Na2S胁迫/诱导下Bacillus vallismortis sp. EPS组分变化及其对铜锌的吸附
2019-11-28李秋华宋卫锋孙梦格李家耀余泽峰
李秋华,宋卫锋,孙梦格,李家耀,余泽峰
Na2S胁迫/诱导下spEPS组分变化及其对铜锌的吸附
李秋华,宋卫锋*,孙梦格,李家耀,余泽峰
(广东工业大学环境科学与工程学院,广东 广州 510006)
为研究胞外聚合物(EPS)对重金属吸附效果的影响,本文通过Na2S胁迫/诱导sp. EPS的化学组成变化来强化其对典型重金属的吸附.结果表明,Na2S胁迫/诱导强度为20mg/L时,S-EPS产量最高,达到105.58mg/gVSS,蛋白质较Control-EPS提高了近一倍,其中巯基含量达到154.36μmol/L,比胁迫前提高了48.2%.在此条件下,S-EPS对Cu(Ⅱ)和Zn(Ⅱ)的吸附效果最好,对重金属离子的吸附与Langmuir等温式拟合较好,拟合理论最大吸附量分别达到1428.57和979.09mg/g EPS,且吸附过程符合二级动力学规律.三维荧光(3D-EEM)和红外光谱(FTIR)分析表明,巯基、蛋白质酰胺I带与酰胺Ⅱ带在吸附Cu(Ⅱ)和Zn(Ⅱ)中起到了主要作用,尤其是对Zn(Ⅱ)的吸附.说明外加硫源提高了巯基蛋白含量,大大提高了重金属去除效果.该生物吸附剂在重金属污染防治中显示出极大的应用前景.
胞外聚合物;重金属;硫化钠;巯基蛋白;吸附
目前,水环境中重金属的污染问题已经成为我国环境污染治理领域研究的重点和难点[1].长时间饮用被重金属污染的水,即使浓度低也可能引起严重疾病[2],如高剂量的铜离子可能导致肝损伤或威尔逊病[3],过量的锌会引起急性肠胃炎等症状,严重时甚至会导致死亡[4].因此,如何高效、低耗地去除水中重金属,最大限度降低重金属污染是当今众多研究者的重要课题.与传统的物理化学法相比,生物吸附技术具有高效、环境友好等优点[5],生物吸附剂来源广泛、丰富廉价,具有广阔的应用前景.其中,胞外聚合物(EPS)成为多数研究者的焦点.
EPS是细菌在不利于自身生存环境中自我保护的屏障之一,主要由多糖、蛋白质、核酸和腐殖质等组成,含有大量负电子基团,是一种良好的重金属吸附剂[6].近年来国内外的研究工作主要集中在EPS对重金属的吸附和机理方面.Harish等[7]在EPS吸附Cr6+的研究中指出,C=O、C—N、—COOH在吸附过程中起了主要作用,吸附机理主要是离子交换和络合作用;Ye等[8]以ps-5.EPS为吸附剂,对Cu2+和Pb2+进行吸附,提出对重金属起作用的是EPS中O—H、C=O、C—O—C、C=O—C官能团,吸附过程以化学作用为主.但关于EPS中各组分与吸附性能之间的关系未进行深入探讨.
EPS的化学组成、官能团种类及浓度对重金属去除具有很大的影响[9].Fang等[10]通过EPS吸附Cu2+的热力学特征研究,发现EPS中的蛋白质和腐殖酸都是Cu2+的强配体,而与蛋白的交互作用要比腐殖质强;Wang等[11]通过. EPS吸附Zn2+和Cu2+的研究,发现EPS中蛋白质对Zn2+和Cu2+均有较强的结合能力,通过三维荧光分析进一步验证了Zn2+可以和蛋白质及多糖作用而Cu2+只与蛋白质作用;简静怡等[12]用微量的Cu2+为胁迫因子,对sp.进行胁迫培养,发现EPS中蛋白质含量明显上升,对Cu(Ⅱ)的吸附效果相比于胁迫前明显提高.可见,蛋白质对重金属的去除起着极其重要的作用,表明了对EPS特定组分进行定向调控,以此增强EPS对金属离子吸附性能的重要性和必要性.但如何实现对EPS组分的定向调控,是当前研究中面临的一个重要问题.
此外,巯基具有良好的吸附重金属性能,可以与重金属结合成稳定的化合物达到去除重金属的目的[13].生物体内重要的重金属解毒因子是含有巯基的分子蛋白,如巯基蛋白.故本实验通过对菌种的外源硫胁迫/诱导培养,定向提高EPS特定组分,尤其是巯基蛋白的含量,提高EPS对典型重金属Cu(Ⅱ)、Zn(Ⅱ)的去除效果,为生物吸附法去除重金属提供数据基础和参考意义.
1 材料与方法
1.1 菌种的活化与培养
本文所用菌株为苯胺黑药高效降解菌,经16S rRNA测序确定其为死谷芽孢杆菌(sp.)[14],从广州市沥滘污水处理厂沉淀池回流污泥中选育并经过分离纯化获得,并用甘油法进行保存.
无机盐培养基:称量磷酸二氢钾1.6g,磷酸氢二钾0.4g,硫酸镁0.06g,氯化钙0.001g,氯化铵1.0g,苯胺黑药0.1g,定容到1000mL容量瓶,未调节pH值.高压灭菌20min,冷却后将解冻的菌株按2%()接种于该培养基中,在35℃、150r/min条件下振荡培养48h.
LB培养基:称量蛋白胨10g,酵母粉5g,氯化钠10g,定容到1000mL容量瓶,调节pH值为(7.2±0.2),高压灭菌20min后进行冷却,然后将培养后的菌液按6%(/)接种至LB培养基中,在35℃、150r/min条件下缺氧培养2h,外加硫源以Na2S溶液形式加入,浓度范围为0~80mg/L,在相同条件下培养22h.以上接种过程均在无菌条件下进行.
1.2 EPS的提取与测定
取30mL活化培养后的菌液在4℃,5000r/min条件下离心15min,收集菌体,用0.9%NaCl溶液重复清洗2遍,制备成菌悬液.用改进的EDTA法提取EPS[15],之后取上清液进行过滤,透析24h后保存待用.
EPS产量用多糖、蛋白质、核酸之和表示,单位为mg/g VSS.测定方法分别为苯酚硫酸法、考马斯亮蓝法[16]和二苯胺法[17],—SH测定方法为DTNB法[18].实验结果均取3次平行试验的平均值.
1.3 吸附实验
将胁迫/诱导前后的sp.EPS分别注入浓度均为20mg/L的Cu(Ⅱ)、Zn(Ⅱ)溶液中,在pH=5.0,35℃,150r/min条件下,振荡吸附2h.然后放入经过预处理的透析袋中,将透析袋放入盛有250mL蒸馏水的烧杯中,室温下在磁力搅拌机上透析12h,然后用火焰原子吸收分光光度计测定透析液中的金属离子浓度.每个样品均设3个平行样,实验数据取其平均值.
EPS吸附量公式如下:
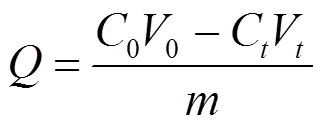
式中:为EPS单位吸附量,mg/g EPS;0为金属离子初始浓度,mg/L;0为吸附前溶液体积,L;C为某时刻金属离子浓度,mg/L;V为透析液体积,L;为EPS质量,g.
1.4 表征分析
胁迫/诱导因子对sp. EPS主要成分的影响以及EPS主要官能团的变化分别用三维荧光(3D-EEM)和傅里叶红外光谱(FTIR)进行分析.
1.5 吸附等温模型
分别配制浓度为10,20,30,50,80,120mg/L的Cu(Ⅱ)、Zn(Ⅱ)溶液,并调节pH=5.0,分别移取20mL于50mL锥形瓶中,加入适量EPS溶液,放于摇床中,在与1.3中同样的条件下进行吸附及透析实验,然后测定透析液中金属离子浓度,按照式(1)计算吸附量,对实验数据进行等温吸附模型拟合.
Langmuir型和Freundlich型吸附等温线是EPS吸附重金属研究中最常见的2种模型,用来反映吸附机制、吸附层结构和吸附剂的宏观表面结构[19].
Langmuir吸附等温线的形式如下所示:
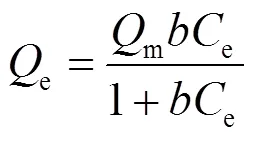
Freundlich模型是一条经验公式,用于非理想条件下的表面吸附和多分子层吸附过程.其等温方程可表示为:
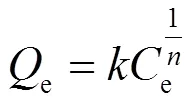

1.6 吸附动力学研究
分别配制浓度为20mg/L的Cu(Ⅱ)、Zn(Ⅱ)溶液,并调节pH=5.0,分别移取20mL于50mL锥形瓶中,加入适量EPS溶液,放于摇床中,在pH=5.0,35℃, 150r/min条件下吸附0~180min,混合溶液透析12h后测定透析液中金属离子浓度,按照式(1)计算吸附量,对实验数据进行动力学模型拟合.
EPS吸附金属离子的过程,分别用准一级动力学模型和准二级动力学模型[21]对时间影响因素的实验数据进行拟合,2种动力学模型的方程式如下:
准一级动力学模型:

准二级动力学模型:
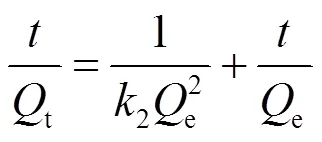
式中:e为平衡吸附量,mg/g;t为时刻吸附量,mg/g;1为准一级吸附速率常数,min-1;2为准二级吸附速率常数,g/(mg·min);为吸附时间,min.
2 结果与讨论
2.1 不同浓度Na2S胁迫诱导对EPS组分及其吸附性能影响
本实验用不同浓度的Na2S作为胁迫/诱导因子(前期尝试了不同价态的硫源,Na2S效果最好.胁迫/诱导下的EPS称为S-EPS,空白样称为Control-EPS). EPS各组分及含量如图1所示.
可以看出,胁迫前后EPS组分多糖和核酸变化不大,而蛋白质在Na2S浓度为20mg/L时含量相比胁迫前增加了近一倍,从21.36增加至36.08mg/g VSS,此时EPS含量达到105.58mg/g VSS.从整体来看,EPS产量随着Na2S的胁迫强度呈先增加后减少的趋势,原因可能是EPS是微生物为适应外部环境变化而产生的,而Na2S对菌株而言是有害物质,会形成对菌株生长不利的环境,为了抵御这种不利条件,菌株需要产生更多的EPS保护自己[5].但当Na2S浓度过高时,微生物活性下降,导致EPS产量下降.
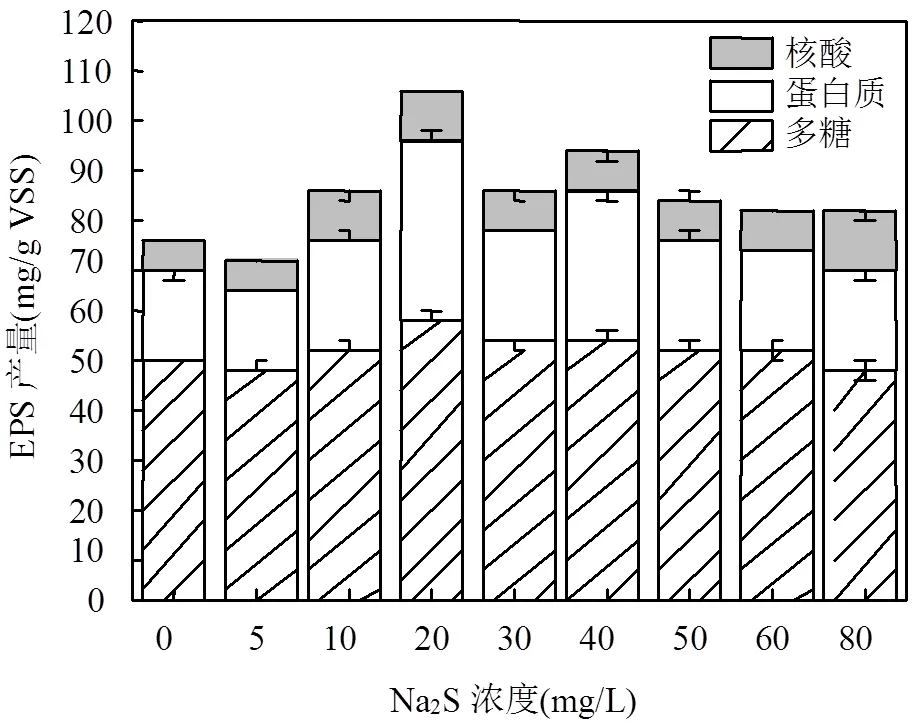
图1 不同强度Na2S胁迫下EPS产量
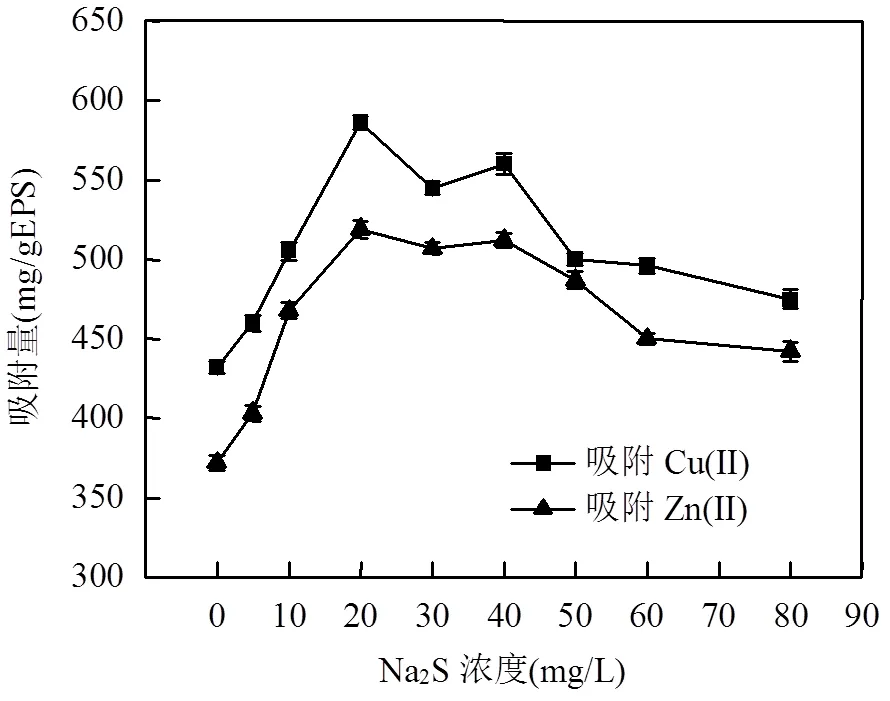
图2 不同强度Na2S胁迫/诱导下EPS对Cu(Ⅱ)和Zn(Ⅱ)的吸附
按照上述胁迫/诱导强度,提取相应的EPS,分别吸附Cu(Ⅱ)和Zn(Ⅱ),吸附量如图2所示.可以看出,胁迫浓度为20mg/L时,EPS对Cu(Ⅱ)和Zn(Ⅱ)的平衡吸附量最高,分别为584.80和519.02mg/g EPS,较未胁迫前吸附量分别提高了35.6%和43.8%.结合前面实验的结果,胁迫/诱导因子强度为20mg/L时, S-EPS中蛋白质含量相比于Control-EPS提高幅度较大,故推测是蛋白质在吸附两种重金属时起了较大作用,其中的—SH、C=O、C—N/N—H等基团数量/浓度增加,使EPS暴露出的点位数量增加,提高了重金属去除效果.表明了外源硫能通过胁迫/诱导菌株产生特异性EPS.以下实验及表征均用浓度为20mg/ L的Na2S.
2.2 两种EPS的三维荧光分析
本实验采用三维荧光光谱进一步研究了Na2S胁迫因子对sp. EPS主要成分的影响.
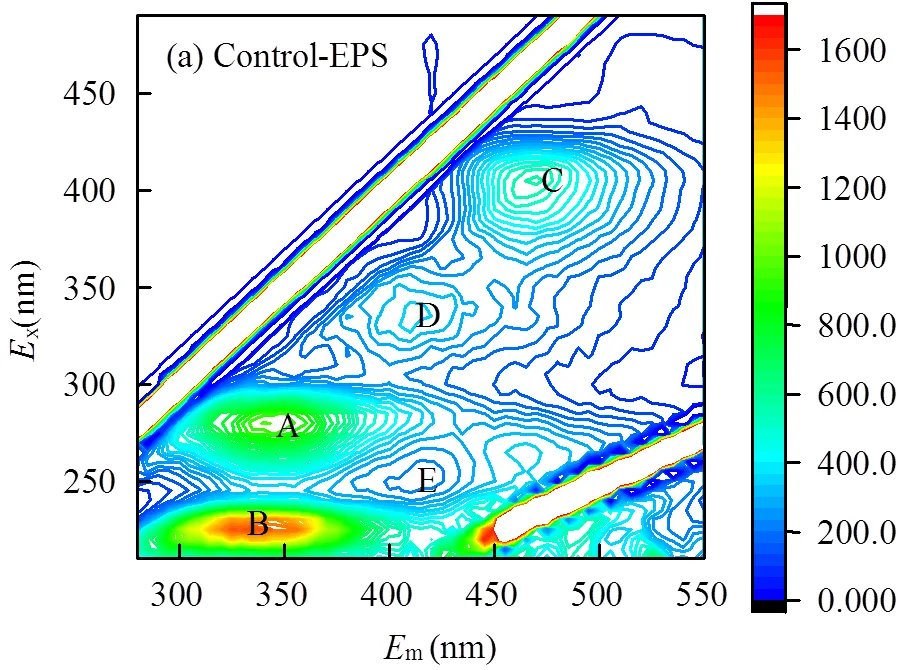
(a), Control-EPS; (b), S-EPS
如图所示,由于含—SH蛋白无发色基团,故2种EPS的官能团种类未发生变化.胁迫前后EPS荧光光谱位置和最大荧光强度的参数见表1.
已有研究指出荧光强度与EPS 的含量具有密切的关系[25].结合3图和表1可看出,峰A和峰B的蛋白质类荧光强度较胁迫前增强最为明显,原因在于部分官能团如C=O、—NH2、—COOH含量增加,这些负电子基团更容易与重金属离子结合,达到去除重金属的目的.以上结果说明,外加硫源胁迫/诱导可以改变EPS成分的含量,使目标物质-蛋白质类含量大幅增加.
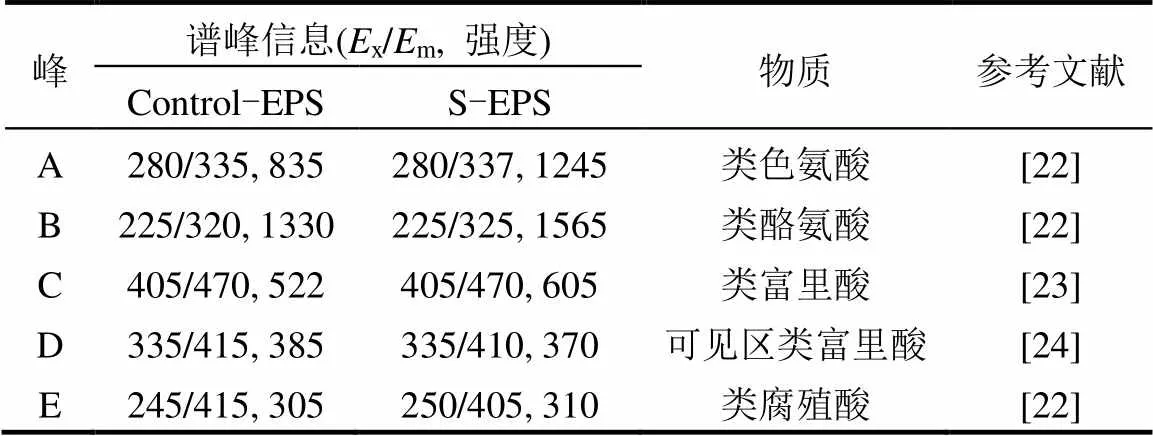
表1 胁迫/诱导前后EPS荧光光谱谱峰信息
2.3 吸附重金属前后EPS的红外光谱分析
spEPS受外加硫源胁迫/诱导前后关键基团峰值和峰位的移动情况可用傅里叶红外光谱进行分析,从而研究其与重金属相互作用机理.胁迫/诱导前后的EPS以及吸附Cu(Ⅱ)和Zn(Ⅱ)后的S-EPS红外谱图见图4.

图4 胁迫/诱导前后和吸附重金属后EPS的红外光谱图
Cu-EPS代表吸附Cu(Ⅱ)之后的EPS,Zn-EPS代表吸附Zn(Ⅱ)之后的EPS
Cu(Ⅱ)和Zn(Ⅱ)之后发生了不同程度的红移, Cu-EPS的红移程度更大,说明C=O参与了对Cu(Ⅱ)的吸附.Zn-EPS的S—O特征峰相较于S-EPS发生了很大程度的红移,说明S—O基团在Zn(Ⅱ)的吸附中发挥了部分作用,猜测可能是外加硫源在此起了部分作用.Cu-EPS在蛋白质酰胺Ⅱ带较S-EPS发生了红移,而在Zn-EPS中酰胺Ⅱ带消失,说明N—H和C—N基团均参与了对Cu(Ⅱ)和Zn(Ⅱ)的吸附.除了个别基团峰发生了漂移和消失外,部分特征峰的强度也存在着明显的不同,说明胁迫后的EPS中部分官能团仍存在某些变化.Jiang等[30]认为谱峰强度与样品所含官能团浓度存在着密切关系,Xu等[31]利用红外光谱研究sp. EPS时,指出峰值强度的大小能反映对应官能团的相对浓度,可看出Cu-EPS和Zn-EPS的特征峰强度均比S-EPS特征峰强度强,验证了前面结论.另外,与糖有关的基团也发生了小幅度的红移.
2.4 吸附等温模型研究
实验采用Langmuir模型和Freundlich模型对Control-EPS、S-EPS分别吸附Cu(Ⅱ)和Zn(Ⅱ)进行拟合,各模型拟合系数见表2.
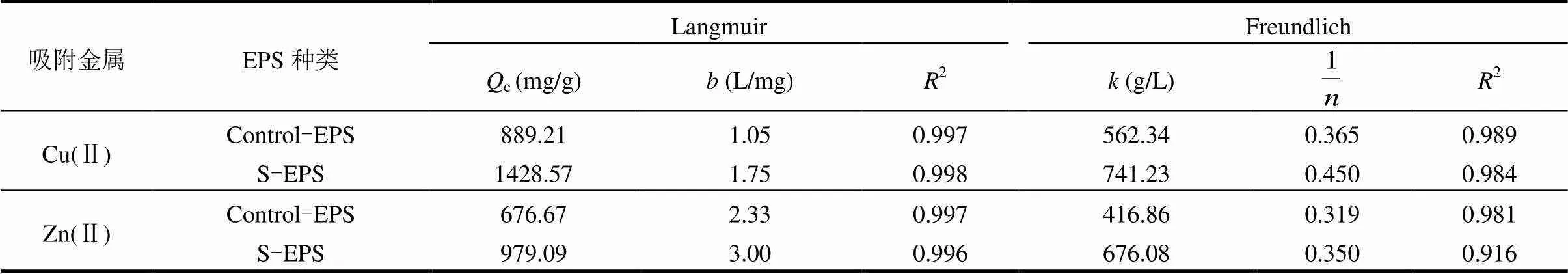
表2 胁迫/诱导前后EPS吸附等温模型拟合
2.5 吸附动力学研究
本研究采用一级动力学模型和二级动力学模型对Control-EPS、S-EPS分别吸附Cu(Ⅱ)和Zn(Ⅱ)进行拟合,各模型拟合系数见表3.
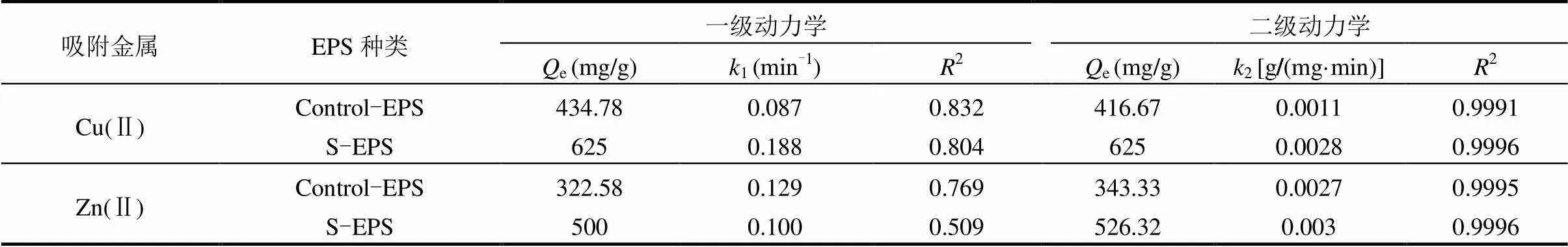
表3 胁迫/诱导前后EPS吸附重金属动力学参数
从表3可以看出,对于2种重金属离子的吸附,Control-EPS和S-EPS的准二级动力学拟合效果均比准一级动力学高,2达到0.99以上,说明2种EPS吸附金属离子的过程采用准二级动力学描述更为准确,其得出的平衡吸附容量e也与实验值接近.准二级动力学模型认为化学反应是控速步骤,可用于多种吸附研究[33-34],表明S-EPS对Zn(Ⅱ)和Cu(Ⅱ)的吸附过程仍由化学反应控制.
3 结论
3.1 在一定范围内,随着胁迫因子的强度增大,spEPS含量总体上呈先上升后降低趋势,并影响其组分含量变化:多糖、核酸含量变化不明显,而对蛋白质影响较大,其含量增加了接近一倍,巯基含量增加48.2%.
3.2 胁迫/诱导浓度为20mg/L时,S-EPS对Cu(Ⅱ)和Zn(Ⅱ)的平衡吸附量最高,分别为584.80和519.02mg/g EPS,较未胁迫前吸附量分别提高了35.6%和43.8%.
3.3 三维荧光和红外光谱结果分析表明,巯基蛋白在S-EPS对Cu(Ⅱ)和Zn(Ⅱ)的吸附中发挥了极为重要的作用,联合其他负电子基团达到去除重金属的目的.
3.4 采用Langmuir模型和Freundlich模型对Control-EPS、S-EPS分别吸附Cu(Ⅱ)和Zn(Ⅱ)数据进行拟合,发现Langmuir模型有更好的模拟效果,对Cu(Ⅱ)和Zn(Ⅱ)的最大吸附量可分别达到1428.57和979.09mg/g EPS.动力学模拟结果显示,二级动力学更符合EPS重金属离子的吸附过程.
[1] 包汉峰,杨维薇,张立秋,等.污泥基活性炭去除水中重金属离子效能与动力学研究 [J]. 中国环境科学, 2013,33(1):69-74. Bao H F, Yang W W, Zhang L Q, et al. Efficiency and kinetics of heavy metal removal from water by sludge-based activated carbon [J]. China Environmental Sciencece, 2013,33(1):69-74.
[2] Finch N C, Syme H M, Elliott J. Association of urinary cadmium excretion with feline hypertension [J]. Veterinary Record, 2012,170(5): 125-U47.
[3] Dal Bosco S M, Jimenez R S, Vignado C, et al. Removal of Mn(II) and Cd(II) from wastewaters by natural and modified clays [J]. Adsorption-Journal of the International Adsorption Society, 2006, 12(2):133-146.
[4] Futalan C M, Kan C C, Dalida M L, et al. Comparative and competitive adsorption of copper, lead, and nickel using chitosan immobilized on bentonite [J]. Carbohydrate Polymers, 2011,83(2): 528-536.
[5] More T T, Yadav J S, Yan S, et al. Extracellular polymeric substances of bacteria and their potential environmental applications [J]. Journal of Environmental Management, 2014,144:1-25.
[6] Wang Y, Qin J, Zhou S, et al. Identification of the function of extracellular polymeric substances (EPS) in denitrifying phosphorus removal sludge in the presence of copper ion [J]. Water Research, 2015,73:252-264.
[7] Harish R, Samuel J, Mishra R, et al. Bio-reduction of Cr(VI) by exopolysaccharides (EPS) from indigenous bacterial species of Sukinda chromite mine, India [J]. Biodegradation, 2012,23(4):487- 496.
[8] Ye S, Zhang M, Yang H, et al. Biosorption of Cu2+, Pb2+and Cr6+by a novel exopolysaccharide fromps-5 [J]. Carbohydrate Polymers, 2014,101:50-56.
[9] Mota R, Pereira S B, Meazzini M, et al. Effects of heavy metals on Cyanothece sp CCY 0110growth, extracellular polymeric substances (EPS) production, ultrastructure and protein profiles [J]. Journal of Proteomics, 2015,120:75-94.
[10] Fang L, Wei X, Cai P, et al. Role of extracellular polymeric substances in Cu(II) adsorption on Bacillus subtilis and Pseudomonas putida [J]. Bioresource Technology, 2011,102(2):1137-1141.
[11] Wang J, Li Q, Li M M, et al. Competitive adsorption of heavy metal by extracellular polymeric substances (EPS) extracted from sulfate reducing bacteria [J]. Bioresource Technology, 2014,163:374-376.
[12] 简静仪,宋卫锋,李海宇,等.锌铜胁迫对EPS组分变化特征及其吸附性能的影响 [J]. 环境科学学报, 2017,37(6): 2099-2106. Jian J Y, Song W F, Li H Y, et al. Influence of the stress of Zn(Ⅱ) and Cu(Ⅱ) on component changes and sorption behavior of extracellular polymeric substances (EPS) from Bacillus vallismortis [J]. Acta Scientiae Circumstantiae, 2017,37(6):2099-2106.
[13] Andersen O. Oral cadmium exposure in mice: toxicokinetics and efficiency of chelating agents [J]. Critical reviews in toxicology, 1989, 20(2):83-112.
[14] 宋卫锋,邓 琪.一株苯胺黑药降解菌的分离鉴定及其降解特性 [J]. 环境科学学报, 2012,41(6):1018-1023. Song W F, Deng Q. An aniline aerofloat-degrading bacterium: Isolation, identification and degradation characteristics [J]. Journal of China University of Mining & Technology. 2012,41(6):1018-1023.
[15] Naik M M, Pandey A, Dubey S K. Biological characterization of lead-enhanced exopolysaccharide produced by a lead resistant Enterobacter cloacae strain P2B [J]. Biodegradation, 2012,23(5): 775-783.
[16] Zhang B, Sun B, Jin M, et al. Extraction and analysis of extracellular polymeric substances in membrane fouling in submerged MBR [J]. Desalination, 2008,227(1-3):286-294.
[17] 陈 哲,张 斌,谌志强,等.1株异养硝化菌胞外聚合物的研究 [J]. 环境科学, 2012,33(4):1318-1322. Chen Z, Zhang B, Shen Z Q, et al. Characteristics of the extracellular polymeric substances of a heterotrophic nitrifying bacterium strain [J]. Environmental Science, 2012,33(4):1318-1322.
[18] Narai-Kanayama A, Hanaishi T K. Mechanistic investigation of capability of enzymatically synthesized polycysteine to cross-link proteins [J]. Biochemistry and biophysics reports, 2016,7:338-346.
[19] Silva S M, Sampaio K A, Ceriani R, et al. Adsorption of carotenes and phosphorus from palm oil onto acid activated bleaching earth: Equilibrium, kinetics and thermodynamics [J]. Journal of Food Engineering, 2013,118(4):341-349.
[20] Sari A, Tuzen M. Kinetic and equilibrium studies of biosorption of Pb(II) and Cd(II) from aqueous solution by macrofungus (Amanita rubescens) biomass [J]. Journal of Hazardous Materials, 2009,164(2/3): 1004-1011.
[21] Yahaya Y A, Don M M, Bhatia S. Biosorption of copper (II) onto immobilized cells of Pycnoporus sanguineus from aqueous solution: Equilibrium and kinetic studies [J]. Journal of Hazardous Materials, 2009,161(1):189-195.
[22] Guo X J, He L S, Li Q, et al. Investigating the spatial variability of dissolved organic matter quantity and composition in Lake Wuliangsuhai [J]. Ecological Engineering, 2014,62:93-101.
[23] 陈诗雨,李 燕,李爱民.溶解性有机物研究中三维荧光光谱分析的应用 [J]. 环境科学与技术, 2015,38(5):64-68. Chen S Y, Li Y, Li A M. Application of Three-dimensional fluorescence spectroscopy in the study of dissolved organic matter [J]. Environmental Science & Technology, 2015,38(5):64-68.
[24] Chen W, Westerhoff P, Leenheer J A, et al. Fluorescence excitation- emission matrix regional integration to quantify spectra for dissolved organic matter [J]. Environmental Science & Technology, 2003,37(24): 5701-5710.
[25] Ma B, Li S, Wang S, et al. Effect of Fe3O4nanoparticles on composition and spectroscopic characteristics of extracellular polymeric substances from activated sludge [J]. Process Biochemistry, 2018,75:212-220.
[26] Kang F, Qu X, Alvarez P J J, et al. Extracellular saccharide-mediated reduction of Au3+to gold nanoparticles: New insights for heavy metals biomineralization on microbial surfaces [J]. Environmental Science & Technology, 2017,51(5):2776-2785.
[27] Shou W, Kang F, Lu J. Nature and value of freely dissolved EPS ecosystem services: Insight into molecular coupling mechanisms for regulating metal toxicity [J]. Environmental Science & Technology, 2018,52(2):457-466.
[28] Li J, Jiang Z, Chen S, et al. Biochemical changes of polysaccharides and proteins within EPS under Pb(II) stress in Rhodotorula mucilaginosa [J]. Ecotoxicology and Environmental Safety, 2019,174: 484-490.
[29] Braissant O, Decho A W, Przekop K M, et al. Characteristics and turnover of exopolymeric substances in a hypersaline microbial mat [J]. Fems Microbiology Ecology, 2009,67(2):293-307.
[30] Jiang W, Saxena A, Song B, et al. Elucidation of functional groups on gram-positive and gram-negative bacterial surfaces using infrared spectroscopy [J]. Langmuir, 2004,20(26):11433-11442.
[31] Xu Y, Hou M, Ruan J, et al. Effect of magnetic field on surface properties of Bacillus cereus CrA and its Extracellular Polymeric Substances (EPS) [J]. Journal of Adhesion Scienceand Technology, 2014,28(21):2196-2208.
[32] Cheng T W, Lee M L, Ko M S, et al. The heavy metal adsorption characteristics on metakaolin-based geopolymer [J]. Applied Clay Science, 2012,56:90-96.
[33] Dragan E S, Humelnicu D, Dinu M V, et al. Kinetics, equilibrium modeling, and thermodynamics on removal of Cr(VI) ions from aqueous solution using novel composites with strong base anion exchanger microspheres embedded into chitosan/poly(vinyl amine) cryogels [J]. Chemical Engineering Journal, 2017,330:675-691.
[34] Fayazi M. Facile Hydrothermal Synthesis of Magnetic Sepiolite Clay for Removal of Pb(II) from Aqueous Solutions [J]. Analytical and Bioanal ytical ChemistryResearch, 2019,6(1):125-136.
Influence of the stress of Na2S on component changes and sorption behavior on Cu (Ⅱ) and Zn (Ⅱ) ofsp. EPS.
LI Qiu-hua, SONG Wei-feng*, SUN Meng-ge, LI Jia-yao, YU Ze-feng
(School of Environmental Science and Engineering of Guangdong University of Technology, Guangzhou 510006, China)., 2019,39(11):4858~4864
In order to study the mechanism of enhanced adsorption of heavy metals by extracellular polymeric substances (EPS), adsorption of typical heavy metals by the EPS fromsp. induced by Na2S was investigated. The results showed that the maximum EPS production of 105.58mg/g VSS coupling with doubled increase in protein in which the contant of -SH increased by 48.2% from 104.15 to 154.36μmol/L were recorded in the presence of 20mg/L Na2S, As a coinstantaneous process, the maximum adsorption of Cu (Ⅱ) and Zn (Ⅱ) by the S-EPS was observed in the presence of 20mg/L Na2S. The kinetics of the adsorption process of Cu (Ⅱ) and Zn (Ⅱ) by the S-EPS can be well fitted by the Langmuir isotherm and the pseudo-second-order model mode and the theoretical maximum adsorption amount of 1428.57 and 979.09mg/g EPS could be obtained, respectively. The results of 3D-EEM and FTIR analyses indicated that the -SH, protein amide I and amide Ⅱ played a major role in the adsorption of Cu (Ⅱ) and Zn (Ⅱ) by the S-EPS, especially for the adsorption of Zn (Ⅱ). The results obtained in this study demonstrated that the addition of sulfur source could increase the content of sulfhydryl protein, and effectively regulate the content of chemical composition, expecially for the sulfhydryl of EPS, and thereby greatly improving the removal efficiency of heavy metals, which showed a great application prospect in the prevention and control of heavy metal pollution.
EPS;heavy metals;Na2S;sulfhydryl protein;asorbent
X172
A
1000-6932(2019)11-4858-07
李秋华(1992-),女,河南商丘人,广东工业大学硕士研究生,主要从事水中重金属去除研究.发表论文1篇.
2019-04-11
广东省科技计划项目(2014A020209077)
* 责任作者, 教授, weifengsong@gdut.edu.cn
