Platinum Decorated Titanium Dioxide Nanosheets for Efficient Photoelectrocatalytic Hydrogen Evolution Reaction
2019-12-16SUKunZHANGYaRuLUFeiZHANGJunWANGXi
SU Kun, ZHANG Ya-Ru, LU Fei, ZHANG Jun, WANG Xi
Platinum Decorated Titanium Dioxide Nanosheets for Efficient Photoelectrocatalytic Hydrogen Evolution Reaction
SU Kun1,2,3, ZHANG Ya-Ru3, LU Fei3, ZHANG Jun1, WANG Xi3
(1. School of Chemistry and Chemical Engineering, Inner Mongolia University, Hohhot 010021, China; 2. School of Pharmacy, Baotou Medical College, Baotou 014040, China; 3. School of Science, Beijing Jiaotong University, Beijing 100044, China)
Two kinds of platinum catalysts with different morphologies were prepared by loading platinum atoms on titanium dioxide nanosheetselectrostatic adsorption. Scanning electron microscope(SEM), X-ray powder diffraction (XRD) and transmission electron microscope (TEM) characterization show that the morphology and structure of platinum could be controlled by changing the platinum loading. With low Pt loading (0.2wt%), the platinum atoms were mainly nanoclusters with a radius of about 2 nm. When the Pt loading increased to 1wt%, the platinum atoms stack into nanoparticles on the titanium dioxide nanosheets. The catalytic hydrogen evolution activity of titanium dioxide nanosheets can be improved obviously by regulating the platinum loading and nanostructure. Under AM1.5 solar light, the Tafel slope of both catalysts were less than 100 mV/dec,56 and 90 mV/dec, respectively. Compared with TiO2-Pt1% catalyst, TiO2-Pt0.2% has a more ideal metal-semiconductor interface, which is favorable for photogenic electrons to migrate to the surface of platinum nanoclusters, and thus perform a higher catalytic activity. This experiment provides a new way for preparing platinum catalysts with high efficiency.
hydrogen evolution reaction (HER); platinum clusters; titanium dioxide; photoelectrocatalysis
Due to high combustion value and environmental friendliness, hydrogen is regarded as one of the cleanest energy sources on the planet[1-4]. An effective and sustainable approach of hydrogen production is water splitting into hydrogen by solar energy. In hydrogen evolution reaction(HER),suitable catalysts, especially photoelectrical catalysts play a crucial role[5]. Highly effective photoelectrochemical catalysts could contribute an extra energy conversion effect compared with individual electrochemical process.
Over the past decades, anatase titanium dioxide has been proven to be a prospective candidate for photoelectric catalysis, considering its relatively high catalytic preference, low toxicity, excellent stability, cost-effectiveness and appropriate band gap[6-9]. Meanwhile, many researchers demonstrated that the morphology characteristics of titania have a significant or even decisive impact on its catalytic performance.
Despite its high cost, platinum, still holds the state-of-art catalytic activity among hydrogen evolution catalysts. By introducing Pt related catalysts, HER can be well performed within an extremely low overpotential and Tafel slope (energetic kinetics)[10-12]. With the aim to promote HER efficiency, platinum benefited with effective electrochemical activity was integrated into efficient photocatalytic titanium dioxide, which could considerably perform a desirable photoelectrochemical catalysis. In this work, sized controlled Pt decorated TiO2was demonstrated, in which electronic state of the loaded Pt was further regulatedsize control to perform distinct catalytic performance.
1 Experimental
1.1 Synthesis of Pt modified titania nanosheets
All chemicals were of analytical grade and used without further purification. Titania nanosheets were preparedthe method reported elsewhere[13]. To decorate nanosheets with Platinum, a conventional impregnation procedure was used. Typically, 40 mg of the as-synthesized titanium dioxide nanosheets was redispersed in 10 mL DI water by ultrasonication for 30 min. 0.2wt% or 1wt% of chloroplatinic acid aqueous solution was carefully dipped into the suspension, which was kept for 2 h in order to make the platinum atoms tightly adsorbed onto the nanosheets. After centrifugal washing with DI water three times, the precipitate was collected and dried at 80 ℃ overnight in vacuum. The catalysts obtained were denoted as TiO2-Pt%, where% indicated weight percentage of platinum on surface of titania nanosheets which was determined by ICP-AES.
1.2 Characterization of the catalysts
XRD pattern was performed using a Bruker D8-Advance with Cu Kα radiation (=0.1542 nm, 40 kV, 40 mA). The morphology and structure of the obtained catalysts were investigateda Hitachi model SU-8010 SEM and JEOL-2100F high-resolution TEM operated at 10 and 200 kV, respectively. The High-Angle Annular Dark Field-Scanning transmission electron microscopy(HAADF-STEM) images were performeda Titan 80-300 scanning/transmission electron microscope FEI Titan G2 80-200 operated at 200 kV, equipped with a probe spherical aberration corrector.
1.3 Measurement of HER activity
The catalyst modified GCE (glassy carbon eletrode) was prepared through drop-casting method. Typically, 5 mg catalyst was suspended in 2 mL water/Nafion solution (9/1,/) under sonication to produce homogeneous ink, which was subsequently coated on a glassy carbon electrode and dried in an ambient environment. All photonic electrochemical experiments were conducted on a CHI660 electrochemical station with a three-electrode system in 0.5 mol/L H2SO4electrolyte. The decorated GCE (3 mm in diameter), a Ag/AgCl (with saturated KCl) electrode and a graphite rod were utilized as working electrode, reference electrode and counter electrode, respectively. The corresponding electrode potential was converted asRHE=Ag/AgCl+0.0591pH+0.197.
Xenon lamp (PLS-SXE300, Beijing Perfect Light) was utilized as the light source. Before the test, the electrolyte was stirred with a magnetic bar for 20 min to make sure the baseline run steady. During the test, a plastic lightproof baffle was put between the light and the electrode. The light was manually switched on and off every 50 s to evaluate the photoelectrocatalytic activity. The cyclic voltammetry was conducted between 0 and −0.4 VReversible Hydrogen Electrode(RHE) at a scan rate of 5 mV/s.
2 Results and discussion
The specific surface area of titanium dioxide has great influence on electron-hole separation and photocatalytic reactivity[14]. Generally, larger surface-to-volume renders the higher catalytic activity. Due to large specific surface area, nanosheets were selected for the substrates of platinum atoms in the experiment. Furthermore, platinum possessed distinct electronic state could dramatically affect catalytic performance of the composite catalyst[15-17].
2.1 SEM micrograph of the catalysts
Initially, the layered morphology of titanium dioxide could be easily demonstratedSEM, as shown in Fig. 1(A). This structure of nanosheets would render large specific surface area and thus provide a flat plane for loading platinum. Furthermore, a desired light absorbed surface could promote the light induced electron-hole conversion efficiency[18]. It should be noted that no obvious particle was recognized, illustrating that the loaded Pt dispersed in a tiny size.
2.2 XRD patterns of the catalysts
XRD was conducted to investigate the crystallinity and lattice structure of the as-synthesized catalysts. Fig. 1(B) reveals that diffraction peaks at 2=25.28°, 37.96°, 48.07°, 53.85°, 55.06° and 62.60° could be well indexed to anatase crystals (JCPDS 21-1272), attributing to crystal planes of (101), (103), (200), (105), (211) and (204) crystal planes, respectively. Apparently, Pt decoration did not change the anatase crystalline, and the crystalline of the loaded Pt was not detected, since relative low amount of Pt was introduced, and long-range order of Pt lattice was undominated.

Fig. 1 (A) SEM image and (B) XRD patterns of Pt modified TiO2
2.3 TEM micrographs of the catalysts
The high-resolution information of the corresponding catalyst was further characterized by STEM/TEM. As shown in the HAADF image, the loaded Pt (Fig. 2(A, C)) with lower amount (0.2wt%) performed as nanoclusters with a diameter less than 2 nm, while the TEM micrograph (Fig. 2(B-D)) of the higher loaded sample (1wt%) was characterized as a predominance of nanoparticles with a diameter around 5 nm. The measured interplanar-spacing of 0.265 nm can be well indexed to (111) crystal plane of crystalline Pt.
2.4 HER performance test of the catalysts
All HER performance tests were conducted at room temperature in a three-electrode cell, which was attached to a CHI-660 workstation. First, current-time curve (-curve) was collected to study the photoelectrocatalytic activity of pure titania nanosheets and platinum decorated ones. As shown in Fig. 3(A), pure titania nanosheets (TNS) exhibited the lowest HER activity in dark, compared with light irradiation condition. It is noted that the tendency of the-curves of both electrodes downwards along the time, while it stables within the irradiation. The photo-induced stabilization of the catalytic current could be attributed to the photosensitivity of TNS, in which the photo-generated electrons provided extra catalytic current and meanwhile filled the decay of the electrocatalytic current. The superior photoelectrocatalytic performance of Pt decorated TNS could be attributed to catalytic superiority of platinum. Correspondingly, a stabilized current within the light irradiation was also observed, in which the photo-generated electrons could energetically transfer to Pt sites and modify the electron cloud of the catalytic Pt[19]. The photoelectrons modified Pt presented a relative lower valence. Thus, the H-intermediates could form in a favorable kinetic path on Pt, which consequently performed a superior hydrogen evolution catalysis. Furthermore, a periodic-curve was obtained (Fig. 3(B)) as intermittent light was introduced onto the electrode. The photo-induced current did not increase immediately when the light was on. This phenomenon might be due to the abundant electronic valence states of the surface of titanium dioxide nanosheets. Photogenerated electrons were probably trapped on the surface of titanium dioxide, which reduced the lifetime of photoelectrons and lead to the delay of photocurrent increase. Furthermore, dramatically enhanced catalytic current (three-fold) of the Pt cluster decorated TNS was demonstrated than that of pure TNS, which presented a prominent light/dark-induced switch ratio.

Fig. 2 HAADF image of (A) TiO2-Pt0.2%, (B) TEM image of TiO2-Pt1%, and the corresponding high-resolution regions (C, D) in (A) and (B), respectively
To get further insight into the mechanism of photo- induced enhancement, electrochemical impedance spectrum was collected. As illustrated in the Nyquist plots (Fig. 4), the charge transfer resistance (ct) of Pt0.2% under irradiation was obviously lower than that in darkness. The reason is that the hot electrons excited from the valance band to the conduction band of TNS could be utilized as supplementary catalytic species. The lowered body resistance of the electrode in light might be attributed to the light-induced hot electrons reduce the interface resistance of the electrode/electrolyte and GCE/ catalyst interfaces. As a corollary, this could dramatically facilitate the hydrogen evolution from the cathode.
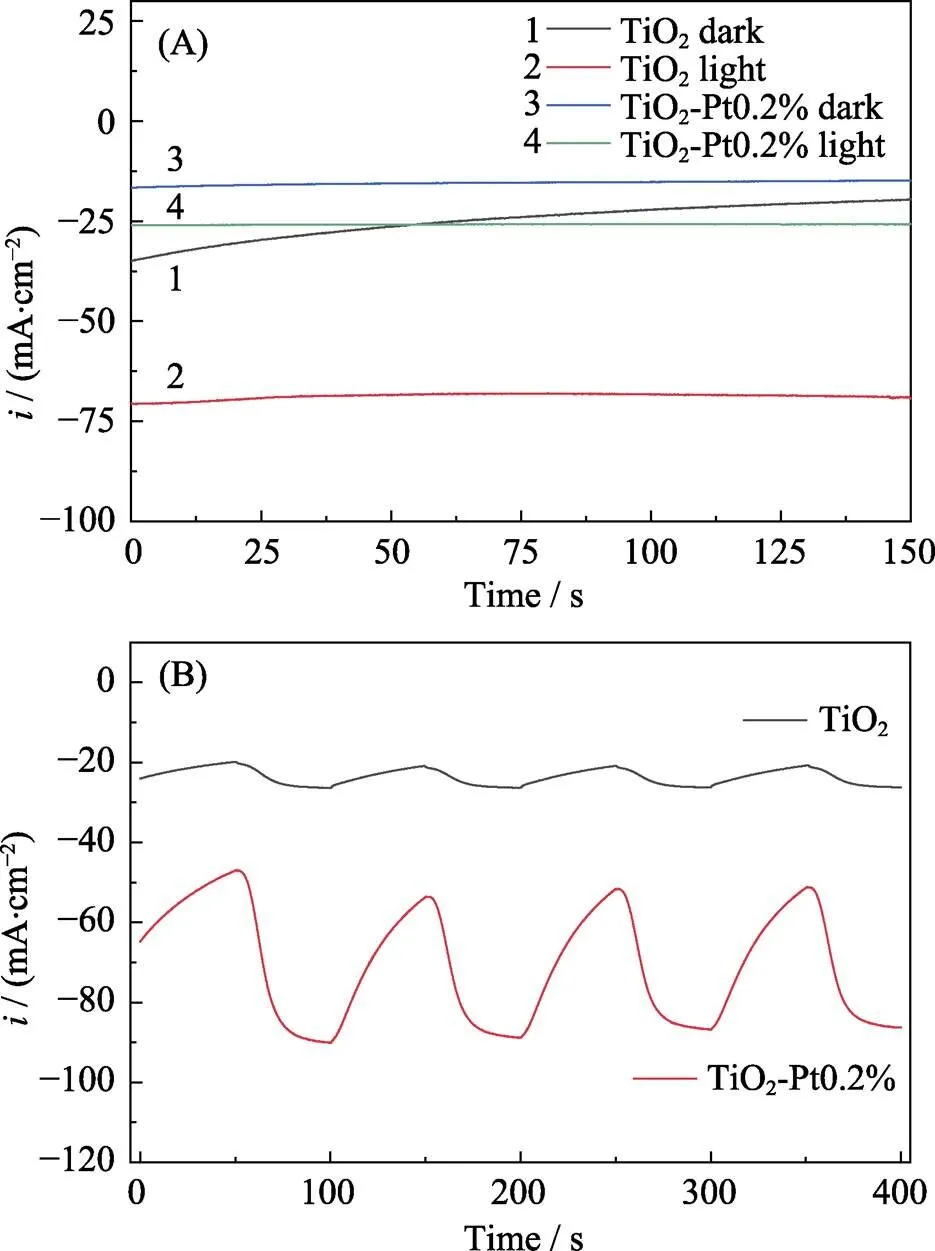
Fig. 3 (A)i-t curves of pure TNS and TiO2-Pt0.2% under continuous light irradiation or in darkness at –0.2 V external bias; (B) Periodic light irradiation induced i-t curves of pure TNS and TiO2-Pt0.2%

Fig. 4 Nyquist plots of glassy carbon electrode modified with TiO2-Pt0.2% under light irradiation (red curve) and darkness (black curve), mass loading of 0.2 mg/cm in 0.5 mol/L H2SO4
To figure out the dependence of the electrochemical performance on Pt atomic configuration, the controlled loading of Pt (0.2wt% and 1wt%) was conducted and the corresponding CV curves were shown in Fig. 5(A). Furthermore, the samples both present a mixed Heyrovsky- Volmer and Tafel-Volmer kinetics. It is worth noted that the TiO2-Pt0.2% performs a preferred Tafel-Volmer kinetics, which derived Tafel slope (56 mV/dec) is more superior than that of TiO2-Pt1% (90 mV/dec) in Fig. 5(B). It’s reasonable that the overpotential is not comparable to the commercial Pt/C, in which the Pt loading amount in TiO2-Pt0.2% was far less than the commercial one. As for TiO2-Pt0.2% and TiO2-Pt1%, these electrodes presented desired photosensitivity. The hot electrons excited from the valence band to the conduction band of TiO2under AM1.5 illumination, and further migrate to the loaded platinum sites for the more preferred hydrogen evolution kinetics; whilst, the holes fill the gap for oxygen evolution reaction on counter electrode, as shown in Fig. 5(C). Interestingly, TiO2-Pt0.2% with lower Pt loading exhibited a higher catalytic preference than TiO2-Pt1%. This is probably because that higher loading of Pt converts the cluster stacking into particles shape, which decreases the exposed catalytic Pt sites, as more Pt atoms are packaged inside[20]. Moreover, the photo-generated electrons transfercould be obstructed, since a relative lower metal-semiconductor interface ratio per Pt atoms could be demonstrated (Fig. 2). Therefore, a mass loading regulated Pt configuration could lever the photoelectrocatalytic preference in the Pt decorated TNS system[21].
3 Conclusion
In summary, titania nanosheets modified with platinum provided enhanced photoelectrocatalytic activity for hydrogen evolution reaction as compared with pure TNS. Furthermore, mass loading was conducted to regulate the atomic configuration of the induced platinum. TiO2- Pt0.2% presented a cluster configuration, while higher loading stack into the nanoparticle framework. The distinct atomic geometry levered the photoelectrochemical catalytic kinetics, and the cluster configuration presented a favored catalytic performance. These results have significant implications for the design and optimization of the atomic configuration for superior electrocatalysis or photoelectrocatalysis.
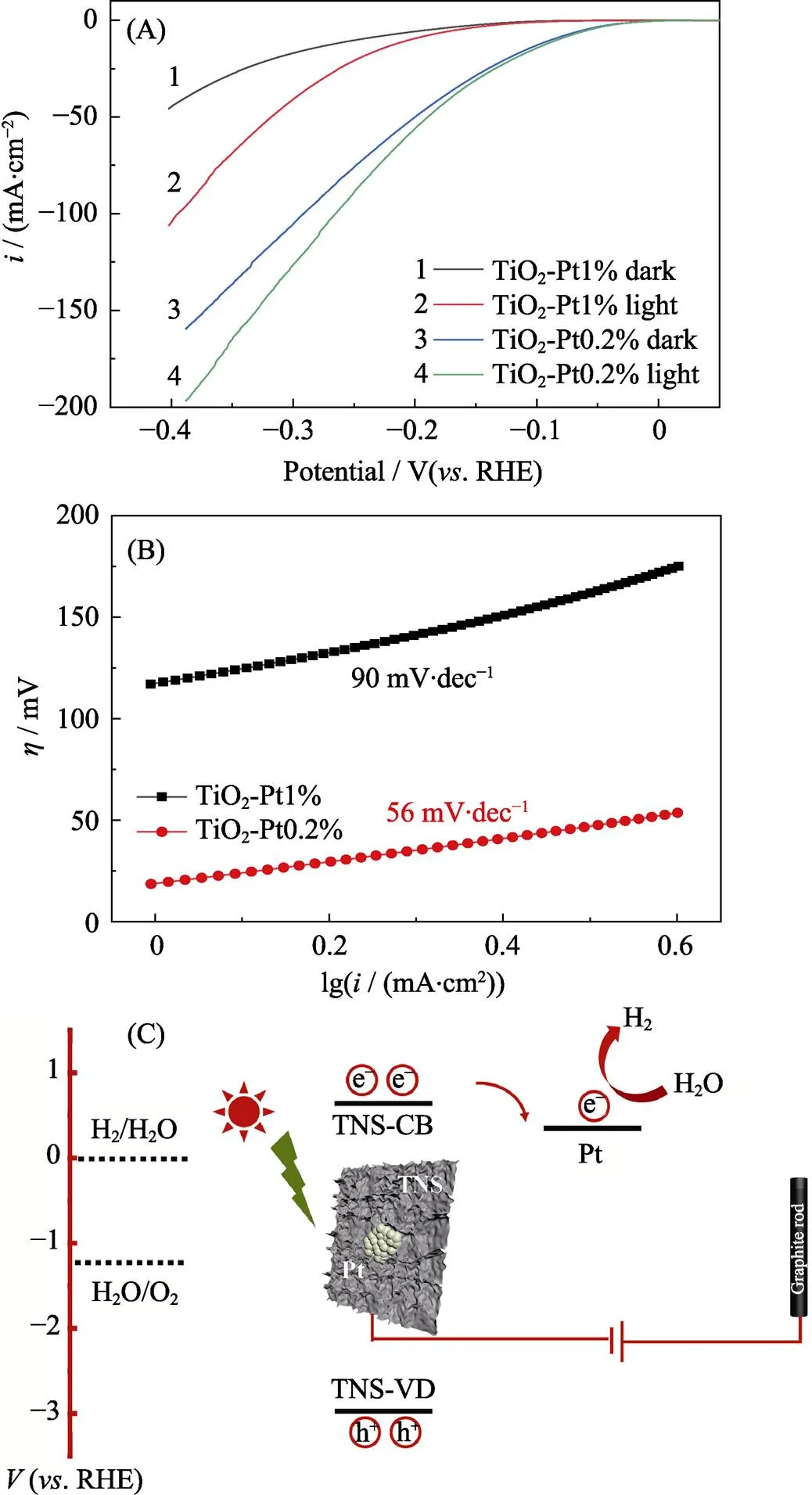
Fig. 5 (A) Linear sweep voltammetry (LSV) curves of glassy carbon electrode modified with pure TiO2-Pt0.2% or TiO2-Pt1% (mass loading of 0.2 mg/cm in 0.5 mol/L H2SO4); (B)corresponding Tafel plots; (C) Proposed schematic route of the photocatalytic process with Pt cluster decorated TiO2 nanosheets as the cathode and graphite rod as the anode
[1] KHAN M, NGO H H, GUO W S,Biohydrogen production from anaerobic digestion and its potential as renewable energy., 2018, 129: 754–768.
[2] GHOSH T, GHOSH P, MAAYAN G. A copper-peptoid as a highly stable, efficient, and reusable homogeneous water oxidation electrocatalyst., 2018, 8(11): 10631–10640.
[3] WENG G M, LI C Y V, CHAN K Y. Hydrogen battery using neutralization energy., 2018, 53: 240–244.
[4] KUMAR G, CHO S K, Sivagurunathan P,. Insights into evolutionary trends in molecular biology tools in microbial screening for biohydrogen production through dark fermentation., 2018, 43(43): 19885–198901.
[5] DING C, SHI J, WANG ZPhotoelectrocatalytic water splitting: significance of cocatalysts. electrolyte, and Interfaces., 2017, 7(1): 675–688.
[6] YANAGIDA S. Nano/microsized TiO2composite photocatalysts for environmental purification., 2018, 126(8): 625–631.
[7] TAN T L, LAI C W, HONG S L. New insights into the photocatalytic endocrine disruptors dimethyl phathalate esters degradation by UV/MWCNTs-TiO2nanocomposites., 2018, 364: 177–189.
[8] YI S S, ZHANG X B, WULAN B R,. Non-noble metals applied to solar water splitting., 2018, 11(11): 3128–3156.
[9] TAYEL A, RAMADAN A R, El SEOUD O A. Titanium dioxide/ graphene and titanium dioxide/graphene oxide nanocomposites: synthesis, characterization and photocatalytic applications for water decontamination., 2018, 8(11): 491–1–45.
[10] EFTEKHARI A. Electrocatalysts for hydrogen evolution reaction., 2017, 42(16): 11053–11077.
[11] TAN H L, DU A, AMAL R,. Decorating platinum on nitrogen- doped graphene sheets: control of the platinum particle size distribution for improved photocatalytic H2generation., 2019, 194: 85–93.
[12] WANG C, SHI H, LIU H,. Quasi-atomic-scale platinum anchored on porous titanium nitride nanorod arrays for highly effi cient hydrogen evolution., 2018, 292: 727–735.
[13] HAN X, KUANG Q, JIN M,. Synthesis of titania nanosheets with a high percentage of exposed (001) facets and related photocatalytic properties.2009, 131(9): 3152–3153.
[14] JIA L, SHU D J, WANG M. Tuning the area percentage of reactive surface of TiO2by strain engineering., 2012, 109(15): 156104
[15] MINGYAEV M E, KORCHAGINA SYA, TAVTORKIN A N,. Crystal structures of mono- and binuclear neodymium diarylphosphate complexes and their catalytic activity in 1,3-diene polymerization., 2018, 29(5): 1475–1487.
[16] NOGUEIRA L S, NEVES P, GOMES A C,. Molybdenum(0) tricarbonyl and tetracarbonyl complexes with a cationic pyrazolylpyridine ligand: synthesis, crystal structures and catalytic performance in olefin epoxidation.,2018, 8(29): 16294–16302.
[17] RAMADAN A E M M, IBRAHIM M M, E L MEHASSEBY I M. New mononuclear copper(I) and copper(II) complexes containing N-4 donors: crystal structure and catechol oxidase biomimetic catalytic activity., 2012, 65(13): 2256–2279.
[18] DI J, YAN C, HANDOKO A D, SEH Z W,Ultrathin two-dimensional materials for photo- and electrocatalytic hydrogen evolution., 2018, 21(7): 749–770.
[19] MOMENI M M, GHAYEB Y, GHONCHEGI Z. Fabrication and characterization of copper doped TiO2nanotube arrays byelectrochemical method as efficient visible-light photocatalyst., 2015, 41(7): 8735–8741.
[20] GUO R, XU X, XIA Y,. Insights into electrocatalytic hydrogen evolution reaction in acidic medium atdispersed Pt atoms on nanoporous gold films., 2018, 368: 379–388.
[21] LIU L L, DU C J, WANG S L,Three new bifunctional additive for safer nickel-cobalt-aluminum based lithium ion batteries., 2018, 29: 1781–1784.
铂修饰二氧化钛纳米片的制备及其光电催化析氢反应研究
苏琨1,2,3, 张亚茹3, 陆飞3, 张君1, 王熙3
(1. 内蒙古大学 化学化工学院, 呼和浩特 010021; 2. 包头医学院 药学院, 包头 014040; 3. 北京交通大学 理学院,北京 100044)
利用静电吸附作用在二氧化钛纳米片上负载铂原子制备了两种不同形态的铂催化剂。SEM、XRD、TEM测试结果表明, 改变铂负载量可以调控铂的形貌结构。在低Pt负载(0.2wt%)下, 铂原子主要是半径约2 nm的纳米簇, 当Pt负载量增加到1wt%时, 铂原子在二氧化钛纳米片上堆积成纳米颗粒。调控Pt负载量和纳米结构, 可以显著提高二氧化钛纳米片催化析氢反应的活性。在AM1.5太阳光照射下, 两种催化剂的塔菲尔斜率都小于100 mV/dec, 分别为56和90 mV/dec。与TiO2-Pt1%催化剂相比, TiO2-Pt0.2%具有更理想的金属–半导体界面, 有利于光生电子迁移至铂纳米簇表面, 因而具有更高的催化活性。本实验为研究更加高效的铂催化剂和其他光电催化剂提供了新的途径。
析氢反应; 铂纳米簇; 二氧化钛; 光电催化
O643
A
date:2019-01-04;
Modified date: 2019-02-07
National Natural Science Foundation of China (21665019, 21165011); Natural Science Foundation of the Inner Mongolia Autonomous Region (2015MS0215); The Fundamental Research Funds for the Central Universities (2018JBZ107, 2018RC022, 2017JBM068, S18I00010, S17I00010, S16C00010); The “1000 Youth Talents plan” Project; The “Excellent One Hundred” Project of Beijing Jiaotong University
SU Kun(1981–), male, candidate of PhD. E-mail: nmsk528@126.com
Corresponding author:ZHANG Jun, professor. E-mail: zhangjundoc@sina.com; WANG Xi, professor. E-mail: xiwang@bjtu.edu.cn
1000-324X(2019)11-1200-05
10.15541/jim20190009
猜你喜欢
杂志排行
无机材料学报的其它文章
- MoS2 Quantum Dots Decorated NH2-MIL-125 Heterojunction: Preparation and Visible Light Photocatalytic Performance
- 仿生超疏水表面的发展及其应用研究进展
- Synthesis of SiC@SiO2 Nanocables via a Catalyst-free Carbothermal Reduction Method
- Deposition Temperature and Heat Treatment on Silicon Nitride Coating Deposited by LPCVD
- Evaluation and Simulation Verification of Thermal Insulation Property of Fiber Fabric Materials in Space Environment
- 单基质白光LED荧光粉研究进展
