Sonochemically assisted synthesis of nano HMX
2020-01-07HemaSinghNileshJahagirdarShaibalBanerjee
Hema Singh,Nilesh Jahagirdar,Shaibal Banerjee
Department of Applied Chemistry,Defence Institute of Advanced Technology(DU),Girinagar,Pune,411025,India
ABSTRACT Nanotechnology has played an influential role in improving the energetic content without subsiding the performance of high energy materials in the current era.In this work,HMX(octahydro-1,3,5,7-tetranitro-1,3,5,7-tetrazocine)nanoparticles were prepared by sonochemically assisted solvent-antisolvent spray technique focussing the reduction in its size so as to improve its energetic properties.In order to fabricate nano HMX various parameters such as different solvents and temperature were investigated.Sonication is one of the strategies recently explored in this regard;so time dependent study of sonication using probe sonicator was performed.It has been postulated that bubble formed during sonication when collapses generate high temperature and many nucleation sites which leads to the formation of uniform spherical particles with small size and fast transition phase.XRD studies depicted phase transformation from α to β as a result of sonication.The TEM images revealed that the rise in the sonication time resulted into decrease in the particle size from 300 to 10 nm.Differential scanning calorimetry(DSC)was employed to determine the heat release of the samples and enhancement in the heat release with the decrease in the particle size.A decrease in the spark sensitivity was observed from 2J(regular HMX)to 50 mJ(nano HMX).
Keywords:Sonication Spray technique Nano HMX Probe sonicator Impact sensitivity
1. Introduction
High energy materials are continually being explored with the intention to enhance their explosive parameters ranging from density,stored energy and transportation etc.One of the most promising compounds holding significant advantages in these aspects is HMX (Octagon). HMX or octahydro-1,3,5,7-tetranitro-1,3,5,7-tetrazocine(C4H8N8O8)is a nitramine based explosive and due to its higher molecular weight,it is one of the most potent explosives to be manufactured[1-4].It has a higher velocity of detonation(VoD)(9100 m s-1Vs 8700 m s-1for RDX Vs 6900 m s-1for TNT).Moreover,it has a detonation pressure higher than conventional explosives(38.7 GPa Vs 34 GPa for RDX Vs 21 GPa for TNT).Its higher density(Specific Gravity:1.91 Vs 1.78 of RDX Vs 1.60 of TNT)[5]permits better loading of explosives especially when volume of the weapons is restricted.Having defined with above advantages,still it has poor sensitivity to impact,friction and electrical sparks thus forestall its use in military and civilian sector[6,7].
In the present era of nanotechnology,it is perceived that the reduction in size of particles lowers the sensitivity of the energetic materials without hampering the energetic content of the materials[8].Consequently,preparation of nano size HMXs is an area of current research in the development of new high-energy materials.Few strategies involved in the fabrication of nanoparticles are solvent-antisolvent[9],recrystallization process[10],spray flash evaporation[11,12],ultrasonication[13]and mechanical demulsification shearing[14].Yongxu[15],Bayat[16]and Kumar[17]sprayed the acetone solution of HMX in a non-solvent(water)to control the mean size of the HMX crystals and thus,this approach seems to be the simplest technique for tuning the size of these nanoparticles. In this process, the solution containing organic compound is sprayed under pressure into an antisolvent(water)which generates high supersaturation resulting in precipitation due to dissociation of the hydrophobic active compound.
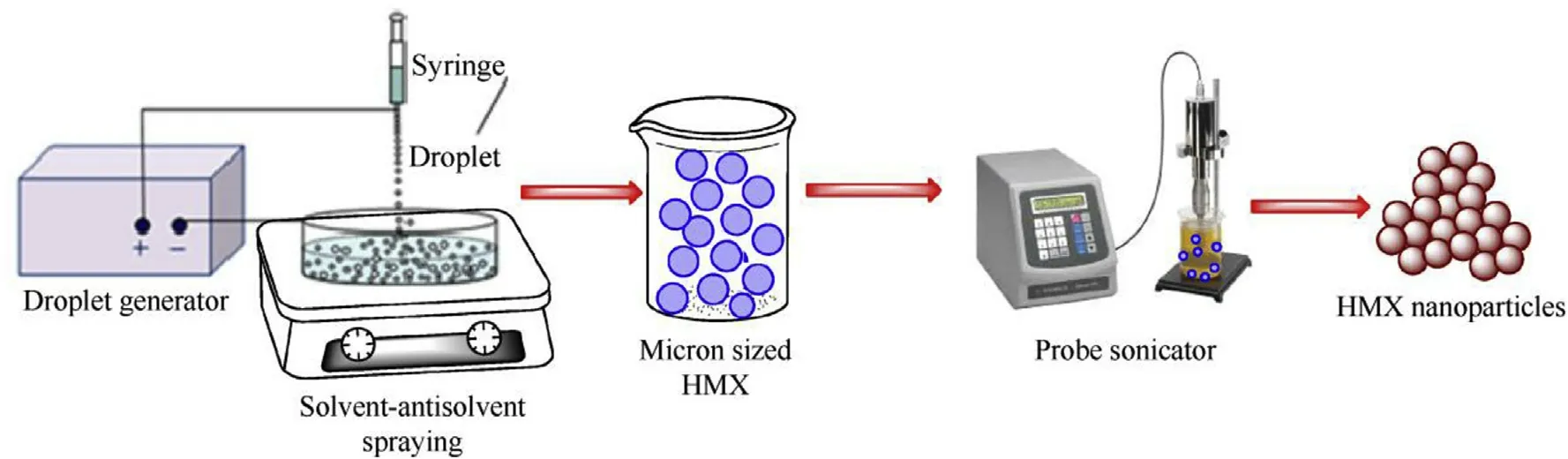
Scheme 1.Schematic preparation of nano HMX.

Fig.1.SEM of HMX particles prepared in different solvent:(a)Acetone,(b)DMSO and(c)DMF.
The spraying may be an interesting method but it usually synthesize micron sized particles and accounts towards the agglomeration and non uniformity of fine particles.Thus,necessity of exploring the techniques which would leads to uniformity and mass transfer in the solution more rapidly than the conventional methods.Ultrasonication finds its inevitable application in synthesis and modifications of nanomaterials.High intensity ultrasound generates large number of bubbles which grows,collapses and produces hot spot with a high transient temperature(5000°C)and pressure(500 atm).The rapid increase in pressure and temperature causes variation in the concentration of solution and temperature,thereby favours the lowering of nucleation[18-22].Although there are reports in which bath sonicator is explored for reducing the size of materials however it has certain limitation such as uneven and uncontrollable cavitations in the bath.While in probe sonicator whole material is processed by same intensity.The power and duration of sonication could be varied so as to tailor the crystallite size distribution.
In this work, nano HMX were prepared by a simple reprecipitation method using solvent and antisolvent method assisted by probe sonicator.The effects of changing experimental parameters such as different solvents,temperature of antisolvent during injection and time of sonication on particle size are accounted.It also includes the effect of the ultrasonically generated acoustic cavitation phenomenon on the solvent-antisolvent process.The size and morphology of these particles were characterized by field emission scanning electron microscopy(FESEM)and TEM.
2. Experimental
2.1. Chemicals
Dimethyl sulphoxide(DMSO),acetone, dimethyl formamide(DMF)of AR grade was purchased and use as supplied without any purification.Ultra-pure water(18.2 MΏ⋅cm)from double stage water purifier(ELGA,PURELAB Option-R7)was used throughout.
2.2. Synthesis of HMX
Accurately weighed HMX was dissolved in three different solvents i.e.DMSO,DMF and acetone up to saturation level.The respective solutions were then quickly sprayed into distilled water(antisolvent)at room temperature through a needle HD 130 having a micronic sized pore.A standard laboratory pump was used to ensure sufficient pressure for the injection.This was done separately for each solvent.
The precipitate of HMX was allowed to settle and the excess water-DMSO/DMF/Acetone solution was drained off.The resulting HMX particles were centrifuged.The three samples that resulted from this experiment were then given for SEM analysis.The size of the HMX particles was not found to be in nano regime from above experimental combinations.Therefore,in order to ensure the formation of nano-sized HMX particles,the following modification was made:Water used for further experimentation was maintained at 5°C instead of room temperature.This was done in order to ensure faster attainment of saturation of water-DMSO solution causing faster precipitation of HMX particles by DMSO being dissolved in water.Further,the water volume was restricted to 50 ml and this was agitated in a Petri dish using a magnetic stirrer at 750 rpm.This solution containing HMX particles suspended in DMSO-water system was sonicated in a probe sonicator with a frequency of 20 kHz and a potency of 750 W for duration of 10,30 and 60 min.This was consisted of multiple cycles,each having sonication of 20 s duration followed by a gap of 5 s.The sonication was at 20 kHz and 750 W.A blank sample without sonication was also prepared(HMX-ws)(see Scheme 1).
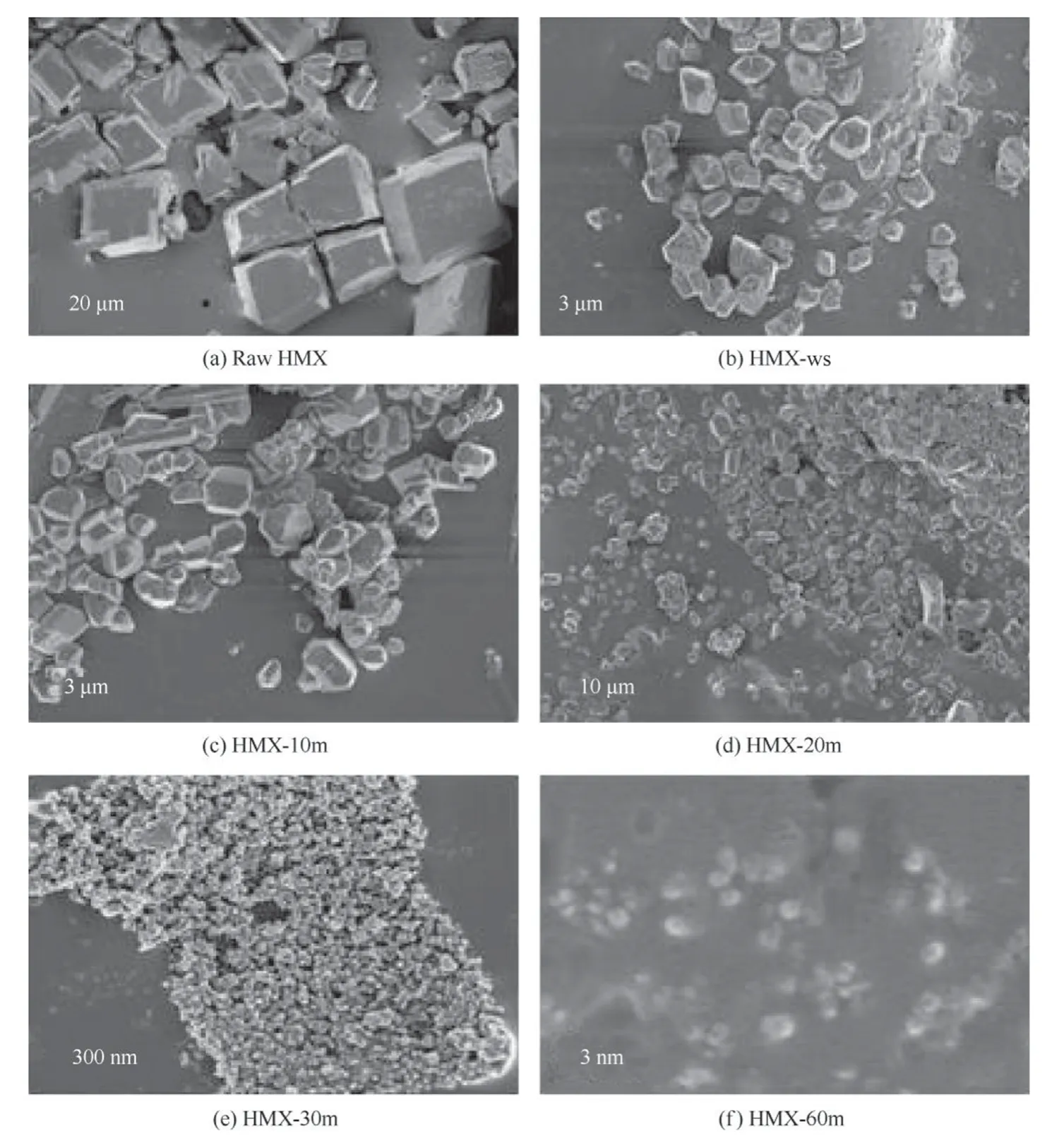
Fig.2.SEM of(a)raw HMX(b)HMX-ws,(c)HMX-10m,(d)HMX-20m,(e)HMX-30m and(f)HMX-60m.
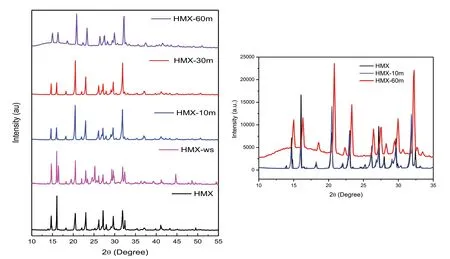
Fig.3.XRD of HMX,HMX-ws,HMX-10m,HMX-20m,HMX-30m and HMX-60m.Inset shows data an expanded form of HMX,HMX-10m and HMX-60m.
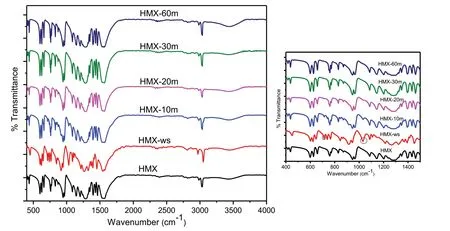
Fig.4.FT-IR of HMX,HMX-ws,HMX-10m,HMX-20m,HMX-30 m and HMX-60m.Inset shows data on expanded form.
2.3. Characterizations
The structural phase analysis of the samples was carried out using Bruker advanced TA with Cu-Kα radiation(λ=1.5406 A°)XRay Powder diffractrometer.The FTIR spectra was recorded by PerkinElmer Carl Leiss Field emission scanning electron microscopy(FESEM)(Sigma 03-18,Germany)was employed to study the morphology of the samples.The TEM measurements were performed using Philips CM-200.The thermal analysis was studied out with PerkinElmer,Pyris DSC-7.The DSC experiments were performed in nitrogen atmosphere with a flow rate of 50 ml min-1and a heating rate of 10 K min-1.The TGA was performed with PerkinElmer STA 6000 in nitrogen atmosphere.The sensitivity of the samples to impact stimuli was determined by the BAM hammer method.
3. Results and discussion
3.1. Effect of organic solvent on the solvent-antisolvent process

Fig.5.TGA curves of HMX,HMX-ws,HMX-30m and HMX-60m
The SEM of HMX samples prepared in presence of acetone,DMSO and DMF-water as solvent-antisolvent using spray technique at room temperature are presented in Fig.1.The particles obtained in presence of acetone depicted rod like morphology along with the spherical particles unlike the particles prepared using DMSO and DMF thus, showing mixed morphology. These results are in agreement with Kim et al. [23] work which reports that the morphology of HMX particles is greatly affected by the organic solvent.The particle size of these particles was observed to be varying widely in the range of 1-10 μm hence,we concluded that the aim of preparing nano HMX was not achieved.
3.2. Effect of time dependent sonication studies
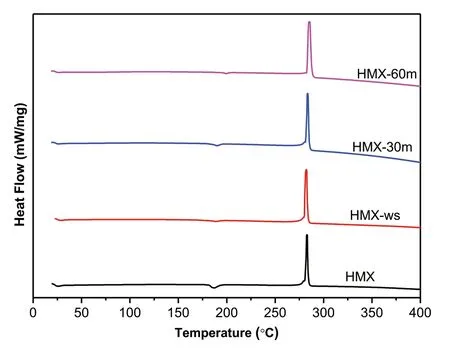
Fig.6.DSC curves of HMX,HMX-ws,HMX-30m and HMX-60m
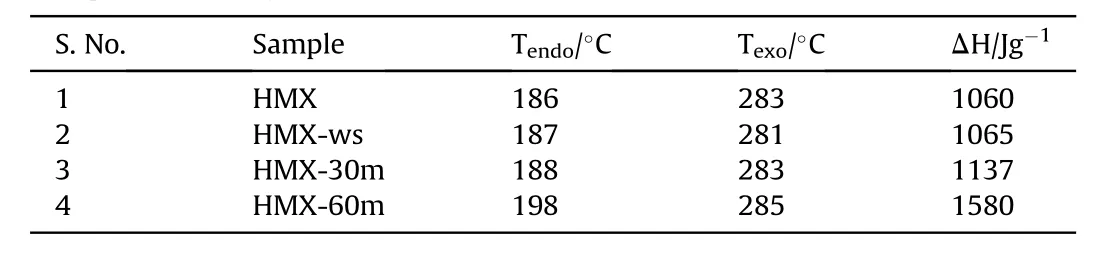
Table 1DSC phenomenological data.
Further,the solvent DMSO was chosen since the solubility of HMX in DMSO was found to be higher in comparison to that in other solvents.The temperature of the antisolvent(water)was lowered to 5°C.The solution containing HMX particles suspended in DMSO-water system was sonicated for 10 min,20 min,30 min and 60 min.Fig.2 illustrates the SEM of raw HMX(HMX),sprayed and without sonicated(HMX-ws)and sonicated for 10 min(HMX-10m),20 min(HMX-20m),30 min(HMX-30m)and 60 min(HMX-60m).The SEM images of all the samples prepared in DMSO indicate cube-like morphology but with different sizes.The raw HMX was found to be 300-450 μm in size while when it was sprayed in cold water the size was reduced to 1-3 μm.Further,the sprayed sample was probe sonicated for 10,20,30 and 60 min and the approximate sizes obtained are 1-2 μm,1-1.5 μm,90-60 nm and 10-40 nm respectively.It was observed that increase the time of sonication,leads to decease in the size of HMX crystals which can be attributed to the bubbles formed during sonication when collapses generate high temperature and creates many nucleation sites which lead to decrease in agglomeration and enhance the controllability of the crystallization process[24].Thus,by tailoring the ultrasonic duration with proper concentration and temperature,the size of the particles would be reduced.The size and the morphology were further confirmed by TEM analysis.
3.3. Composition analysis of HMX samples
HMX crystals exist in four different polymorphs:α(orthorhombic),β(monoclinic),γ(monoclinic)and δ(hexagonal)phases[25].β-HMX is the most preferred from military point of view due to its highest density,impact sensitivity and detonation velocity in comparison with other polymorphs[26].The XRD pattern of raw HMX shows peaks with 2θ values at 13.84°,14.82°,16.16°,20.55°,22.99°,26.17°,29.66°,31.88°and 32.11°which are assigned to(220),(040),(111),(131),(400),(080),(171),(022)and(202)planes respectively for α-HMX(JCPDS no.00-025-1748).Similarly,XRD pattern of HMX sprayed in presence of DMSO(HMX-ws)was obtained but the peak at 2θ=16°seem to split into two peaks(2θ=16.00°and 16.32°)along with some additional peaks.This indicate that along with α-HMX some amount of γ-form may also be present which is consistent with the Lee et al.[27]report which states that the gamma polymorph is obtained by cold antisolvent precipitation.On the other hand,XRD of samples sonicated at 10 min,30 min and 60 min samples depict peaks at 2θ=14.8°,16.0°,18.33°,20.57°,21.83°,22.81°,26.15°,27.00°,29.66°and 31.83°corresponding to(011),(020),(110),(02),(12),(120),(012)(031)(020) and (32) Miller indices for β-HMX form (JCPDS no.45-1539).Soni et al.have reported that converting fine particles of γ to β form is a great challenge.The polymorphic transformation is reported in the literature by heating or pressing of pellets of nanosize materials[28].In another work,Lee et al.have found that β-HMX would be formed by recrystallizing HMX from acetone[29].In the present case,we have found that ultrasonication resulted in the phase transformation.The slight increase in the width of the peaks of 60 min sonicated sample(Fig.3(inset))implies a reduction in the particle size.
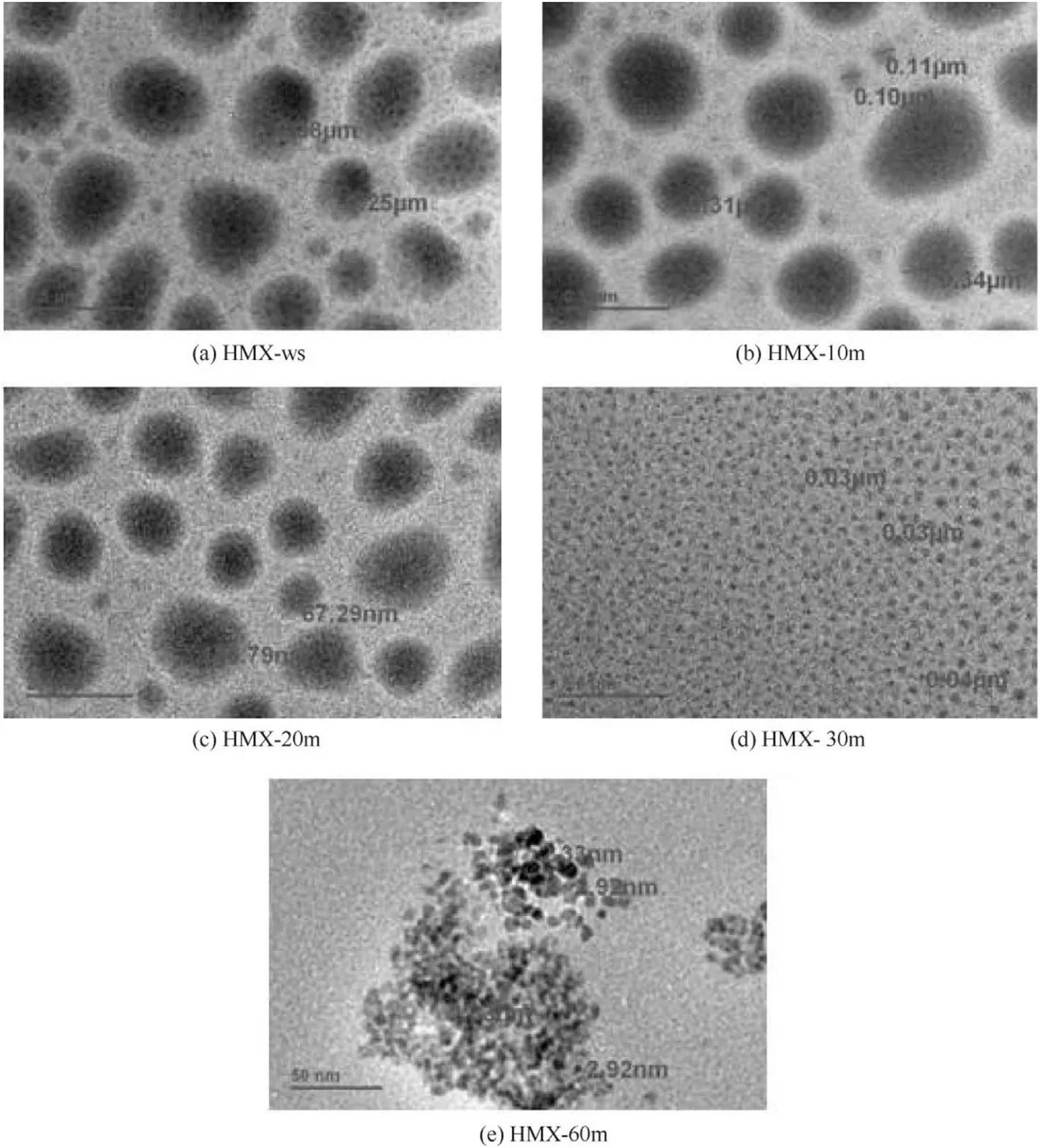
Fig.7.TEM of(a)HMX-ws,(b)HMX-10m,(c)HMX-20m,(d)HMX-30m and(e)HMX-60m.
The FT-IR analysis of raw HMX,HMX-ws,HMX-10m,HMX-20m,HMX-30m and HMX-60m are shown in Fig.4.The major IR bands for HMX samples are at 1564 cm-1, 1145 cm-1, 964 cm-1and 946 cm-1, 843 cm-1and 761 cm-1, 625 cm-1and 600 cm-1assigned to the characteristic vibrations of νsNO2,νsNO2ν ring,ring stretching band,δ and γ-NO2and τ+γ NO2respectively[17].It is noteworthy that the sample HMX-ws shows peaks at 1027 cm-1and 709 cm-1typical signatures corresponding to the γ polymorph of HMX which is consistent with the XRD data.Further,absence of these peaks in sonicated samples confirms the formation of β-form of HMX[30].Additionally,a peak 1140 cm-1is observed in HMX-ws indicating the presence of residual DMSO which on sonication fades away.
Kinetic crystallization such as spray method produces mostly metastable phases.In case of HMX,gamma form is the kinetically stable form that is formed during spraying onto water.Further,when it is ultra sonicated in a medium which contains DMSO,it can assist desolution of gamma phase and appearance of thermodynamically stable beta phase.This is a solution mediated phase transformation[31].
3.4. Thermal decomposition studies of HMX samples
Thermal study is a vital parameter from the energetic perspective as it elaborates the energy liberated by the material.The TGDSC of raw HMX,HMX-ws,HMX-30m and HMX-60m were performed.It is noted in the TGA graph(Fig.5)that the decomposition temperature of the 60 m sonicated sample is lower than the other samples,which can be ascribed to nano size of HMX-60m sample.The HMX-ws sample shows that percentage of residual is high which may be due to the presence of absorbed DMSO in the sample which is consistent with the FT-IR results.The DSC thermal curve of HMX shows first endothermic peak at 189°C which is due to the phase transformation,small second endothermic peak at 278°C corresponds to melting of HMX followed by an exothermic decomposition at 287°C(see Fig.6).
The corresponding phenomenological data is summarized in Table 1.In the DSC thermal curve,the endothermic phase transformation peak in HMX-60m sample is observed at higher temperature(198°C)as compared to raw HMX(186°C).This result shows that the phase transformation takes place at high temperature with the reduction of particle size. Another interesting finding was an increase in heat release(ΔH)with the increase in the sonication time was observed from DSC.This implies that simple ultrasonic strategy can play an important role in increasing the heat release and alter the thermal parameters of the sample.
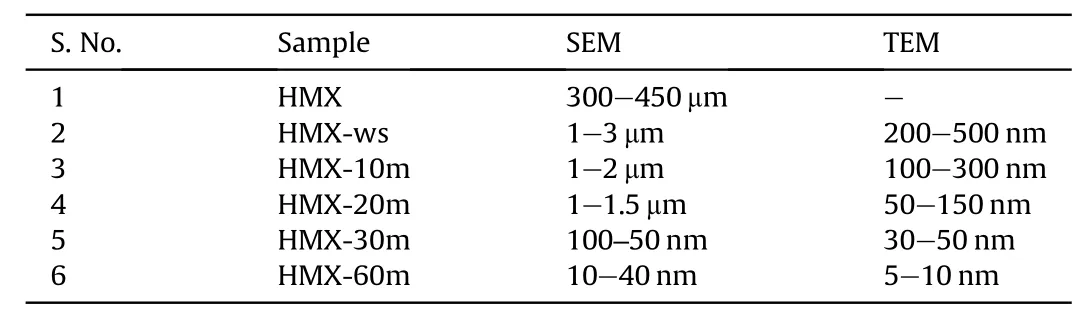
Table 2Sizes of samples by SEM and TEM.
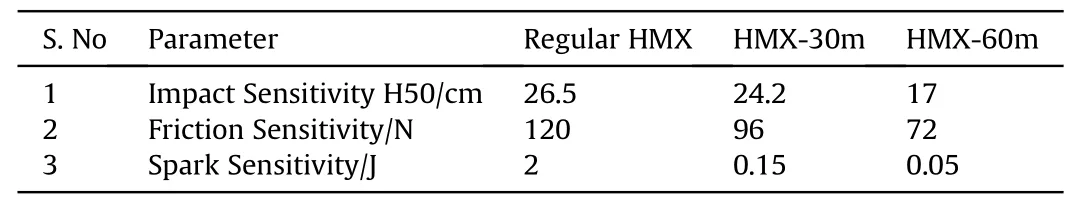
Table 3Sensitivities of nano HMX and regular HMX.
3.5. Size dependent studies of HMX samples
The TEM micrographs of HMX-ws,HMX-10m,HMX-20m HMX-30m and HMX-60m are displayed in Fig.7.Presence of spherical particles can be observed from the micrographs of all the samples.The size of the sample obtained by spraying in presence of DMSO is 200-500 nm but when the sample was sonicated at different duration of time a decrease in size is observed.The sizes of the particles are presented in Table 2.The TEM analysis also confirmed that sonication reduces nano cluster formation.
3.6. Sensitivities of nano HMX sample and regular HMX
Further,the impact,friction and spark sensitivity of nano HMX(HMX-30m and HMX-60m)and regular HMX were investigated and the results are tabulated in Table 3.It was revealed that the friction and spark sensitivity were remarkably reduced with the decreasing of the crystal size.The reduction in the friction sensitivity is in line with theoretical postulates and similar results are reported in the literature also for HMX[32]and RDX[33]while the crystal entered into the nano domain.It may be attributed to the fact that decrease in the size reduces the internal defects but may lead to enhancement in surface imperfections.While,a decrease in the spark sensitivity may be ascribed to high surface area of the nanomaterials which can be easily stimulated[34].
4. Conclusion
We have successfully developed a synthetic path for preparing HMX nanoparticles using solvent-antisolvent assisted probe sonication process.The study includes the effect of the ultrasonically generated acoustic cavitation phenomenon on the solventantisolvent process.The observed results demonstrated the time dependent effect of sonication on the reduction of crystal size(300-10 nm).It was noteworthy to find that sonication resulted in a phase change from α to β form of HMX.A signifciant enhancement in the heat release was noted in the DSC thermogram due to sonication.In the end ultrasound technique owing to its advantages in the production of crystals with improved habit and reduced size marks the key area of research which can potentially be explored industrially. Sensitivity measurement was performed and a decrease in the spark sensitivity was observed from 2J(regular HMX)to 50 mJ(nano HMX).
Acknowledgments
The authors thank the Vice Chancellor,DIAT,for giving us support towards the publication.We thank ER&IPR,DRDO,New Delhi for funding the project “DRDO-DIAT Programme on Nanomaterials”.Authors are also thankful to DST/DBT-BIRAC supported Venture Center,at CSIR-NCL,Pune,India for thermogravimetric analysis(TGA).
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.dt.2019.04.010.
杂志排行
Defence Technology的其它文章
- Round robin using the depth of penetration test method on an armour grade alumina
- Effect of particle gradation of aluminum on the explosion field pressure and temperature of RDX-based explosives in vacuum and air atmosphere
- FEM analysis and experimental investigation of force and chip formation on hot turning of Inconel 625
- Study on liquid-filled structure target with shaped charge vertical penetration
- Thermal decomposition of ammonium perchlorate catalyzed with CuO nanoparticles
- Investigating the dynamic mechanical behaviors of polyurea through experimentation and modeling
