion reaction of elemental explosives and the other active components.They react quickly(less than 200 ns)and more easily.The second stage is the stage in which the aluminum powder participates in the reaction.The aluminum powder cannot react directly with the explosives but needs to react with the products of the explosives and oxygen in the environment at the high temperatures and pressures caused by the reaction of the explosives.The second phase is divided into the post-detonation early expansion part(4-10 ms),and the late reaction part,which contributes to the blast effects(1-100 ms)[12,24].
Then the samples are detonated in air.The results are shown in Fig.6.
From Fig.6,we can see that the voltage signal of the sample detonated in air is significantly enhanced compared with that of the sample detonated in vacuum.The explosion equilibrium temperature of the aluminum powder with different particle sizes detonated in air is higher than that of the aluminum powder with different particle sizes detonated in vacuum The equilibrium temperatures of the samples in descending order are R1(Al6)>R4(Al6+Al flake)>R2(Al6+Al13)>R3(Al6+Al24).In addition to reacting with the gaseous explosion products when detonated in air,the aluminum powder reacts with oxygen to release a large amount of heat.As shown by the explosion temperature versus time curves,after gradating the aluminum flake with the Al6 aluminum powder,the temperature increases significantly,indicating that it reacts better with oxygen present in the air.
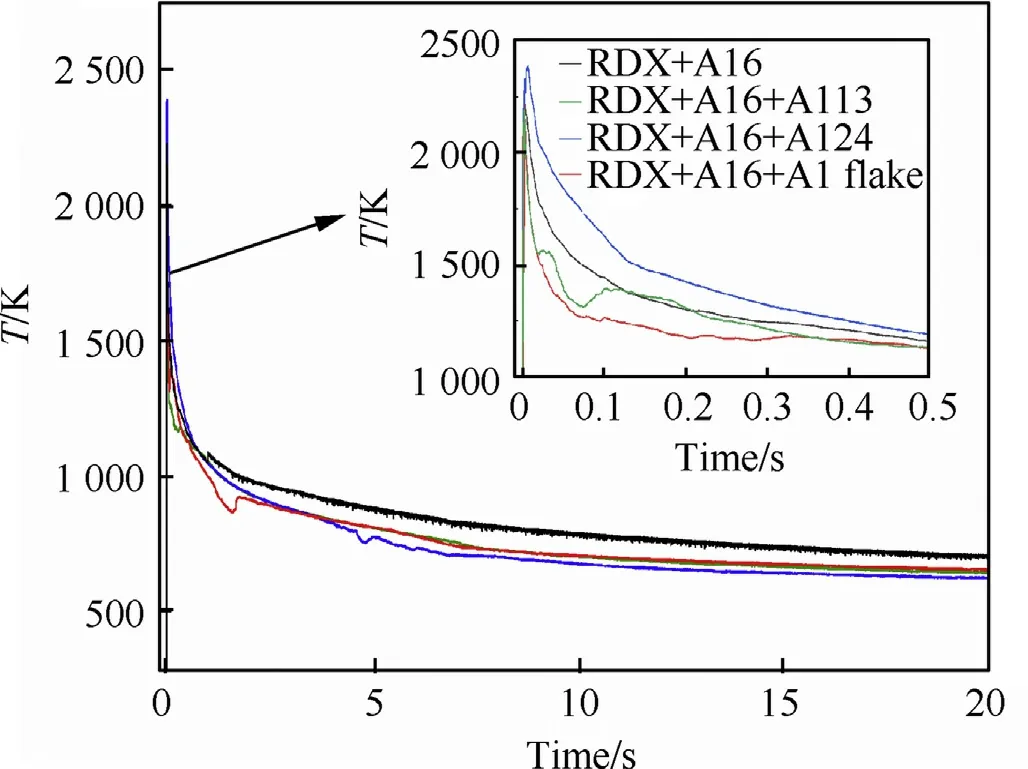
Fig.6.Temperature profile of different particle gradation of aluminum powder in air within 20.0 s of the explosion.
Previous research results indicate that aluminum powder with a smaller particle size has a better thermal effect because of its relatively larger specifci surface area.Therefore,in order to further explore the relationship between equilibrium temperature and particle size of aluminum powder after gradation,specific surface area of the four particle gradation samples was measured by nitrogen adsorption experiment (ASAP 2000 surface area and porosity analyzer produced by American micrometrics company).Nitrogen was used as the adsorbate,the adsorption temperature and degassing temperature was 77 K and 433.15 K, and the degassing time was 300 min.The nitrogen adsorption curve is shown in the Fig.7.
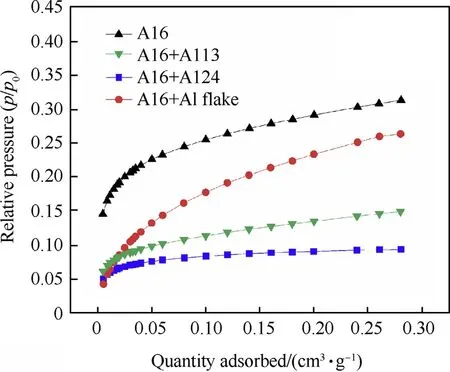
Fig.7.Nitrogen adsorption curve of four particle gradation aluminum powder.
As shown in Fig.7,the adsorption capacities of the four samples of nitrogen are Al6>Al6+Al flake>Al6+Al13>Al6+Al24.The specific surface area of each sample can be obtained from the amount of nitrogen adsorbed in Fig.7.The results show that the specific surface areas of the four particle gradation samples are Al6:0.9940 m2/g,Al6+Al flake:0.9214 m2/g,Al6+Al13:0.4812 m2/g,Al6+Al24:0.2899 m2/g,which are consistent with the equilibrium temperature rules of the four particle gradation aluminum powders in air(R1>R4>R2>R3).The exploratory studies[25]show that the decrease of particle size will result in the increase of the specific surface area of aluminum,what's more,the oxidation rate of aluminum powder is improved.In an air atmosphere,a sample with a higher specific surface area has a higher oxidation rate(as shown in Fig.10)of aluminum powder thus more heat is released,which leads to an increase in the equilibrium temperature of the explosion field.This indicating that the larger the specific surface area of the particle gradation of aluminum powder in air,the higher the equilibrium temperature of the explosion field.
3.3. Products analysis
To analyze the gaseous products quantitatively,an external standard method of gas chromatography was used.In the experiment,a thermal conductivity detector(TCD)was used with a Clarus 500 gas chromatograph produced by PERKINELMER.The results are shown in Table 3.
First,the relationship between the peak area and the concentration of the four gases(CH4,CO,CO2,N2)at different concentrations was calibrated using standard gases.Then,the samples were detected,and the peak area of the gaseous explosion products at the corresponding retention time were obtained.Last,the gas concentration was calculated.What's more,the relative standard deviation(RSD)is used in the measurement to reflect the degree of precision of the gas products analysis results.The RSD of CH4,CO,CO2,N2are lower than 3.1%,2.8%,1.4%and 2.5%,respectively.The relative error of gas content is controlled within 1%.
The total number of moles of gas is obtained from the ideal gas equation of state:

wherePis the equilibrium pressure,Tis the equilibrium temperature,Vis the volume of the explosion tank,andRis the ideal gas constant.
In terms of the quasi-static pressure and temperature treatment,the voltage obtained in the last 1 s of the 20 s time period(average of 10000 points)is used;the voltage signal is converted to the corresponding pressure and temperature.The results are shown in Table 4.
To study the composition of the solid products,X-ray diffraction(XRD)spectra were collected(D500 Diffractometer,Empyrean of Malvern,Cu Kα radiation)for 2θ in the range of 5°-90°.In ourexperiments,the explosive column was contained in a ceramic sleeve,which resulted in the appearance of ceramic diffraction peaks.Therefore,in addition to the XRD analysis of the residue extracted from the device both in vacuum and in air,XRD analysis of a fine ceramic powder was performed.The XRD patterns are shown in Fig.8.
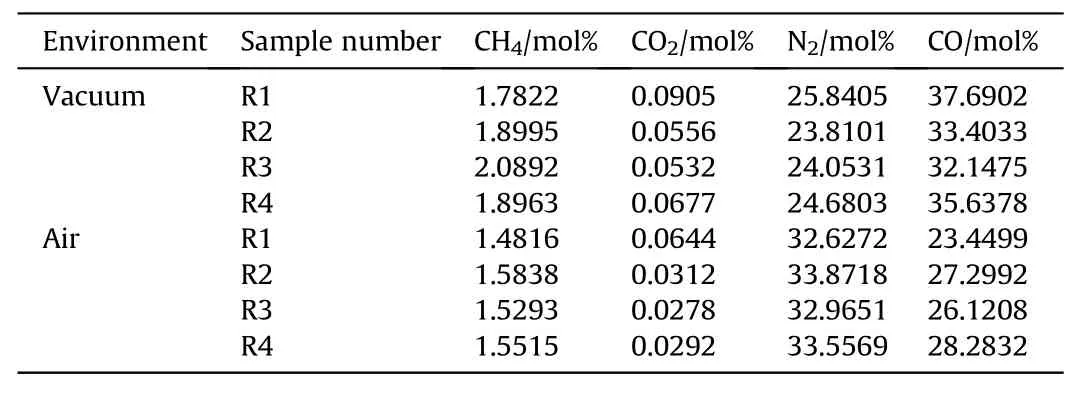
Table 3Gaseous products after detonation of aluminized explosives with different particle gradations.

Table 4Equilibrium pressure,equilibrium temperature and calculated moles of gas products.
The XRD pattern of the ceramic background indicates that it contains SiO2(Pro No.1-649)and Al2SiO5(Pro No.1-627).Due to a small amount of corundum in the ceramic,Al2O3(Pro No.1-1303)diffraction peaks were also present.As for the residue extracted from the device after detonation in vacuum and in air,a new phase of γ-Al2O3(Pro No.1-1303)formed,which indicates the oxidation of the aluminum powder.The unreacted Al(Pro No.1-1179)has a relatively weak diffraction peak,which may be due to the influence of the ceramic background.Therefore,the residue consists mainly of Al,Al2O3,and amorphous C.The amorphous carbon has a melting point of more than 3000 K because its crystal structure is similar to that of graphite.The melting points of Al and Al2O3are 933 K[26]and 2303.15 K[27],respectively.At temperatures ranging from 480 to 710 K at atmospheric pressure,Al,Al2O3and C exist in the solid phase.The melting point increased when the pressure increased to 2.7×105-4.3×105Pa.Therefore,Al,Al2O3and C exist in the solid phase based on our calculations.Aluminum oxide nitride was not found in the solid residues,which is different from the results of Trzcinski[7,28].Also,aluminum was completely oxidized to Al2O3in air of his experiment.The reason is that the experimental condition and atmosphere were different.Our experiment was carried out in vacuum and air but Trzcinski's research was carried out in nitrogen,argon and air.What's more,the volume of his device is 24.9 times larger while the charge mass is only about 2 times larger.Consequently,the conditions make the energy after the explosion more concentrated so that the system has a higher temperature which can promote the oxidation of Al.Secondly,it is expected that aluminum oxide nitride will oxidize to Al2O3given sufficient time in the presence of sustained high temperature and oxygen[29].

Fig.8.XRD pattern of the 60RDX/35Al(14%Al6+Al24)/5binder residue after detonation in vacuum and in air.
The calculations are carried out in an equilibrium state,as shown in Fig.9(where the temperature ranged from 480 to 710 K and the pressure ranged from 2.7×105-4.3×105Pa).The operating conditions of water in the equilibrium state are represented by the red area of the phase diagram shown in Fig.9.Under equilibrium conditions of the experiment,H2O exists in the vapor phase.Therefore,in the calculation,we regard H2O as a vapor.
The gaseous product is dried using silica gel to obtain the molar amount of water vapor in the explosive product,as shown in Table 5.
Based on the quantitative analysis of the gases,and XRD results of solid products,and the following assumptions,we can determine the complete analysis of the reaction ratio of the aluminum powder with different particle gradations.
N is present in the form of N2.
H exists in H2,CH4and H2O vapor.
O exists in the form of CO,CO2,H2O and Al2O3.
C exists in the form of CH4,CO,CO2and C.
Al exists in the solid phase as Al and Al2O3.
NOxgas products are very limited,which can be ignored.
The number of moles of other gaseous products can be calculated according to the following formula,whereand ωCOrepresent the mole percent of each gas in the gas phase.
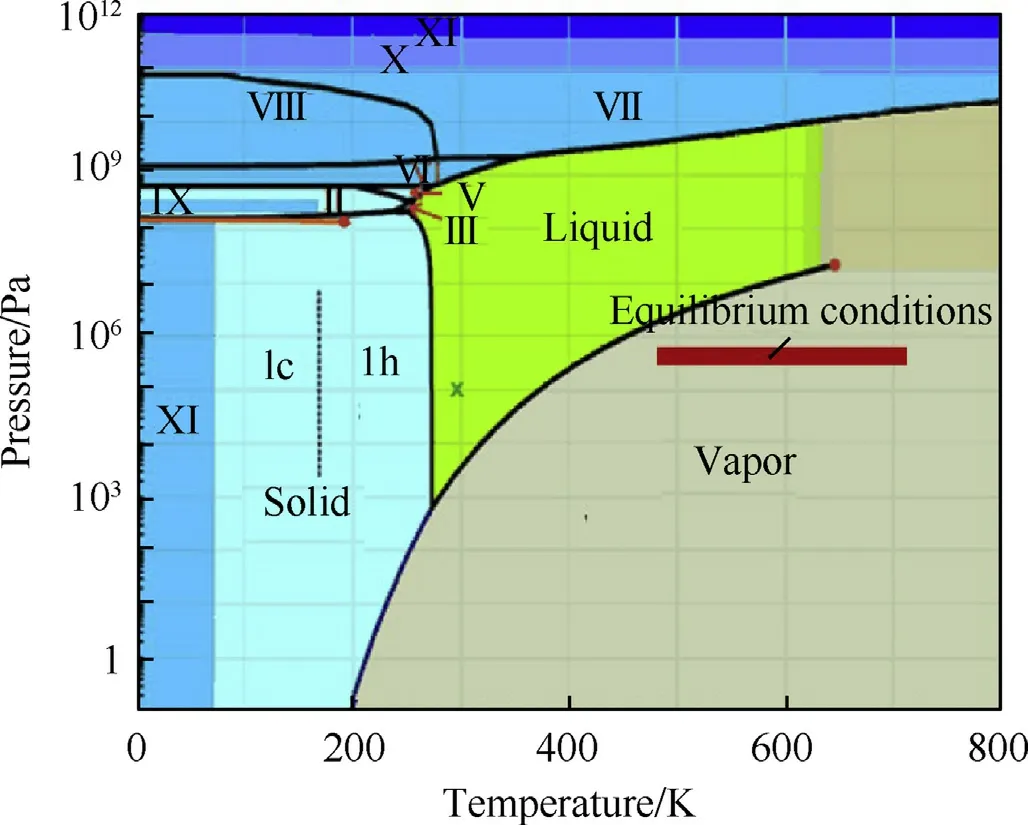
Fig.9.The state of H2O in the water phase diagram under equilibrium conditions.
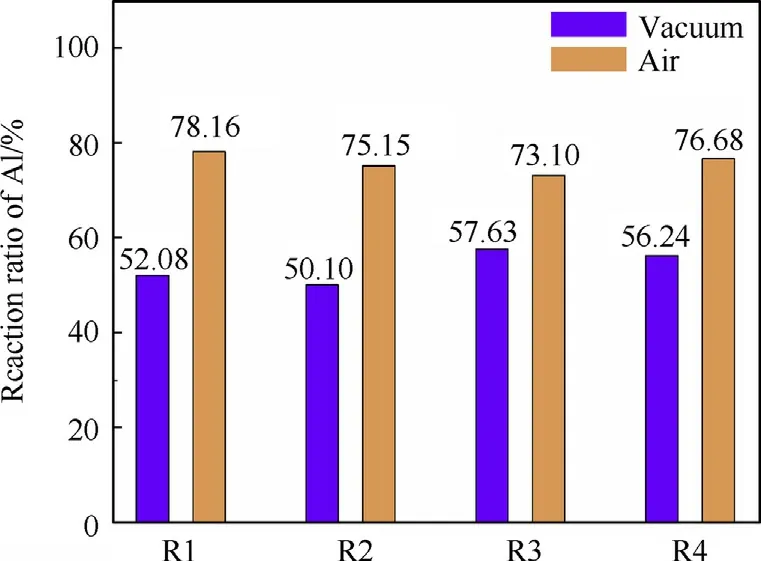
Fig.10.The reaction ratio of Al for aluminum samples containing different particle gradations reacted in vacuum and in air.

Then the number of moles of H2is:
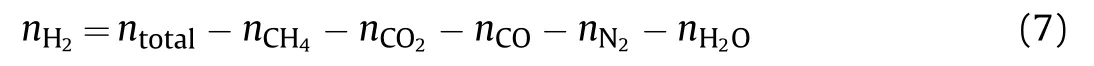
Since the oxygen content is conserved,the total molar amount of oxygennOtotalbefore the reaction is:

In our assumptions,O exists in the form of CO,CO2,H2O and Al2O3,and consequently,the numbers of moles of Al2O3can be calculated according to the conservation of O.
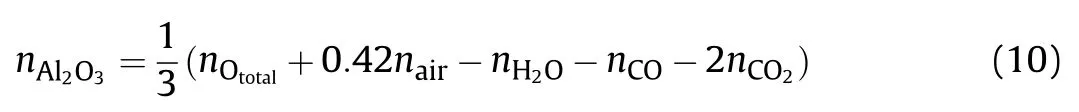
Finally,the unreacted Al can be calculated according to the conservation of Al:

From what has previously been discussed,the number of moles of different particle gradation aluminized explosives reacted in vacuum and in air can be calculated.The results are shown in Table 5.
The binder that accounts for 5%of the mass fraction was ignored in our algorithm,so a very small mass has been neglected in the balance calculation of hydrogen.However,this does not affect the overall results of the calculation(because the value is relatively small).From the remaining Al content,the reaction ratio of Al(φ)in the explosion can be calculated as shown in Eq.(12).

The reaction ratio of Al calculated for the aluminum samples containing different particle gradations in vacuum and in air is shown in Fig.10.
The reaction ratio of aluminum powder has an important influence on the energy output of the explosion process.As shown in Fig.10.The completeness of the reactions of the aluminum samples containing different particle size gradations in air is about 20%higher than that of the samples detonated in vacuum.After gradating the aluminum powder composed of Al24 with the aluminum powder composed of Al6 and gradating the aluminum powder composed of the aluminum flake with the aluminum powder composed of Al6,the reaction ratio of the aluminized explosive detonated in vacuum was increased by 10.6%and 8.0%,respectively.The reaction ratios of the aluminum powder detonated in vacuum and in air in descending order are R3>R4>R1>R2 and R1>R4>R2>R3,which are consistent with the experimentally obtained equilibrium temperature.Therefore,the more complete the reaction of the aluminum powder is,the more energy that is released from the detonation,which results in a temperature increase of the system.
In air atmosphere,the particle size gradation does not increase the reaction rate of the aluminum powder,which may be due to the relatively small explosion space of the device.According to the quantitative analysis of gas products,the content of each component of the explosion product of aluminized explosive and the equation of explosion reaction can be calculated.
The amount of RDX and aluminum powder in the explosive is certain and RDX reacts completely after detonation, so the completeness of aluminum reaction has significant influence on the energy output of the system.Through quantitative analysis of gaseous and calculation of solid phase products,the reaction ratio of the aluminum powder with different particle gradation is consistent with the equilibrium temperature of the explosion field,indicating the feasibility of this method.What's more,the equilibrium pressure of the explosion field is not related to the equilibrium temperature,which is consistent with the experimental results of Huang[17].
Since we are concerned with the reaction ratio of Al,on the left side of the equation we convert RDX and aluminum into molar mass ratio(1:6.395)according to the mass ratio in the formula(60:35).The right side of the equation is the gas obtained by quantitative analysis and the solid product obtained by thecalculation.We focus on the main product of the explosion since the explosion product is a relatively complicated.Therefore,the explosion reaction equation can be relatively close to describe the process of the explosion reaction.Considering the temperature has an important influence on the phase state of the substances generated during the reaction,the lower limit of our temperature range is taken as the equilibrium temperature after the explosion reaction.The upper limit is taken as the melting point of Al 933 K(the actual melting point of Al will be higher than 933 K when the pressure is higher than normal pressure)because Al is considered as a solid phase in the reaction equation.
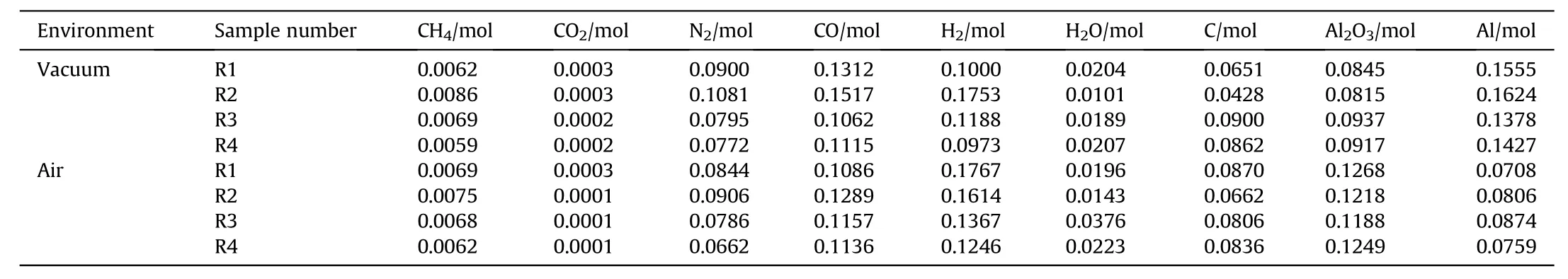
Table 5Number of moles of the explosive products following the reaction of the aluminized explosives composed of different particle gradations.
In vacuum atmosphere,the explosion reaction equations are as follows:
R1:
C3H6N6O6Al6.395→0.122CH4+0.006CO2+1.773N2+2.586CO-+1.971H2+0.402H2O+1.284C+1.665Al2O3+3.065Al(610.43-933 K)
R2:
C3H6N6O6Al6.395→0.170H4+0.005CO2+2.125N2+2.981CO+3.446-H2+0.119H2O+0.842C+1.602Al2O3+3.191Al(481.13-933 K)
R3:
C3H6N6O6Al6.395→0.136CH4+0.003CO2+1.563N2+2.089CO-+2.336H2+0.372H2O+1.769C+1.843Al2O3+2.710Al(633.89-933 K)
R4:
C3H6N6O6Al6.395→0.116CH4+0.004CO2+1.514N2+2.186CO+1.908-H2+0.406H2O+1.691C+1.798Al2O3+2.798Al(617.93-933 K)
Under air conditions,in addition to the RDX and Al on the left side of the reaction equation,there is oxygen present by air,the oxygen volume is converted to the number of moles added to the left side of the equation.The explosion reaction equations are as follows:
R1:
C3H6N6O6Al6.395+0.0518O2→0.135CH4+0.006CO2+1.664N2-+2.142CO+3.484H2+0.386H2O+1.715C+2.499Al2O3+1.396Al(644.01-933 K)
R2:
C3H6N6O6Al6.395+0.0518O2→0.147CH4+0.003CO2+1.787N2-+2.542CO+3.183H2+0.282H2O+1.306C+2.403Al2O3+1.589Al(623.99-933 K)
R3:
C3H6N6O6Al6.395+0.0518O2→0.133CH4+0.002CO2+1.546N2-+2.277CO+2.690H2+0.740H2O+1.586C+2.337Al2O3+1.720Al(623.99-933 K)
R4:
C3H6N6O6Al6.395+0.0518O2→0.122CH4+0.002CO2+1.299N2-+2.231CO+2.447H2+0.438H2O+1.642C+2.452Al2O3+1.491Al(706.57-933 K)
Yang[16]et al.used a German testo 350 sensor to detect the explosion gaseous products and calculated the explosion reaction equations,but the sensor only detected CO2and CO.Compared with his experiment,the quantitative detection of gases by gas chromatography is much more accurate and sensitive.Therefore,the explosion reaction equations calculated by this method are more reliable and are valuable as references for theoretical calculations.
4. Conclusion
(1)In a confined environment,the quasi-static pressure generated by an explosion occurring in air is higher than that occurring in vacuum.In vacuum,the quasi-static pressures of the samples in descending order are R1>R3>R4>R2.In air,the quasi-static pressures in descending order are R1>R2>R4>R3.Four aluminum powders with different gradations were prepared,and their effect on the explosion field equilibrium temperatures in vacuum and in air are R3>R4>R1>R2 and R1>R4>R2>R3 respectively.Samples of R4(Al6+Al flake)and R3(Al6+Al26)have relatively higher temperatures after detonation,which shows that the particle gradation method can enhance the reaction energy output of aluminum during the initial reaction stage of the explosion.
(2)The completeness of the reactions of the four aluminum powder samples in vacuum and in air are R3>R4>R1>R2 and R1>R4>R2>R3,respectively.In vacuum,after gradating the aluminum powder composed of Al24 with the aluminum powder composed of Al6 and gradating the aluminum powder composed of the aluminum flake with the aluminum powder composed of Al6,the reaction ratio of the aluminized explosive in vacuum increased by 10.6%and 8.0%,respectively.In air,the reaction ratio of the Al6 aluminum powder can reach as high as 78.16%,and the reaction ratio is slightly reduced after particle gradation.
(3)The reaction equation of the explosives in vacuum and in air is calculated by quantitative analysis of the explosion products(see section 3.3),which provides a powerful basis for the research of RDX-based explosive reactions.
Acknowledgement
This work was supported by National Natural Science Foundation of China(Grant no.11502194).
杂志排行
Defence Technology的其它文章
- Round robin using the depth of penetration test method on an armour grade alumina
- Sonochemically assisted synthesis of nano HMX
- FEM analysis and experimental investigation of force and chip formation on hot turning of Inconel 625
- Study on liquid-filled structure target with shaped charge vertical penetration
- Thermal decomposition of ammonium perchlorate catalyzed with CuO nanoparticles
- Investigating the dynamic mechanical behaviors of polyurea through experimentation and modeling
