MoS2 Quantum Dots Decorated NH2-MIL-125 Heterojunction: Preparation and Visible Light Photocatalytic Performance
2019-12-16HANLiZHANGXiaoMinWUDeYong
HAN Li, ZHANG Xiao-Min, WU De-Yong
MoS2Quantum Dots Decorated NH2-MIL-125 Heterojunction: Preparation and Visible Light Photocatalytic Performance
HAN Li, ZHANG Xiao-Min, WU De-Yong
(Hubei Key Laboratory of Biological Resources Protection and Utilization, School of Chemical and Environmental Engineering, Hubei Minzu University, Enshi 445000, China)
MoS2quantum dots (QDs) decorated NH2-MIL-125 (MoS2QDs/NH2-MIL-125) heterostructures were successfully fabricated by a facile method. XRD results exhibit that NH2-MIL-125 and MoS2form crystals in the synthesis process of MoS2QDs/NH2-MIL-125 heterojunctions. TEM results demonstrated clearly that the MoS2quantum dots with size of about 4 nm successfully disperse on the surface of NH2-MIL-125 plate. Compared with bulk MoS2and NH2-MIL-125, the MoS2QDs/NH2-MIL-125 heterostructures exhibit enhanced photocatalytic performance in degradation of methyl orange under visible light irradiation, about 5.8 and 7.4 times higher than that of pure bulk MoS2and NH2-MIL-125, respectively. Meanwhile, MoS2QDs/NH2-MIL-125 composites exhibit good stability and reusability during cycle experiment. The excellent photocatalytic activity of MoS2QDs/NH2-MIL-125 heterostructures is attributed to the formation of heterojunctions between MoS2QDs and NH2-MIL-125, facilitating separation of the photogenerated charge carries. PL results prove that MoS2QDs/NH2-MIL-125 composites own lower recombination of photogenerated electrons and holes, resulting in superior photocatalytic ability.
MoS2quantum dots(QDs); metal-organic frameworks; heterojunction; photocatalysis
Due to the permanent porosity, high surface area, and diverse structures, metal-organic frameworks (MOFs) have gathered increasing attention in the field of sensing[1], absorption[2]and catalysis[3-5]. NH2-MIL-125(Ti) with visible light absorption ability has been applied in the degradation of organic pollutants[6]. Moreover, constructing heterojunction between semiconductor and NH2-MIL-125 is an effective strategy to further improve the photocatalytic activity of NH2-MIL-125. Because the heterojunction can improve the separation of photogenerated charge carries, resulting in the superior photocatalytic activity[7-8]. For example, as compared with pure Ag3PO4, NH2-MIL-125 and P25, Ag3PO4@NH2-MIL-125 photocatalyst exhibited enhanced visible-light-induced activity toward MB and RhB[9]. Bi2S3@NH2-MIL-125(Ti) composites showed higher activity toward Cr(VI) reduction and RhB degradation than pure NH2-MIL-125(Ti) and Bi2S3[10]. BiOBr/NH2-MIL-125 composites also exhibited enhanced photocatalytic performance for RhBdegradation under visible light irradiation[11].
Herein, we prepared a new hybrid photocatalyst by coupling MoS2QDs and NH2-MIL-125(Ti). Due to the unique advantages of small sizes and short charge-transfer lengths, semiconductive QDs have attracted more and more interest[7,12]. However, pure QDs are easy to self-aggregate and have rapid recombination of the photogenerated charge carriers[13]. To solve these problems, loading QDs onto other materials has been proved as an effective strategy[14]. MoS2QDs can trap the photo-induced electrons and efficiently improve the photogenerated charge carriers separation efficiency, consequentially increase the photocatalytic performance[13,15]. For example, MoS2QDs can improve the photocatalytic detoxification and disinfection of Bi2WO6in the wastewater[15], and also enhance the photocatalytic activity of g-C3N4toward the degradation of MO and phenol under visible light irradiation[13]. In this report, MoS2QDs decorated NH2-MIL-125 heterostructures were synthesized by a two-step method, and the synthesized MoS2QDs/NH2-MIL-125 composites exhibited significantly enhanced photocatalytic performance for the degradation of MO under visible light irradiation.
1 Experimental
1.1 Preparation of MoS2 QDs/NH2-MIL-125
Metal-organic frameworks NH2-MIL-125 were prepared according to the reference [16], and the MoS2QDs solution was synthesized through one-step solvothermal route reported in reference [17]. MoS2QDs/NH2-MIL-125 composites were constructed as follows: NH2-MIL-125 powders (0.50 g) were ultrasonically dispersed in ethanol (40 mL), and as-prepared MoS2QDs solution (0.514 mL) was added dropwise under ultrasonication into the NH2-MIL-125 suspension. The mixed suspension was magnetically stirred at room temperature until the solvent was naturally evaporated, and finally dried at 80 ℃ for 12 h to get MoS2QDs/NH2-MIL-125 composites.
1.2 Characterization
The morphology of the catalyst was observed by scanning electron microscope (SEM, JEOL JSM-7001F, Japan) and transmission electron microscope (TEM, JEOL-100CX, Japan). The crystallinity of powder was characterized by powder X-ray diffraction (XRD, D/max-2200/PC, Rigaku Corporation, Japan) with Cu Karadiation. A diffuse reflectance UV-Vis spectrophotometer (DRS, TU-1901) was used to obtain the absorption spectra of samples. The photoluminescence (PL) spectra of the samples were obtained by using a Varian Cary Eclipse spectrometer with an excitation wavelength of 325 nm. The visible light photocatalytic activity of catalyst was evaluated according to the reference [18].
2 Results and discussion
Fig. 1 shows the TEM images of MoS2QDs/NH2- MIL-125 composites. The size of NH2-MIL-125 is about 1.5–3mm (Fig. 1(a)), and MoS2QDs are dispersed uniformly on the surface of NH2-MIL-125 plate (Fig. 1(b)). Observed from the high-magnification TEM images (Fig. 1(c-d)), the size of MoS2QDs is about 4 nm, an interplanar spacing of 0.23 nm is corresponded to the (103) planes of the MoS2QDs13]. And the elemental mapping analysis (Fig. 1(e-f)) suggest the presence and uniform distribution of Mo and S in the MoS2QDs/NH2-MIL-125 composites.
XRD patterns of the bulk MoS2, NH2-MIL-125 and MoS2QDs/NH2-MIL-125 are shown in Fig. 2. The peaks at 2=14.43o, 32.75o, 39.58o, 44.18oand 49.86oare attributed to the (002), (100), (103), (006) and (105) planes of bulk MoS2, which match the standard pattern of MoS2(JCPDF 37-1492)[13,17]. The XRD pattern of NH2-MIL- 125(Ti) was in accordance with simulated XRD patterns[6,19], indicating the formation of NH2-MIL-125(Ti) crystal. From the XRD pattern of MoS2QDs/NH2-MIL- 125, the peak at 2= 14.43oof MoS2can be observed, but other peaks of MoS2don’t emerge.

Fig. 1 TEM images (a, b), HRTEM images (c, d) and the partial element mappings (e, f) of MoS2 QDs/NH2-MIL-125 composites

Fig. 2 XRD patterns of bulk MoS2, NH2-MIL-125 and MoS2 QDs/NH2-MIL-125 composites
The photocatalytic activities of the bulk MoS2, NH2-MIL-125 and MoS2QDs/NH2-MIL-125 were investigated through photodegradation of MO under visible light irradiation (>420 nm). As shown in Fig. 3(a), bulk MoS2and NH2-MIL-125 removed about 21% and 16% of MO, respectively. Satisfactorily, QDs/NH2-MIL-125 composites exhibited enhanced photocatalytic performance, and degraded 81% MO over 120 min. Generally, the photocatalytic degradation of pollutants follows pseudo-first-order reaction<[10]. The degradation rate constant () can be calculated by the equation: ln(/0) =-, where0andare the initial concentration of MO and the concentration of MO at a reaction time of, respectively. Fig. 3(b) shows the kinetic fitting curves for the MO degradation with different samples. The photocatalytic degradation rates are 0.00198, 0.00157 and 0.01157 min-1for bulk MoS2, NH2-MIL-125 and MoS2QDs/NH2-MIL-125, respectively. The rate of MoS2QDs/NH2-MIL-125 is 5.8 and 7.4 times of pure bulk MoS2and NH2-MIL-125, respectively.
The enhanced photocatalytic activity of MoS2QDs/NH2-MIL-125 should be attributed to the formation of heterojunctions between MoS2QDs and NH2-MIL- 125, not to the light absorption of MoS2QDs. In Fig. 4(a), it was clearly observed that MoS2QDs have little effect on the light absorption ability of NH2-MIL-125. However, the heterojunctions between MoS2QDs and NH2-MIL-125 are of great benefit to the photo-generated charge carries separation[20]. The PL spectra always be utilized to analyze the photogenerated electron-hole recombination efficiency. Fig. 4(b) shows the PL spectra of pure NH2-MIL-125 and the MoS2QDs/NH2-MIL-125 composites. NH2-MIL-125 has stronger PL peak, and MoS2QDs/NH2-MIL-125 shows lower PL peak. This results indicate that MoS2QDs/NH2-MIL-125 composites own lower recombination of the photo-generated electrons and holes[21-22]. As a result, MoS2QDs/NH2-MIL-125 composite exhibits a better visible-light-induced photocatalytic activity. Meanwhile, the stability of MoS2QDs/NH2-MIL-125 composites was investigated by repeating the photocatalytic degradation of MO for five cycles under the same conditions. As shown in Fig. 5, there is no obvious inactivation in the photocatalytic activity after five recycles, indicating the composites have good stability and reusability.
3 Conclusions
In summary, MoS2QDs/NH2-MIL-125 composites were successfully prepared by a facile method. MoS2QDs with size of about 4 nm dispersed uniformly on the surface of NH2-MIL-125 plate. Compared with pure bulk MoS2and NH2-MIL-125, MoS2QDs/NH2-MIL-125 photocatalysts display greatly improved photocatalytic activity over methyl orange under visible light irradiation. The photocatalytic degradation rate of MoS2QDs/NH2-MIL-125 is 5.8 and 7.4 times of pure bulk MoS2and NH2-MIL-125, respectively. This excellent property should be attributed to the formation of heterojunction between MoS2QDs and NH2-MIL-125, which can lower the recombination of the photo-generated electrons and holes. This work could provide a novel strategy for designing semiconductor/MOF heterostructured photocatalysts.

Fig. 3 (a) Photocatalytic activity and (b) plot of –ln(C/C0) of bulk MoS2, NH2-MIL-125 and MoS2 QDs/NH2-MIL-125 composites over MO as a function of irradiation time
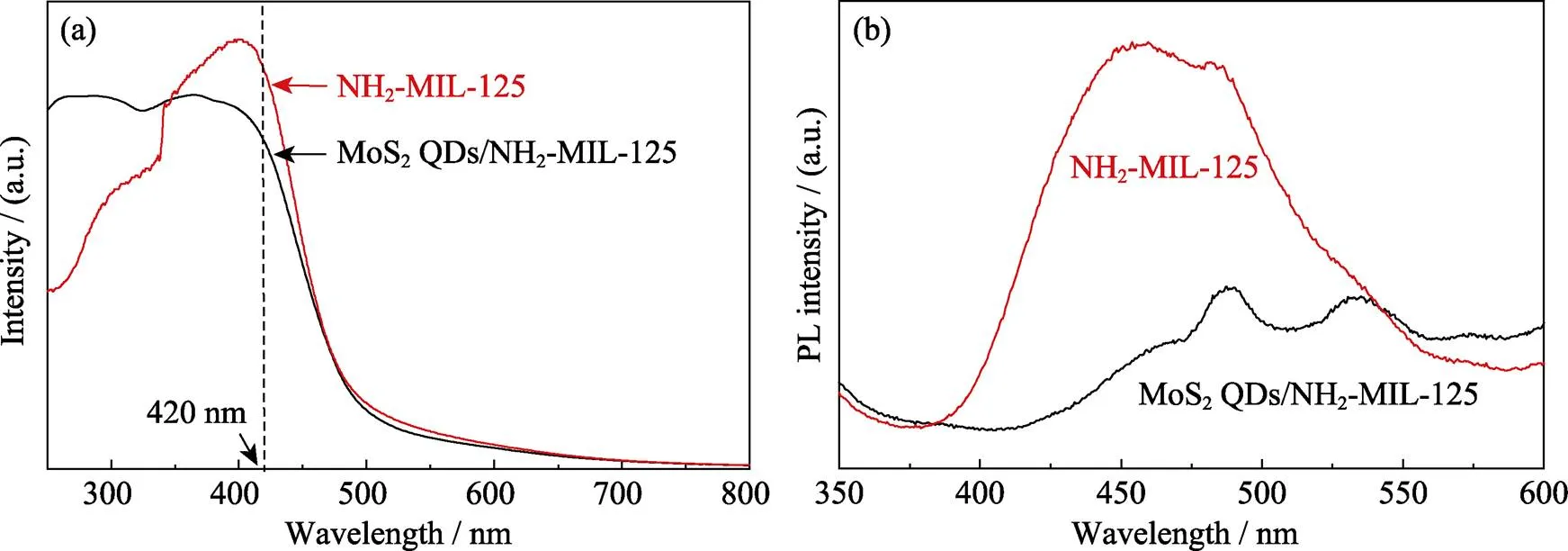
Fig. 4 UV-Vis diffuse reflectance spectra (a) and PL spectra (b) of NH2-MIL-125 and MoS2 QDs/NH2-MIL-125 composites

Fig. 5 Stability test of MoS2 QDs/NH2-MIL-125 composites for MO degradation under visible light irradiation
[1] GOGOI C, YOUSUFUDDIN M, BISWAS S. A new 3D luminescent Zn(II)-organic framework containing a quinoline-2,6-dicarboxylate linker for the highly selective sensing of Fe(III) ions.., 2019, 48(5): 1766–1773.
[2] LIU D X, GU J J, LIU Q L,Metal-organic frameworks reactivate deceased diatoms to be efficient CO2absorbents., 2014, 26(8): 1229–1234.
[3] JIANG D, XU P, WANG H,Strategies to improve metal organic frameworks photocatalyst's performance for degradation of organic pollutants., 2018, 376: 449–466.
[4] YANG Y F, WANG W J, LI H,NH2-MIL-53(Al) nanocrystals anchored on the surface of RGO hollow spheres and its visible light degradation of methylene blue., 2017, 197: 17–20.
[5] KHANDAN F M, AFZALI D, SARGAZI G,Novel uranyl-curcumin-MOF photocatalysts with highly performance photocatalytic activity toward the degradation of phenol red from aqueous solution: effective synthesis route, design and a controllable systematic study..,2018, 29(21): 18600–18613.
[6] HU S, LIU M, LI K Y,Surfactant-assisted synthesis of hierarchical NH2-MIL-125 for the removal of organic dyes., 2017, 7(1): 581–587.
[7] ZHANG Z J, XU J Y, ZENG H B,Carbon quantum dots/BiPO4nanocomposites with enhanced visible-light absorption and charge separation., 2018, 33(5): 582–586.
[8] CORRIEA F C, CALHEIROS M, MARQUES J,Synthesis of Bi2O3/TiO2nanostructured films for photocatalytic applications., 2018, 44(18): 22638–22644.
[9] ABDLHAMEED R M, TOBALDI D M, KARMAOUI M. 2018. Engineering highly effective and stable nanocomposite photocatalyst based on NH2-MIL-125 encirclement with Ag3PO4nanoparticles.a, 2018, 351: 50–58.
[10] WANG M, YANG L, YUAN J,Heterostructured Bi2S3@NH2-MIL-125(Ti) nanocomposite as a bifunctional photocatalyst for Cr(VI) reduction and rhodamine B degradation under visible light., 2018, 8(22): 12459–12470.
[11] ZHU S R, LIU P F, W M K,Enhanced photocatalytic performance of BiOBr/NH2-MIL-125(Ti) composite for dye degradation under visible light.., 2016, 45(43):17521–17529.
[12] CHEN W, GU J, LIU Q,Quantum dots of 1T phase transitional metal dichalcogenides generatedelectrochemical Li intercalation., 2018, 12(1): 308–316.
[13] SHI L, HE Z, LIU S Q. MoS2quantum dots embedded in g-C3N4frameworks: a hybrid 0D-2D heterojunction as an efficient visible- light driven photocatalyst., 2018, 457: 30–40.
[14] ZHAO F F, RONG Y F, WAN J M,MoS2quantum dots@TiO2nanotube composites with enhanced photoexcited charge separation and high-efficiency visible-light driven photocatalysis., 2018, 29(10): 105403.
[15] MENG X C, LI Z Z, ZENG H M,MoS2quantum dots-interspersed Bi2WO6heterostructures for visible light-induced detoxification and disinfection.., 2017, 210: 160–172.
[16] ZHANG B X, ZHANG J L, TAN X N,MIL-125-NH2@TiO2core-shell particles produced by a post-solvothermal route for high-performance photocatalytic H2production., 2018, 10(19): 16418–16423.
[17] GU W, YAN Y, CAO X,A facile and one-step ethanol-thermal synthesis of MoS2quantum dots for two-photon fluorescence imaging., 2016, 4(1): 27–31.
[18] WU D, WU C, TAN H. Facile synthesis of novel I-doped Bi4O5Br2nanosheets with enhanced visible light photocatalytic activity., 2018, 29(13): 11090–11095.
[19] WU Z Y, HUANG X B, ZHENG H Y,Aromatic heterocycle- grafted NH2-MIL-125(Ti)conjugated linker with enhanced photocatalytic activity for selective oxidation of alcohols under visible light., 2018, 224: 479–487.
[20] CARVALHO K T G, NOGUEIRA A E, LOPES O F,Synthesis of g-C3N4/Nb2O5heterostructures and their application in the removal of organic pollutants under visible and ultraviolet irradiation., 2017, 43(4): 3521–3530.
[21] YE F, LI H F, YU H T,Hydrothermal fabrication of few-layer MoS2nanosheets within nanopores on TiO2derived from MIL-125(Ti) for efficient photocatalytic H2evolution.., 2017, 426: 177–184.
[22] WANG F Z, LI W J, GU S N,Facile fabrication of direct Z-scheme MoS2/Bi2WO6heterojunction photocatalyst with superior photocatalytic performance under visible light irradiation., 2017, 335: 140–148.
具有可见光催化活性的MoS2量子点/NH2-MIL-125复合材料的制备及性能表征
韩丽, 张晓敏, 吴德勇
(湖北民族大学 化学与环境工程学院, 湖北省生物资源保护与利用重点实验室, 恩施 445000)
采用水热法合成金属–有机骨架材料NH2-MIL-125, 并修饰硫化钼量子点, 从而构筑具有增强电荷分离的二硫化钼量子点/NH2-MIL-125复合光催化材料(MoS2QDs/NH2-MIL-125)。利用XRD、HRTEM、DRS、PL对材料性能进行分析, 通过降解甲基橙MO染料测试MoS2QDs/NH2-MIL-125复合材料的光催化性能。结果表明:尺寸约 4 nm的MoS2QDs均匀分散在NH2-MIL-125上, 在可见光照射下, MoS2QDs/NH2-MIL-125复合材料的光催化性能极大优于单一的Bulk MoS2和NH2-MIL-125, 降解常数分别是它们的5.8和7.4倍。循环光催化实验结果表明, MoS2QDs/NH2-MIL-125复合材料的光催化能力具有良好的稳定性。DRS和PL的测试结果表明, MoS2QDs/NH2-MIL-125复合材料优异的可见光催化性能主要归因于异质结构的形成, 抑制了光生电子–空穴的复合, 进而提高了光催化性能。
二硫化钼量子点; 金属–有机骨架材料; 异质结构; 光催化
TB34
A
date:2019-01-04;
Modified date: 2019-04-12
National Natural Science Foundation of China (21767009); China Scholarship Council (201808420149); Hubei Key Laboratory of Biological Resources Protection and Utilization (PKLHB1908); Undergraduate Innovation Program (201910517006)
HAN Li (1996-), female, undergraduate. E-mail: 2213651218@qq.com
WU De-Yong, associate professor. E-mail: wdy001815@126.com
1000-324X(2019)11-1205-05
10.15541/jim20190010
猜你喜欢
杂志排行
无机材料学报的其它文章
- Platinum Decorated Titanium Dioxide Nanosheets for Efficient Photoelectrocatalytic Hydrogen Evolution Reaction
- 仿生超疏水表面的发展及其应用研究进展
- Synthesis of SiC@SiO2 Nanocables via a Catalyst-free Carbothermal Reduction Method
- Deposition Temperature and Heat Treatment on Silicon Nitride Coating Deposited by LPCVD
- Evaluation and Simulation Verification of Thermal Insulation Property of Fiber Fabric Materials in Space Environment
- 单基质白光LED荧光粉研究进展
