Relationship Between π-conjugated Bridge and Photophysical Properties of Blue Light-emitting Fluorescent Materials with Twisting A-π-D-π-A Configuration
2019-11-19LUGuojingLIAOXiaoqingLILuGUOZhenGAOLongMIAOYanqinLIJieGUOKunpengXUHuixiaWANGHua
LU Guo-jing, LIAO Xiao-qing, LI Lu, GUO Zhen, GAO Long, MIAO Yan-qin, LI Jie, GUO Kun-peng, XU Hui-xia, WANG Hua*
(1. Key Laboratory of Interface Science and Engineering in Advanced Materials, Ministry of Education,Taiyuan University of Technology, Taiyuan 030024, China;2. Co-innovation Center for Micro/Nano Optoelectronic Materials and Devices,Research Institute for New Materials Technology, Chongqing University of Arts and Science, Chongqing 402160, China;3. College of Material Science & Engineering, Taiyuan University of Technology, Taiyuan 030024, China)*Corresponding Author, E-mail: wanghua001@tyut.edu.cn
Abstract: Blue light-emitting fluorescent materials have great commercial application potential as blue-light emitter for organic light-emitting devices(OLEDs). In this work, a series of blue light-emitting fluorescent materials with twisting A-π-D-π-A configuration were designed and synthesized, in which 4-(9H-carbazol-9-yl)benzeneamine(CzPA) as electron donor unit and trifluoromethylphenyl (FMP) as electron acceptor unit. To investigate the relationship between π-conjugated bridge and photophysical properties, the benzene, 9,9′-dioctyl-9H-fluorene and di(9,9′-dioctyl-9H-fluorene) are introduced as π-conjugated bridge between CzPA and FMP, which are named as CzPA-B-FMP, CzPA-F-FMP, CzPA-DF-FMP, respectively. By comparing photophysical properties in detail, it can be concluded that combining with charge transfer characteristic, the local excitation characteristic of excited state can be enhanced with increasing length of π-conjugated bridge between CzPA and FMP, which can improve the fluorescent quantum efficiency of these materials and external quantum efficiency of blue light OLED. But, too longer π-conjugated units will also deteriorate photophysical properties for bigger intermolecular conjugating effect.
Key words: OLED; blue light-emitting; fluorescent materials; charge transfer; EQE
1 Introduction
At present,the organic light-emitting devices (OLEDs) have been utilized as displays of smart phones, TV, and wearable devices. In this case, it possesses excellent application prospect[1-9]. Up to now, the commercial organic phosphorescent materials used in OLEDs are mainly focusing on the iridium(Ⅲ) complexes, which can fully utilize the singlet state and triplet excitons to achieve nearly 100% internal quantum efficiency(IQE)[2,10-14]. However, the storage of iridium in earth is quite rare, resulting in high cost of iridium(Ⅲ) complexes. Thus, it is necessary to design novel organic fluorescent materials for replacing phosphorescent materials based on iridium(Ⅲ) complexes. Different from phosphorescent materials, organic fluorescent materialsis iridium-free, leading to the low cost of OLEDs. If the IQE of organic fluorescent materials can exceed the theoretical limitation of 25%, they will be expected to become the attractive replacement of phosphorescent materials[15-28].
Recently, the thermally activated delayed fluorescence(TADF)[19-21]materials can achieve a complete separation between the highest occupied molecular orbital(HOMO) and the lowest unoccupied molecular orbital (LUMO), which induces in smaller energy gap (ΔEST) between the singlet and triplet excited states (S1and T1). Therefore, the triplet excitons can transform to singlet excitons by possible reverse intersystem crossing (RISC)[22-23]from T1to S1, inducing in high exciton utilizing efficiency (ηS) above upper limit of 25% and high external quantum efficiency(EQE) eventually. However, due to strong intramolecular charge transfer (CT) in these TADF materials, their broad emission spectra and reddish shift are not conducive to obtaining deep blue electroluminescence (EL) emission (CIEx<0.15,CIEy<0.10).
Meanwhile, CT emission also leads to low fluorescent quantum efficiency(ηPL) because of total separation between HOMO and LUMO. This ideal blue EL emission need to consist of locally-excited (LE) and CT components, which is advantageous to combination of highηPLand highηS. This type of material was first proposed by Ma’s group and named by hybrid locally excited and charge transfer (HLCT) material[23-26]. The earliest reported HLCT material relies on the twisting D-A structure, for example, blue fluorescent material of TPMCN with triphenylamine as electron donor(D) unit and phenanthrene imidazole as electron acceptor (A) unit[26]. TheηSof TPMCN is 85%, however, theηPLof TPMCN is only 13% due to excessive CT characteristic, which induce in lower EQE of 2.2%. So, they designed and prepared the blue HLCT material of TBPMCN, in which the benzene ring is introduced as π-conjugated unit between triphenylamine and phenanthroline for increasing overlap between HOMO and LUMO. It can strengthen LE characteristic of excited state of HLCT materials in EL emission, which can raiseηPLto 40%,ηSto 97% and improve EQE to 7.8%. In addition, the EL emission color of TBPMCN is bluer than that of TPMCN, owing to rather smaller partial ratio CT characteristic of TBPMCN relative to that of TPMCN. Hence, it can be suggested the π-conjugated bridge inserted between the donor and acceptor in HLCT materials expresses close relationship with their luminous efficiency and emission color.
Based on the above consideration, we select the benzene ring (B), 9,9′-dioctyl-9H-fluorene (F) and di(9,9′-dioctyl-9H-fluorene)(DF) as π-conjugated bridge between donor of 4-(9H-carbazol-9-yl)benzeneamine (CzPA) and acceptor of trifluoromethylphenyl (FMP), and designed a series of blue fluorescent materials with twisting A-π-D-π-A configuration[20,31], namely CzPA-B-FMP, CzPA-F-FMP and CzPA-DF-FMP, respectively. By comparing their photophysical properties, the influence of π-conjugated bridge on the device performance was investigated in detail.
2 Experiments
2.1 Synthetic Section
Fig.1 shows the synthetic routes of target materials. All solvents and reagents referred to in this work were commercially available and were used without further purification. All operations involving air-sensitive reagents were carried out under dry nitrogen protection. The chemical structures of all materials were characterized by1HNMR,13C NMR,19F NMR, that used tetramethylsilane (TMS) as internal standard and deuterated chloroform (CDCl3) as the solvent[27-29]. Also, they were characterized by mass spectrometry and elemental analysis fully.

Fig.1 Synthetic routes in this work
2.1.1 CzPA-B-FMP
(1) CzPA-B-Br
The CzPA (2 g, 10 mmol), 1,4-dibromobenzene (7.08 g, 30 mmol), sodium tert-butoxide (1.92 g, 10 mmol), 1,1′-bis(diphenylphosphino)ferrocene (dppf) (0.05 g, 0.09 mmol), tris(dibenzylideneacetone)dipalladium (Pd2(dba)3) (0.05 g, 0.05 mmol) were added into toluene(30 mL). Under N2atmosphere, the mixture was stirred at 90 ℃ for 18 h. After the reaction was completed, the resulting mixture was poured into water and extracted with dichloromethane. The collected organic layer was dried with anhydrous MgSO4, filtered, and then reduced pressure distilled to remove the solvent. Finally, the residue was purified by column chromatography on silicagel eluted with petroleum ether to afford CzPA-B-Br as white powder(2.83 g, 50%).1H NMR(600 MHz, CDCl3)δ8.13(ddd,J=7.7, 4.3, 3.4 Hz, 2H), 7.45-7.32(m, 10H), 7.31-7.25(m, 3H), 7.21-7.19 (m, 2H),7.06-6.99(m, 3H).
(2) CzPA-B-FMP
The CzPA-B-Br (0.56 g, 1 mmol), 4-trifluoromethylphenylboric acid (0.66 g, 3 mmol), tetrakis(triphenylphosphine)platinum (Pd(PPh3)4) (0.058 g, 0.05 mmol), 2 mol/L potassium carbonate aqueous solution (20 mL), toluene (40 mL) were mixed and being refluxing (90 ℃) for 24 h under nitrogen protection. After the reaction was completed, the mixture was poured into water and extracted with dichloromethane. The collected organic layer was dried with anhydrous MgSO4, filtered, and then reduced pressure distilled to remove the solvent. Finally, the residue was purified by column chromatography on silicagel eluted with ethyl acetate/petroleum ether (1∶20) to afford CzPA-B-FMP as the pale yellow powder (0.24 g,36.9%).1H NMR (600 MHz, CDCl3)δ8.14-8.16(ddd,J=5.7, 3.3, 0.9 Hz, 2H), 7.67 (d,J=5.0 Hz, 6H), 7.58-7.54(m, 2H), 7.45-7.38 (m, 10H), 7.31-7.26 (m, 6H), 7.24(dd,J=8.9, 2.4 Hz, 2H).13C NMR (151 MHz, CDCl3)δ144.33, 143.07, 142.18, 141.43, 132.77, 130.98, 128.58, 128.47, 126.84, 126.02, 125.90, 125.88, 123.36, 120.44, 120.39, 119.88, 119.77, 119.05, 118.34, 109.94, 109.90.19F NMR (376 MHz,CDCl3)δ62.28(s). MS(MALDI-TOF,m/z), Found: [M+ matrix]+ 698.232 0; Calcd for C44H28F6N2: [M+matrix]+698.215 7. Elemental anal, Found: C, 75.31; H, 4.56; N, 4.14%. Calcd for C44H28F6N2: C, 75.64; H, 4.04; N, 4.01%.
2.1.2 CzPA-F-FMP
(1) CzPA-F-Br
The 4-(9H-carbazol-9-yl)benzeneamine (CzPA) (0.92 g, 4 mmol), 9,9-dioctyl-2,7-dibromofluorene (8.8 g, 16 mmol), sodium tert-butoxide (1.92 g, 10 mmol), dppf (0.05 g, 0.09 mmol) and Pd2(dba)3(0.05 g, 0.05 mmol) were added into toluene (12 mL). Finally, the mixture was degassed and stirred at 110 ℃under N2atmosphere for 18 h. After the reaction was completed, the mixture was extracted with dichloromethane and saturated brine system, the organic layer was collected and dried with anhydrous magnesium sulfate, filtered, then reduced pressure distillation to remove the organic solvent[30-31]. The crude product was purified by column chromatography on silicagel eluted with dichloromethane/petroleum ether (1∶25) to afford CzPA-F-Br as yellow-green solid (1.28 g, 30%).1H NMR(600 MHz, CDCl3)δ8.16(d,J=7.7 Hz, 2H), 7.61(d,J=8.2 Hz, 2H), 7.50-7.42(m, 10H), 7.33-7.27(m, 4H), 7.25-7.22(m, 2H), 7.17(dd,J=14.3, 7.4 Hz, 4H), 1.95-1.84 (m, 4H), 1.21-1.05 (m, 22H), 0.83-0.75 (m, 6H), 0.69 (d,J=11.1 Hz, 4H).
(2) CzPA-F-FMP
The CzPA-F-Br(0.59 g, 0.5 mmol), 4-trifluoromethylphenylboronic acid (0.54 g, 2 mmol), Pd(PPh3)4(0.058 g, 0.05 mmol), 2 mol/L potassium carbonate aqueous solution (20 mL), toluene (40 mL) were added mixed and degassed. Under N2protection, the mixture was stirred at 110 ℃for 20 h. After reaction, the mixture was poured into water and extracted with dichloromethane. Collected the organic layer, dried over anhydrous MgSO4, filtered and concentrated under reduced pressure to obtain crude product. The crude product was purified by column chromatography on silicagel eluted with dichloromethane/petroleum ether (1∶40) to afford CzPA-F-FMP as yellow-green powder (0.5 g, 23%).1H NMR (600 MHz, CDCl3)δ8.16 (d,J=7.8 Hz, 2H), 7.77 (d,J=8.2 Hz, 4H), 7.72(m, 6H), 7.67 (m, 2H), 7.59 (dd,J=7.9, 1.6 Hz, 2H), 7.54 (d,J=1.5 Hz, 2H), 7.50 (dd,J=8.1, 3.7 Hz, 2H), 7.44 (ddd,J=6.5, 5.6, 5.2 Hz, 4H), 7.39-7.35 (m, 2H), 7.30 (ddd,J=7.5, 5.6, 2.4 Hz, 4H), 7.19 (dd,J=8.2, 2.0 Hz, 2H), 1.97 (m, 8H), 1.19-1.08(m, 40H), 0.80-0.69 (m, 20H).13C NMR (151 MHz, CDCl3)δ152.64, 151.51, 147.01, 145.14, 141.02, 140.98, 137.89, 136.06, 134.95, 127.70, 127.30, 126.24, 125.82, 125.65, 125.62, 125.23, 123.71, 123.43, 123.29, 121.46, 120.80, 120.28, 119.78, 119.61, 119.29, 109.72, 55.29, 40.26, 31.74, 29.99, 29.30, 29.18, 26.88, 24.81, 23.96, 22.55, 13.95.19F NMR (376 MHz, CDCl3)δ62.31(s). MS (MALDI-TOF,m/z),Found: [M+matrix]+1 322.677 0; Calcd for C90H100F6N2: [M+matrix]+1 322.779 1. Elemental anal, Found: C, 81.99; H, 8.149; N, 1.94%. Calcd for C90H100-F6N2: C, 81.66; H, 7.61; N, 2.12%.
2.1.3 CzPA-DF-FMP
(1) Br-F-FMP
The 4-trifluoromethyl benzeneboronic acid (0.756 g, 0.04 mmol), 9,9′-dioctylindole-2,7-dibromoindole (2.2 g, 0.04 mmol), 2 mol/L K2CO3(aq, 20 mL), Pd(PPh3)4(0.05 g, 0.023 mmol), toluene (20 mL) were added into a two-necked flask, and then heated to 60 ℃ for 5 h within the nitrogen atmosphere. After cooling to room temperature, the extraction, filtration operation reference the synthesis of CzPA-F-FMP. The crude product was purified by column chromatography on silicagel eluted with hexane to afford Br-F-FMP as white powder(0.163 g, 55%).1H NMR (600 MHz, CDCl3)δ7.76-7.69(m, 5H), 7.62-7.57(m, 2H), 7.52 (d,J=8.0 Hz, 1H), 7.49-7.45(m, 2H), 2.03-1.88 (m, 4H), 1.38-0.93(m, 23H), 0.84-0.78 (m, 6H), 0.61 (d,J=38.0 Hz, 4H).
(2) Be-F-FMP
The Br-F-FMP(1.22 g, 2 mmol), boronic acid pinacol ester (10 g, 4 mmol), potassium acetate (0.98 g, 10 mmol), [1,1′-bis(diphenylphosphine)ferrocene]palladium dichloride (Pd(dppf)-Cl2) (0.052 g, 0.066 mmol) were dissolved in DMF (40 mL). The temperature of this mixture was raised to 65 ℃under nitrogen atmosphere. After heating for 20 h, the mixture was cooled to room temperature. And, the extraction, filtration operation reference the synthesis of CzPA-F-FMP. The crude product was purified by column chromatography on silicagel eluted with dichloromethane/hexane (1∶35) to afford Be-F-FMP as white powder(0.63 g, 45%).1H NMR(600 MHz, CDCl3)δ7.82(d,J=7.7 Hz, 2H),7.79-7.68(m, 6H), 7.58(dd,J=7.8, 1.5 Hz, 1H),7.55 (s, 1H), 2.01(ddt,J=13.0, 10.0, 6.5 Hz, 4H),1.39 (d,J=6.1 Hz,12H),1.20-1.15 (m,4H),1.13-1.00 (m,16H), 0.79 (m,6H), 0.70-0.56 (m,4H).
(3) CzPA-DF-FMP
The CzPA-F-Br (0.59 g,0.5 mmol),2-(9,9′-dioctyl-7-(4-(trifluoromethyl)phenyl)-9H-fluoren-2-yl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane (Be-F-FMP)(1.32 g,2 mmol),Pd(PPh3)4(0.058 g, 0.05 mmol), 2 mol/L potassium carbonate aqueous solution (20 mL),toluene (40 mL) were added into a two-necked flask and being refluxing (at 90 ℃) for 24 h under nitrogen protection. After cooling to room temperature, the extraction,filtration operation reference the synthesis of CzPA-F-FMP. The crude product was purified by column chromatography on silicagel eluted with dichloromethane/hexane (1∶50) to afford CzPA-DF-FMP as yellow-green powder (0.5 g,23%).1H NMR (600 MHz,CDCl3)δ8.16(d,J=7.7 Hz, 2H),7.80(d,J=7.6 Hz, 7H), 7.76-7.57(m, 20H), 7.52(d,J=8.1 Hz, 2H), 7.46(d,J=7.0 Hz, 4H), 7.41-7.26(m, 7H), 7.22(d,J=7.7 Hz, 2H), 2.13-1.89(m, 16H), 1.14(m, 80H), 0.84-0.71(m, 40H).13C NMR(151 MHz, CDCl3)δ152.81, 152.11, 152.01, 151.62, 147.60, 146.96, 145.40, 141.26, 141.23, 141.09, 140.33, 140.01, 139.74, 138.61, 136.67, 127.89, 127.58, 126.43, 126.37, 126.02, 125.87, 125.85, 123.99, 123.65, 123.49, 121.84, 121.57, 121.50, 120.80, 120.48, 120.33, 119.96, 119.69, 119.63, 109.97, 55.57, 55.47, 40.52, 31.98, 31.92, 30.27, 30.14, 29.56, 29.44, 29.33, 24.24, 24.02, 22.78, 22.74, 14.19, 14.17.19F NMR(376 MHz,CDCl3)δ62.30(s). MS(MALDI-TOF,m/z),Found: [M+matrix]+2100.4740; Calcd for C148H180F6N2: [M+matrix]+2 100.408 4. Elemental anal, Found: C,84.32; H,8.66; N,1.47%. Calcd for C148H180F6N2:C,84.61; H, 8.64; N, 1.33%.
2.2 Measurements Section
The ultraviolet visible(UV-vis) absorption spectrum and the photoluminescence (PL) spectrum were recorded with Hitachi U-3900 UV-Vis spectrophotometer and Horiba Fluoromax-4 fluorescence spectrophotometer,respectively. The HOMO and LUMO energy levels of the materials were determined by cyclic voltammetry (CV) and spectrometry. The CV was measured in CIE 660E electrochemical, and tetrabutyl ammonium perchlorate-acetonitrile solution with a concentration of 0.1 mol/L was used as the electrolyte, the measurements were working at a scan rate of 100 mV/s with a conventional three-electrode configuration consisting of a glassy carbon working electrode,a platinum wire auxiliary electrode, and a saturated calomel reference electrode calibrated with ferrocene/ferrocenium (Fc/Fc+).
2.3 Fabrication of OLEDs

3 Results and Discussion
3.1 UV-Vis Absorption Spectra
To study the effect of different π-conjugated bridge on the ground state electronic transition,we tested the UV-vis spectra of all materials in the dilute solvent (THF, 10-6mol/L). As shown in Fig.2, all materials exhibit almost same absorption bands in the short-wavelength region, the absorption peak at 200-250 nm corresponding to n-π*electronic transition around the nitrogen atom, the absorption peak at 250-300 nm corresponding to the π-π*absorption band of benzene rings. Moreover, all materials exhibit a weak absorption band in the long-wavelength region, which corresponds to CT from D unit of CzPA to A unit of FMP. It can be seen in Fig.2 and Tab.1 that the absorption band at 300-450 nm of three materials shows red-shift with increasing length of π-conjugated bridge between CzPA and FMP.

Fig.2 UV-Vis absorption spectra of materials in THF solution with concentration of 1.0×10-6mol/L
3.2 PL Spectra
To acquire a better understanding of its excited state, properties of materials were investigated by characterizing its photoluminescence (PL) spectra in dilute solution(THF, 10-6mol/L). As shown in Fig.3, the PL spectra of CzPA-B-FMP, CzPA-F-FMP and CzPA-DF-FMP in THF solution show the blue emission peaks (λPL) located at 438, 467, 456 nm, respectively. Among them, CzPA-B-FMP exhibits smallerλPLrelative to those of CzPA-F-FMP and CzPA-DF-FMP. It is because of weaker intermolecular π-conjugation effect in CzPA-B-FMP molecules comparing with those in CzPA-F-FMP molecules and CzPA-DF-FMP molecules. So, it can be suggested that prolonging the π-conjugated bridge between donor and acceptor can enhance intermolecular π-conjugation effect and lead to red-shift ofλPLin PL spectra of blue fluorescent materials with twisting A-π-D-π-A configuration.

Fig.3 PL spectra of three materials measured in THF solution (10-6mol/L)
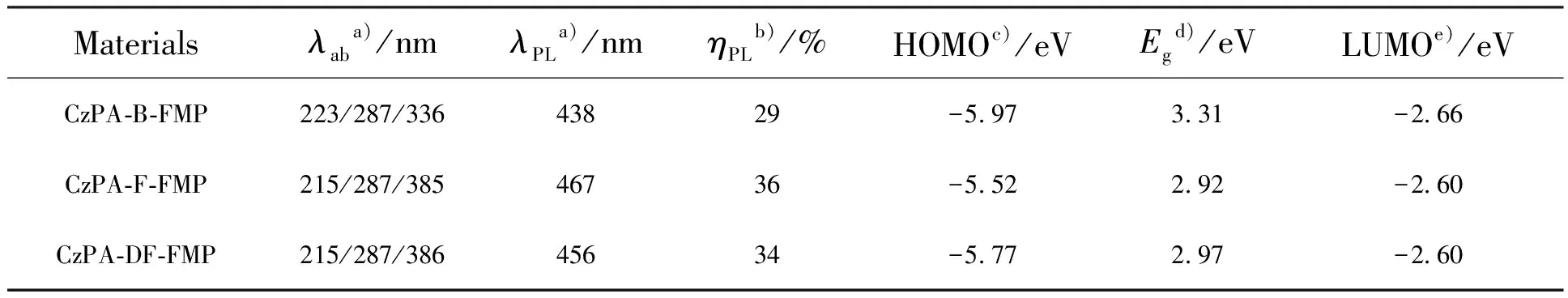
Tab.1 Photophysical properties data of materials
a)Measured in solution.b)Absolute PL quantum yield in film by using an integrating sphere.c)Detectedviacyclic voltammetry.d)Estimated by the UV-Vis and PL spectrum.e)Estimated by deductingEgfrom HOMO levels.
3.3 Solvation Effect
In order to investigate the solvatochromic effect of all materials, the PL spectra of all materials in different polar solvents were measured. As shown in Fig.4, all materials showed remarkable solvatochromic effect. With the increasing of solvent polarity from n-hexane(f=0.001 2) to DMSO(f=0.305), theλPLin PL spectra of CzPA-B-FMP, CzPA-F-FMP and CzPA-DF-FMP exhibits bathochromic shift for 79, 86, 77 nm, respectively. It can be deduced that excited state of three materials shows typical CT characteristics.

Fig.4 PL spectra of materials in different polar solvents with concentration of 1.0×10-6mol/L. (a) CzPA-B-FMP. (b) CzPA-F-FMP. (c) CzPA-DF-FMP.
For further study on CT characteristic of excited states, the relationship between Stokes shift (νa-νf) and the orientation polarizability of solventf(ε,n) was investigated to estimate the excited-state dipole moment(μe), according to Lippert-Matagamodel[31-32].
(1)

As shown in Fig.5(a), the fitting correlation of Stokes shift againstf(ε,n) of CzPA-B-FMP is in straight line (The specific data were shown in Tab.2), which means that theμe(1.348×10-29C·m) of CzPA-B-FMP doesn’t vary with the increasing of solvent polarityf(ε,n). It is identified that excited state of CzPA-B-FMP exhibits only CT characteristic.
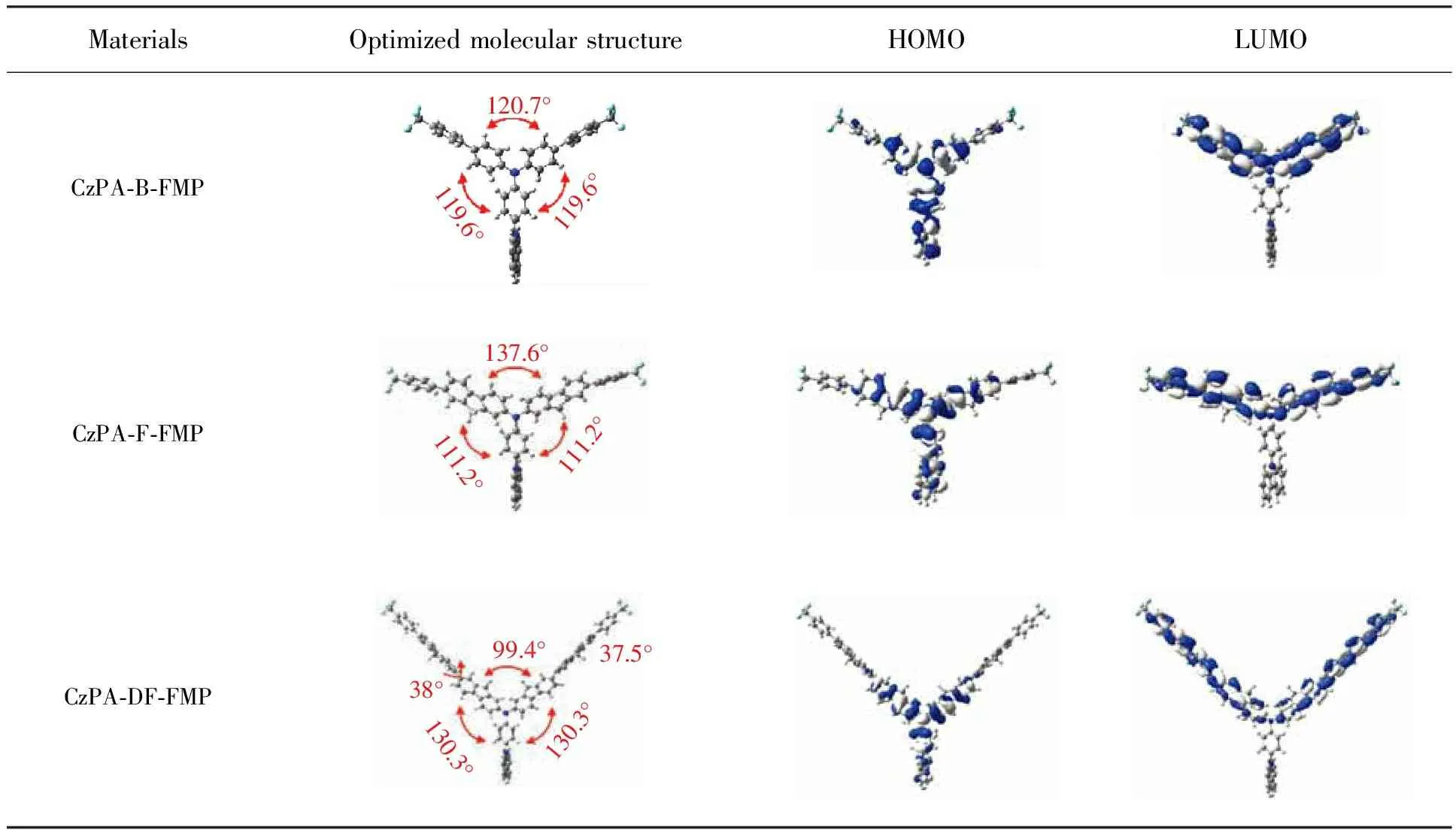
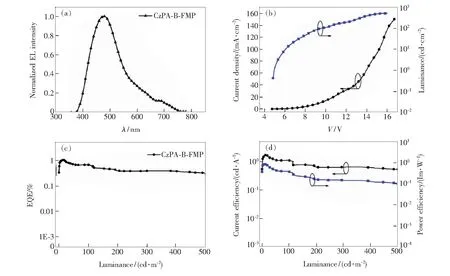
As depicted in Fig.5(b) and Fig.5(c), for CzPA-F-FMP and CzPA-DF-FMP, the slope of Stokes shift againstf(ε,n) in low polar and high polar solvents is different (The specific datas were shown in Tab.2). In low-polar solvents (f<0.096),Stokes shift shows little change with the increasing of solvent polarity, and theμeof CzPA-F-FMP and CzPA-DF-FMP was calculated to be 3.583×10-29C·m and 5.928×10-29C·m, respectively. In the high-polar solvent (f>0.149), the Stokes shift rises linearly with the increasing of solvent polarity, and theμeof CzPA-F-FMP and CzPA-DF-FMP is calculated to be 1.075×10-28C·m and 1.314×10-28C·m, respectively. It is indicates that excited state of CzPA-F-FMP and CzPA-DF-FMP exhibits intercrossed LE characteristic and CT characteristic: LE characteristic dominates excited state in low-polarity solvents (f<0.096), CT characteristic dominates excited state in high-polarity solvents (f>0.149), and hybrid LE characteristic and CT characteristic in moderate polarity solution (0.096 Fig.5 Fitted correlation of the Stokes shift as a function of solvent polarity. (a) CzPA-B-FMP. (b) CzPA-F-FMP. (c) CzPA-DF-FMP. In addition, it also can be seen that theμeof CzPA-DF-FMP is much larger than that of CzPA-F-FMP, which is due to the larger distance between CzPA and FMP for longer π-conjugated bridge in CzPA-DF-FMP relative to those in CzPA-F-FMP. It will lead rather lower CT efficiency in CzPA-DF-FMP than that in CzPA-F-FMP, which induces in lowerηSand EQE eventually. Tab.2 Photophysical properties of the materials in various solvents The molecular geometries and the frontier molecular orbitals of three materials were optimized by density functional theory(DFT) calculations[36]at the B3LYP/6-311G*(d,p) level. As shown in Fig.6, all materials adopted moderately twisting A-π-D-π-A configuration. It is observed that the dihedral angles between the two π-conjugated bridges(i.e. B, F and DF) are 120.74°, 137.60° and 99.41° in CzPA-B-FMP, CzPA-F-FMP, CzPA-DF-FMP, respectively. The dihedral angles between FMP and π-conjugated bridge(i.e. B, F and DF) are 37.5°, 38.5°, 38.3° in CzPA-B-FMP, CzPA-F-FMP CzPA-DF-FMP, respectively. It is important that above twisting A-π-D-π-A configuration causes spatial separation between HOMO and LUMO for achieving CT from CzPAF to TMP, resulting in possible RISC from Tmto Sn. So, all materials can achieve highηSabove upper limit of 25%. MaterialsOptimized molecular structureHOMOLUMOCzPA-B-FMP119.6°120.7°119.6°CzPA-F-FMP111.2°137.6°111.2°CzPA-DF-FMP130.3°130.3°38°37.5°99.4° Fig.6 Optimized structure and the calculated HOMO/LUMO spatial distributions of all materials It can be seen in Fig.6 that the molecular steric structures of all materials vary with increasing length of π-conjugated bridge. In CzPA-B-FMP, the B unit as π-conjugated bridge is the shortest, which induces in stiff molecular structure with “Y” shape and smaller overlap of 0.443 9 between HOMO and LUMO. It is speculated that CT characteristic is major in excited state of CzPA-B-FMP. Relative to CzPA-B-FMP, the F unit as π-conjugated bridge in CzPA-F-FMP is longer inducing rather planar structure with “T” shape, which leads to bigger overlap of 0.521 6 between HOMO and LUMO. It is speculated that excited state of CzPA-F-FMP emerges LE characteristic. Furthermore, the length of DF unit as π-conjugated bridge in CzPA-DF-FMP is double that of F unit, which leads to stiff molecular structure with “wing” shape. As expressed in Fig.6, it can be found that the overlap part between HOMO and LUMO is nearly identical with that of CzPA-F-FMP, which mainly distributes on amine with adjacent two fluorene units. But, the distance between CzPA and FMP is the longest among three materials, which results in the lowest CT efficiency from CzPA to FMP. So, it is suggested that excited state of CzPA-DF-FMP expresses lower LE characteristic relative to that of CzPA-F-FMP. So, it is concluded that the partial ratio of LE characteristic to CT characteristic in excited state of blue fluorescence materials with twisting A-π-D-π-A configuration increases with the prolonging of π-conjugated bridge between D unit and A unit. To identify the energy level, we acquired theEHOMOof three materials by cyclic voltammetry. TheEHOMOof CzPA-B-FMP, CzPA-F-FMP, CzPA-DF-FMP is -5.97, -5.52, -5.77 eV, respectively(Fig.7). The optical band gap (Eg) was obtained byEg=1240/λedge. And theEgwas calculated to be 3.31, 2.92,2.97 eV for CzPA-B-FMP, CzPA-F-FMP and CzPA-DF-FMP. Accordingly, it can be determined that theELUMOof CzPA-B-FMP,CzPA-F-FMP, CzPA-DF-FMP is -2.66, -2.60, -2.60 eV, respectively. It implies that the increasing of π-conjugated unit between CzPA and FMP can reduce theEg, which shows litter influence onELUMO. Fig.7 Cyclic voltammograms in tetrahydrofuran solution of all materials Furthermore, the blue light OLEDs based on three materials as emitter were fabricated. The device configuration of OLEDs were ITO/PEDOT∶PSS(40 nm)/PVK∶OXD-7∶(CzPA-B-FMP, CzPA-F-FMP, CzPA-DF-FMP) (50∶40∶10, 50 nm)/TPBi (30 nm)/LiF(1 nm)/Al(100 nm), which named with DeviceⅠ(for CzPA-B-FMP), DeviceⅡ(for CzPA-F-FMP) and Device Ⅲ(for CzPA-DF-FMP). As shown in Fig.8 and Fig.9, it can be seen that the emission peaks (λEL)in EL spectra of DeviceⅠ, DeviceⅡand Device Ⅲ locates at 479, 453, 443 nm, respectively. It also can be found that theλELof DeviceⅠis the longest, owing to major CT characteristics of CzPA-B-FMP different from CzPA-F-FMP and CzPA-DF-FMP. And theλELof DeviceⅡis longer than that of Device Ⅲ, attributing to lower CT characteristics in CzPA-DF-FMP than that in CzPA-F-FMP. So, it can be suggested that the π-conjugated bridge between the CzPA and FMP shows close relationship with EL spectra. When the length of π-conjugated bridge is too short, the EL emission of material exhibits major CT characteristic, which brings the red shift and broadening of blue-emission peak in EL spectra,e.g. CzPA-B-FMP. When the π-conjugated bridge gets longer, the EL emission of material exhibits CT characteristic combining with LE characteristic, which induces in the blue shift and narrowing of blue-emission peak in EL spectra,e.g. CzPA-F-FMP and CzPA-DF-FMP. So, it can be deduced proper length of π-conjugated bridge between D unit and A unit can realize pure blue-light emission of blue fluorescent compounds with twisting A-π-D-π-A configuration. Fig.8 EL performance of DeviceⅠ(CzPA-B-FMP). (a) EL spectrum at 7 V. (b)J-V-Lcurve. (c) EQE-Lcurve. (d) CE-L-PE curve. Tab.3 EL performance data in this work Fig.9 EL performance of Device Ⅱ(CzPA-F-FMP) and Device Ⅲ (CzPA-DF-FMP). ( a) EL spectra at 7 V. (b)J-V-Lcurve. (c) EQE-Lcurve. (d) CE-L-PE curve. As expressed in Fig.8 and Fig.9, the DeviceⅡexhibits the best device performance among three devices, for example, the highest EQE of 5.31%. It is since that the proper length of π-conjugated bridge between CzPA and FMP can realize effective equilibrium of LE characteristic and CT characteristic during EL emission of CzPA-F-FMP, which will ensure higherηPLon the premise of highηS. And the smallest EQE of DeviceⅠ(1.14%) is because of smallestηPLof CzPA-B-FMP, owing to the shortest π-conjugated bridge of benzene unit. But, the EQE of Device Ⅲ(3.41%) is smaller than that of DeviceⅡ, due to more nonradiative transition originated from intermolecular conjugating effect in CzPA-DF-FMP relative to that in CzPA-F-FMP. In addition, theηSof three materials was calculated by the following formula: ηEQE=γ×ηS×ηPL×ηout, (2) Whereηoutis light out-coupling efficiency(≈ 20%),γis the ideal recombination efficiency of excitons (≈100%)[37-38]. According to the above formula, theηSof CzPA-B-FMP, CzPA-F-FMP and CzPA-DF-FMP is estimated to be 19.6%, 72.1% and 40.9%, respectively. Among them,ηSof CzPA-F-FMP and CzPA-DF-FMP is much higher than upper limit ofηS(25%) of traditional fluorescent materials, however, theηSof CzPA-B-FMP is rather lower. Because theηSof fluorescent material with twisting D-A configuration is determined by such factors as the RISC efficiency, radiative transition efficiency and nonradiative transition efficiency, it is expected that the π-conjugated bridge also influenced above exciton dynamic process[39-40]. It need further work on disclosing the relationship between π-conjugated bridge andηS, which will be discussed in our future work. In summary, a series of blue fluorescent materials with twisting A-π-D-π-A configuration (i.e. CzPA-B-FMP, CzPA-F-FMP, CzPA-DF-FMP) was designed and prepared. The photophysical properties of three materials were investigated in detail. And, the relationship between π-conjugated units and photophysical properties was discussed. With increasing the length of π-conjugated bridge between CzPA and FMP, the LE characteristic of excited state enhanced combining with CT characteristic. It can improve the blue EL emission, highηPLand EQE. But, too longer π-conjugated bridge will also deteriorate blue EL emission for longer intermolecular conjugating effect. Therefore, based on the above conclusion, the blue OLED with the CzPA-F-FMP as emitter exhibits EL emission peak located at 443 nm, higher EQE of 5.31%, and highηSof 72.1%. This conclusion implies that by adjusting the length of π-conjugated bridge between donor and acceptor in fluorescent materials with twisting A-π-D-π-A configuration can realize the control of photophysical properties, and the proper length π-conjugated bridge will give rise to good photophysical properties.
3.4 Time-dependent Density Function(TD-DFT) Calculation
3.5 Energy Level Structure

3.6 Electroluminescence(EL) Performance


4 Conclusion
