Bridgman Growth and Spectral Properties of Nd3+∶YCa4O(BO3)3 Single Crystal
2019-12-23-,-,n,,-
-, -, n, , -
(State Key Laboratory Base of Novel Functional Materials & Preparation Science, Key Laboratory of Photoelectric detection Materials & Devices of Zhejiang Province, Faculty of Materials Science & Chemical Engineering, Ningbo University, Ningbo 315211, China)
Abstract:Nd3+-doped YCOB single crystal is a valuable self-double-frequency optical material applied in laser modulation technique. Nd3+∶YCOB polycrystalline powder was initially synthesized by solid-state reaction at elevated temperature. High purity Nd3+∶YCOB crystal grain was prepared from the polycrystalline powder by zone melting process. A series of transparent Nd3+∶YCOB single crystals, with 1mol%, 2mol% and 5mol% nominal Nd3+ dopant concentration, had been grown by means of vertical Bridgman method with optimized conditions. The spectral properties of as-grown crystals were characterized by measuring the absorption spectra, fluorescence spectra and fluorescence lifetime. It shows that Nd3+∶YCOB crystal wafers exhibit a strong fluorescence emission centered at 1064 nm wavelength with a fluorescence lifetime of 157-162 μs upon photonic excitation with 808 nm infrared light.
Key words:Nd3+∶YCOB; crystal growth; bridgman method; spectral property
1 Introduction
The oxyborate crystal YCa4O(BO3)3(abb. YCOB) was discovered as an excellent nonlinear optical material in twenty years ago[1-4]. YCOB single crystal had been investigated to exhibit a series of superior performances such adequate birefringence, high nonlinear optical coefficient, high damage threshold as well as its nonhygroscopic[5-8]. The unique properties enable the oxyborate crystal a promising candidate for amplifications in high-power ultra-short laser pulse and near infrared laser[9-12]. The near infrared laser with wavelength of 1.064 μm, produced by Nd3+-activated single crystals such as Nd3+∶YAG and Nd3+∶YVO, have been using as dominant solid-state laser sources in the past decades. The ion Y3+in YCOB crystal lattice can be easily replaced and near infrared laser[9-12]. The near infrared laser with wavelength of 1.064 μm, produced by Nd3+-activated single crystals such as Nd3+∶YAG and Nd3+∶YVO, have been using as dominant solid-state laser sources in the past decades. The ion Y3+in YCOB crystal lattice can be easily replaced with other rare earth ions owing to their same electric valence and close ion radius. The previous literature reported Nd3+-activated laser crystal Nd3+∶YCOB by doping Nd3+ions into the matrix crystal lattice with an appropriate ratio. It has been verified that 0.532 μm green laser can be acquired from Nd3+∶YCOB single crystal by converting 1.064 μm fundamental laser due to the self-frequency-doubling effect[13-15].
In recent years, our group has been devoting much efforts to explore the crystal growth technique so as to acquire large-size YCOB single crystals for high-power laser devices application[16-18]. Using the polycrystalline materials with stoichiometric composition, YCOB single crystals had been grown by means of the Czochralski or Bridgman method in the previous work. In this work, our group presents a two step preparation route to obtain high purity Nd3+∶YCOB crystal material for crystal growth. Nd3+∶YCOB polycrystalline powder was firstly synthesized by solid-state reaction at elevated temperature, and then the initially synthesized polycrystalline powder was subjected a vertical zone melting process so as to acquire high-purity Nd3+∶YCOB crystal grain. A series of transparent Nd3+∶YCOB single crystals with different dopant concentration had been grown by means of vertical Bridgman method. The spectral properties of as-grown crystals were characterized by measuring the absorption spectra, fluorescence spectra and fluorescence lifetime.
2 Experimental
2.1 Feed materials preparation
The reagents of CaCO3(4N), H3BO3(4N), Y2O3(4N) and Nd2O3(4N) were used as the initial materials and Nd3+-doped YCOB polycrystalline powder were synthesized by solid-state reaction at elevated temperature. According to the stoichiometric molar ratio of 8CaCO3∶6H3BO3∶(1-x)Y2O3∶xNd2O3= 8∶6∶1-x∶x, wherex= 0.01, 0.02, 0.05, the complex powder was prepared by fully mixing the weighed reagents in a grinding miller. The complex powder was added with 1mol% excess H3BO3so as to compensate the volatilization of H3BO3in the sintering process. Nd3+∶YCOB polycrystalline powder was prepared via solid-state reaction by sintering the complex powder continuously at 1250 ℃ for 24 h. Nd3+∶YCOB polycrystalline charge obtained by the solid-state reaction was further subjected to the recrystallization process in a vertical zone melting furnace so as to prepare high purity crystal grain for Nd3+∶YCOB crystal growth.
2.2 Crystal growth
Using the high purity crystal grain obtained by zone melting process, Nd3+∶YCOB single crystal was grown by vertical Bridgman process. A self-design vertical Bridgman furnace, installed with MoSi2bars as the heating elements, possess three temperature zones in the furnace chamber. i.e. high temperature zone, gradient zone and low temperature zone. A platinum crucible used in the crystal growth has a cylindrical chamber with a dimension of φ25-30×200-240 mm with a seed well of 8-10 mm in diameter at the conical bottom. The oriented crystal growth was performed by using a seed crystal with the crystallographic direction <010>installed in the seed well. 200-600 g crystal grain was charge into the crucible chamber after the seed crystal was installed in the seed well properly. After the crucible filled with crystal grain was sealed, it was placed in a suitable height in the vertical Bridgman furnace. In the crystal growth process, the temperature gradient of solid-liquid interface was adjusted at 30-40 ℃/cm under the controlled temperature of high temperature zone at 1570-1590 ℃. The crystal growth was performed through descending the crucibles at a rate of 0.3 mm/h or so, using an automatic lowering apparatus controlled with a computer. Once the growth process lasting for 10-15 d had been finished, the furnace chamber was cooled to ambient temperature at a rate of 30-60 ℃/h. As the crucible loading crystal was taken out from the alumina tube, a transparent Nd3+∶YCOB single crystal boule was obtained from the stripped crucible.
2.3 Characterizations
The crystallography phase of the polycrystalline powder and as-grown crystals were characterized using X-ray diffraction analysis, which was recorded in the 2θrange from 10° to 80° by using a Bruker D8 Focus diffractometer with Cu Kαradiation.A series of polished crystal wafers with 1.5 mm thickness were fabricated form as-grown crystal boules with different Nd3+dopant concentration. The absorption spectra of the crystal wafers were measured in the wavelength range from 400 nm to 1000 nm by a Lambda 35 UV/Vis spectrometer. The fluorescence spectra were measured under 808 nm photonic excitation by a French JY Triax 320 fluorescence spectrometer. The fluorescence decay time curve was measured by a British Scitec Model 300CD optical chopper with a pulse frequency of 20 Hz together with an Agilent's Infiniium 54833 D oscilloscope.
3 Results and discussion
3.1 Feed materials preparation
It had been discovered that the high purity polycrystalline material with stoichometric composition was essential for Nd3+∶YCOB crystal growth with high optical humogeneity. However, the polycrystalline powder synthesized by solid-state reaction usually contains the impurity compositions such as CaO, B2O3and Y2O3due to the uncompleted synthetic reaction. Using the polycrystalline powder synthesized by solid-state reaction, as-grown crystal boule usually contains much inclusions which bring out serious optical scattering. Unfortunately, so far no wet-chemical synthesis route in aqueous solution could be used to synthesize the composite oxyborate compound YCa4O(BO3)3with stoichiometric composition. In this work, the high-purity polycrystalline material with the stoichiometric composition of NdxY1-xCa4O(BO3)3could be prepared by our proposed process.
Firstly, Nd3+∶YCOB polycrystalline powder was synthesized from the initial reagents by the following solid-state reaction at elevated temperature. The complex powder was prepared basically according to the stoichiometric composition, while H3BO3was added with 1wt% excess so as to compensate for the volatilization in the sintering process. Nd3+∶YCOB polycrystalline materials were synthesized with a nominal Nd3+dopant concentration of 1mol%, 2mol% and 5mol%, where the doped ions Nd3+partially replace Y3+ions in the crystal lattice.
xNd2O3+(1-x)Y2O3+8CaCO3+6H3BO3=2NdxY1-xCa4O(BO3)3+8CO2+9H2O
Secondly, Nd3+∶YCOB polycrystalline powder obtained above was further subjected to the zone melting treatment, where the main impurities were expelled from the powder charge by the recrystallization process. In the zone melting process, the temperature around the solid-liquid interface was adjusted as high as -60 ℃/cm so as to remove the impurities effectively. Fig.1(a) shows the nearly transparent Nd3+∶YCOB crystal grain with high purity prepared by zone melting process.
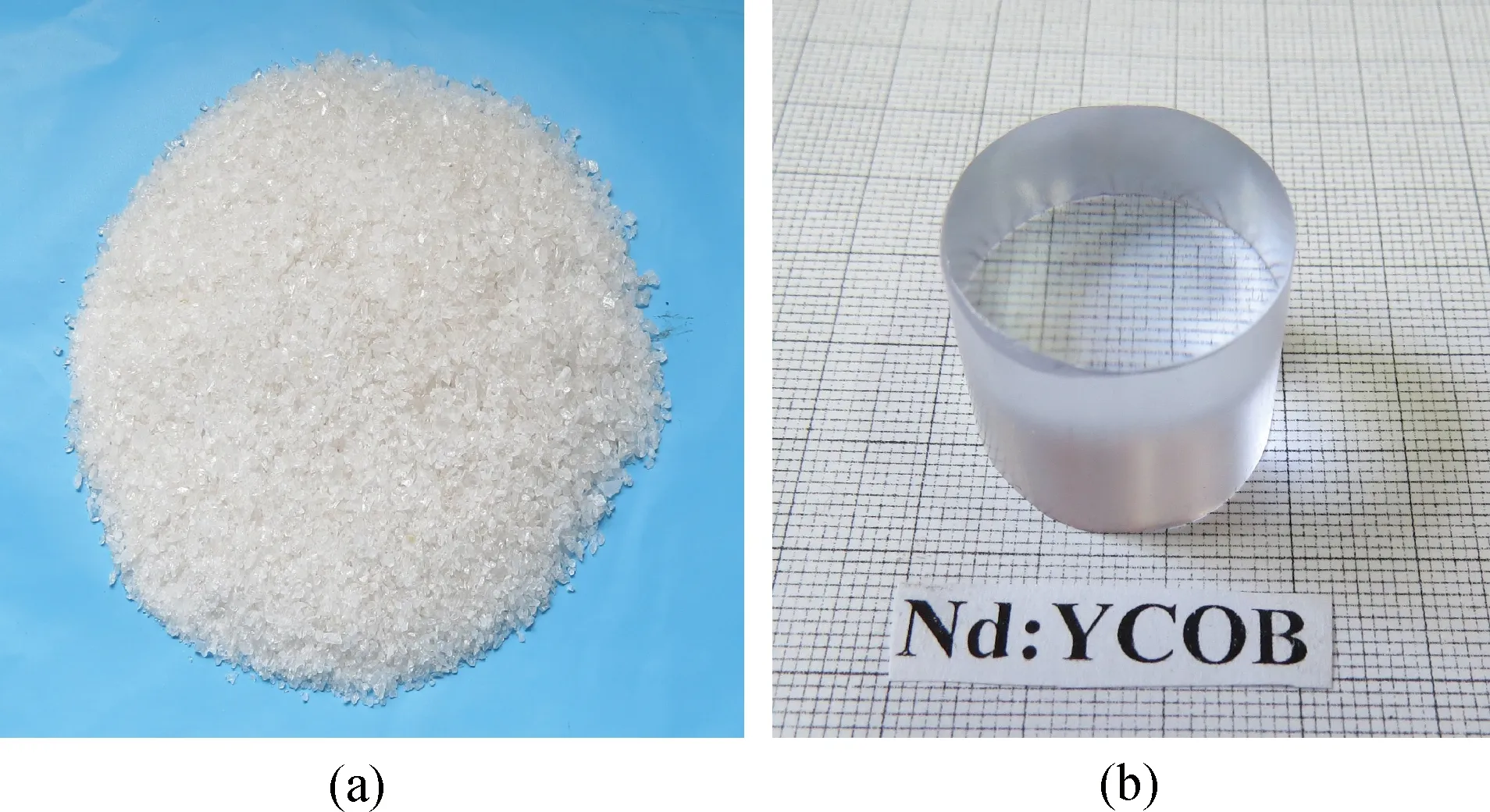
Fig.1 (a)The crystal grain prepared by zone melting process; (b)Nd3+∶YCOB single crystal grown by vertical Bridgman process
3.2 Crystal growth
Using the crystal grain prepared with crystallization process, Nd3+∶YCOB single crystal was grown by vertical Bridgman process as described above. In vertical Bridgman process, the furnace chamber was controlled at the temperature range at 1570-1590 ℃ in the high temperature zone, which was 60-80 ℃ higher than the melting point of the oxyborate crystal. The oriented crystal growth along <010> crystallographic direction was executed by using a seed crystal installed in the crucible bottom. After the furnace chamber was heated to the controlled temperature, the seeding operation was performed by adjusting the crucible to a suitable height so that only the upper part was melted together with the melts in the crucible chamber. Considering the oxyborate crystal materials possesses a higher melts viscosity, an appropriate crystallization rate was performed with a slower crucible descending rate of 0.3 mm/h or so. If the crystallization rate was carried out with too fast crucible descending rate, as-grown crystal exhibited some optical scattering caused from the inclusion inside the crystal medium. The platinum crucible charged with crystal grain was sealed so as to avoid the melt volatilization in the crystal growth.
3.3 Crystallographic characterizations
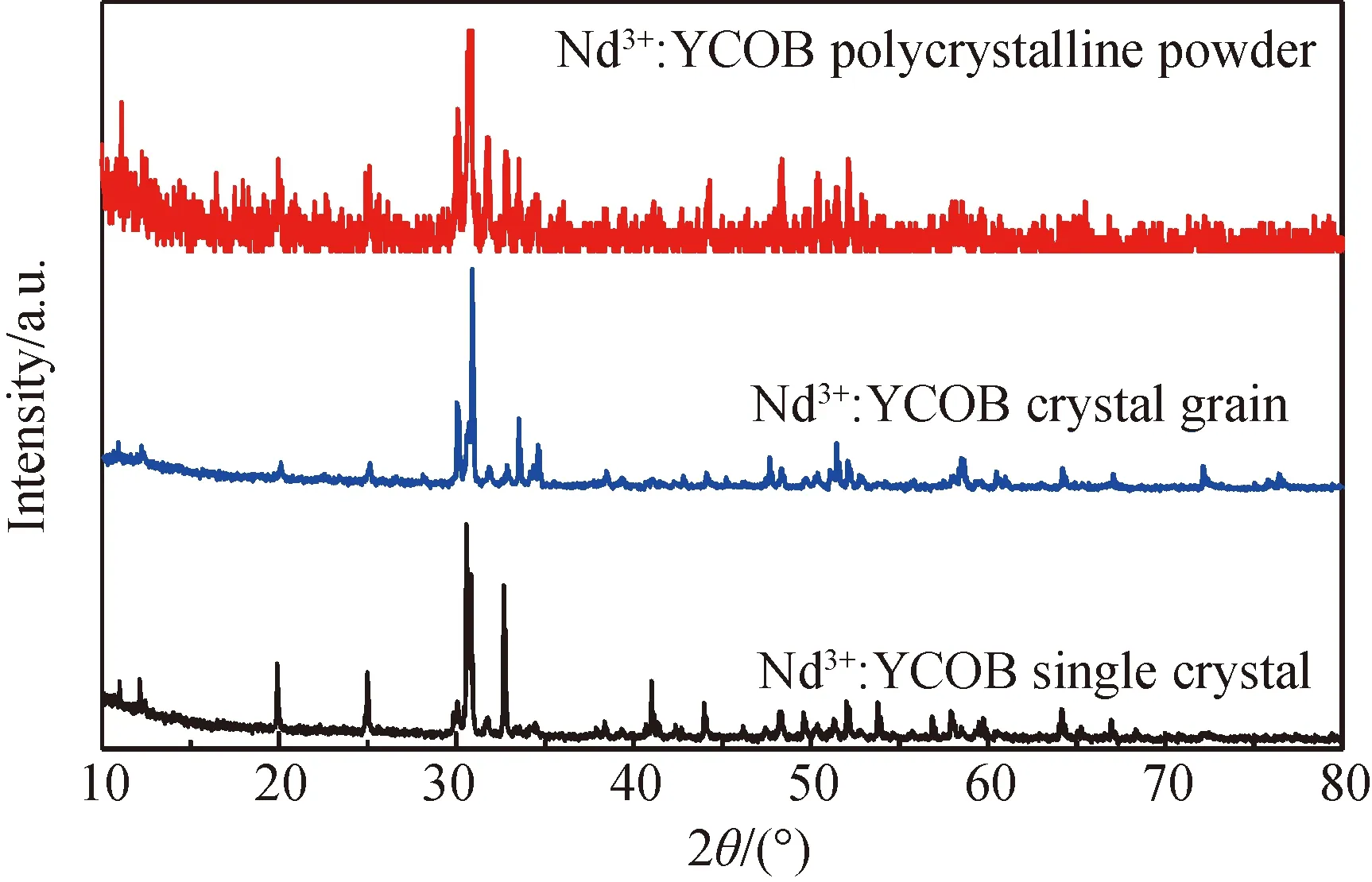
Fig.2 XRD patterns of (a) polycrystalline powder synthesized by solid-state reaction; (b)crystal grain prepared by zone melting process; (c) Nd3+∶ YCOB single crystal grown by vertical Bridgman process
Fig.1(b) shows Nd3+∶YCOB crystal sample with dimension ofφ25×30 mm obtained by vertical Bridgman process described above. The cylindrical crystal sample with light purple color exhibits an excellent optical transmission. The single crystal was examined to be free of optical scattering inside by a He-Ne laser beam. Based on X-ray diffraction rocking curve measured with (010) oriented crystal wafer, the crystallization quality of the single crystal was proved to be desirable by the fact that the crystal wafer exhibited a FWHM value less 50 arc sec. Fig.2 presents X-ray powder diffraction patterns of the three samples, i.e. Nd3+∶YCOB polycrystalline powder, the purified crystal grain and as-grown single crystal. The crystal grain and single crystal have been verified to be the compound of ReCa4O(BO3)3with monoclinic structure as the diffraction peaks of XRD pattern accords with the standard data of JCPDF-50-0403. However, XRD pattern of the polycrystalline powder shows many minor diffraction peaks, which indicates a small amount of oxide compositions such as CaO, B2O3and Y2O3remained in the polycrystalline powder.
3.4 Spectral properties
Fig.3 shows the absorption spectra measured in the wavelength range of 400 nm to 1000 nm for Nd3+∶YCOB crystal wafers doped with different Nd3+concentrations. The typical absorptions peaks exhibited in the absorption spectra prove that Nd3+ions have been doped into the crystal lattice. It can be seen that the absorption peaks intensity increases evidently as Nd3+dopant concentration increases from 1mol% to 5mol%. The absorption spectra present five strong absorption peaks in the range of 400-1000 nm, which are located around 520 nm, 580 nm, 740 nm, 800 nm and 860 nm, respectively. These absorption peaks can be attributed to Nd3+ion characteristic transitions from the ground state to the excited states of4I9/2→4G9/2+4G7/2,4I9/2→2G7/2+4G5/2,4I9/2→4S3/2+4F7/2,4I9/2→2H9/2+4F5/2and4I9/2→4F3/2. Since Nd3+ion possess a rich energy level structure with narrow energy levels gaps, the absorption peaks appear to be somewhat overlapped.
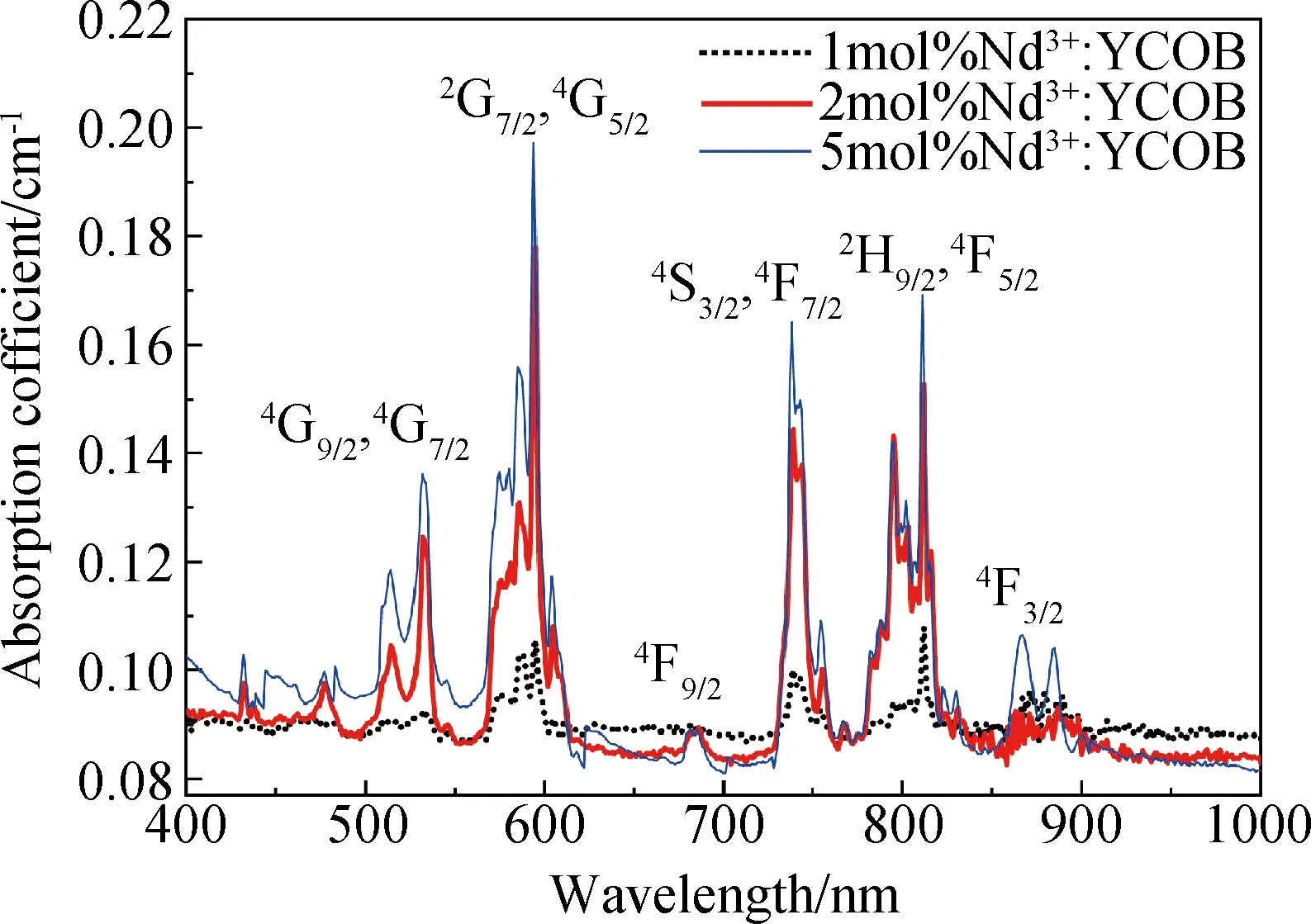
Fig.3 Absorption spectra of Nd3+∶YCOB crystal with different Nd3+dopant concentration
As the absorption spectra of Nd3+∶YCOB crystal exhibits a strong absorption peak around 800 nm, the fluorescence spectra were measured with an infrared laser centered at 808 nm as the excitation source. Fig.4 shows the fluorescence spectra measured in the range of 850-1500 nm for Nd3+∶YCOB crystal wafers with different Nd3+dopant concentration. There are three distinct fluorescence emission peaks located at 890 nm, 1064 nm and 1323 nm, which are attributed to the transition of4F3/2→4I9/2,4F3/2→4I11/2and4F3/2→4I13/2, respectively. It can be seen that the fluorescence intensity increases evidently as Nd3+dopant concentration increases from 1mol% to 5mol%. Under the photonic excitation with an infrared laser centered at 808 nm, the strong fluorescence output with a central wavelength of 1064 nm can be acquired by the transition of4F3/2→4I11/2. The energy level diagram of Nd3+ion showed in Fig.5 exhibits the transition process for 1064 nm strong fluorescence emission. As Nd3+∶YCOB crystal is pumped by 808 nm photonic excitation, the active ions Nd3+on the ground state4I9/2absorb the pump light and jump to the excited state of4F3/2and2H9/2. Once passing very quickly the non-radiative transition to the metastable state4F3/2, the active ions on4F3/2state falls to the lower level4I11/2and then returns to the ground state4I9/2via the non-radiative transition. Under 808 nm infrared photonic excitation, the fluorescence decay curves of 1064 nm emission were also measured. Fig.6 shows the fluorescence decay curves of Nd3+∶YCOB crystal wafers with 1mol%, 2mol% and 5mol% dopant concentration. According to the fitted fluorescence decay curves, the fluorescence lifetime of Nd3+∶YCOB crystals are determined to be 157-162 μs.
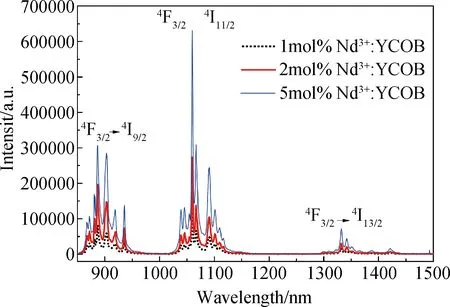
Fig.4 Fluorescence spectra of Nd3+∶YCOB crystal with different Nd3+dopant concentration under 808 nm photonic excitation
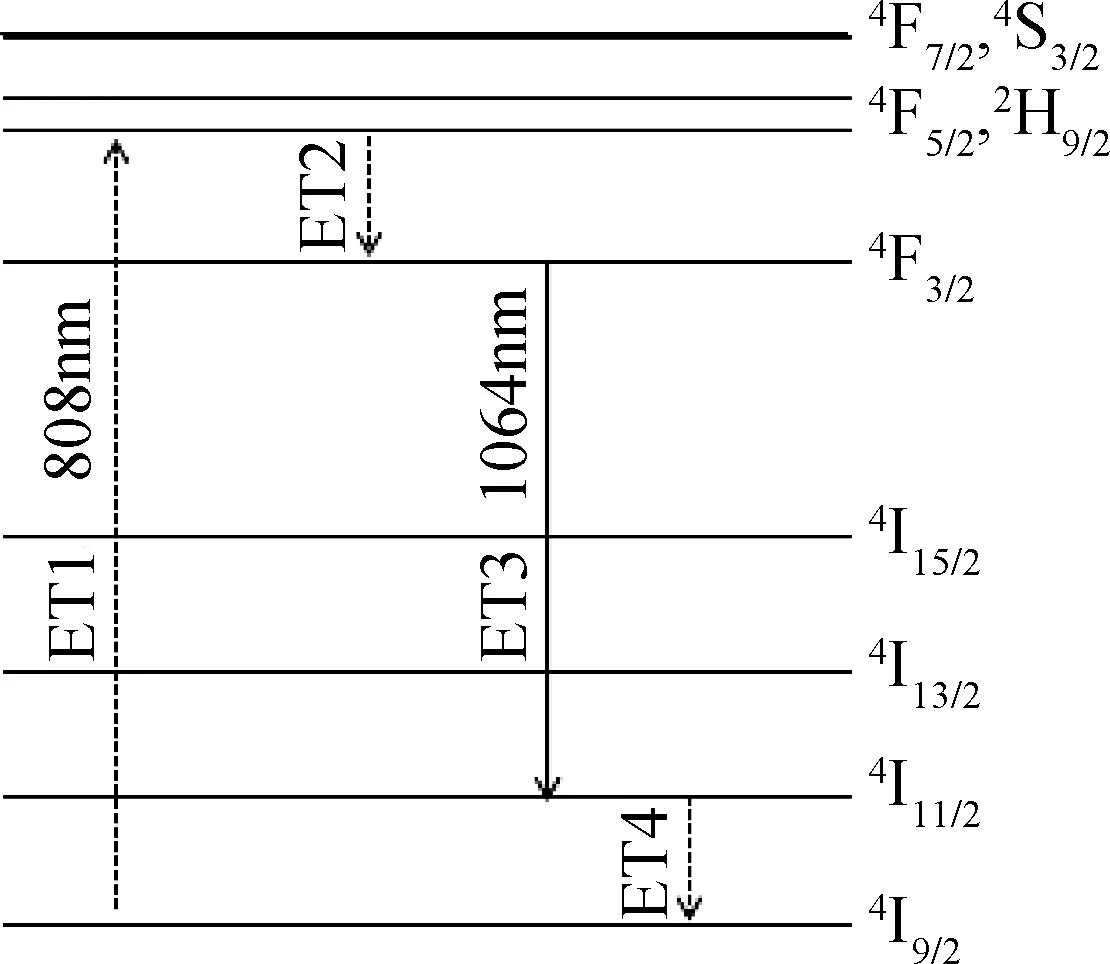
Fig.5 Energy level diagram of Nd3+ ion doped in crystal lattice
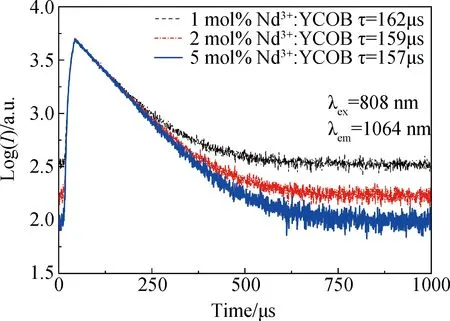
Fig.6 Fluorescence decay curves of 1064 nm emission for Nd3+∶YCOB crystal with different concentration under 808 nm photonic excitation
4 Conclusion
High purity Nd3+-doped YCOB crystal grain was prepared by zone melting process from the polycrystalline powder initially synthesized by solid-state reaction. Using the purified crystal grain, Nd3+∶YCOB single crystals with nominal Nd3+dopant concentration of 1mol%, 2mol% and 5mol% had been grown by means of vertical Bridgman method with optimized conditions. X-ray powder diffraction analysis proves that Nd3+ions have been doped into the crystal lattice and the absorption spectra exhibits the typical absorption peaks corresponding Nd3+ions. Upon photonic excitation with 808 nm infrared light, Nd3+∶YCOB single crystals produce a strong fluorescence emission centered at 1064 nm wavelength with a fluorescence lifetime of 157-162 μs. The intensities of absorption peaks and the fluorescence emissions increase evidently with the increasing Nd3+dopant concentration in 1mol%-5mol% doping range in this work.
