大鼠脑损伤联合他克莫司促进坐骨神经再生的效果
2019-11-14何新泽林书卿杨涛王成刚李芹于立志孙金占王培
何新泽 林书卿 杨涛 王成刚 李芹 于立志 孙金占 王培
[摘要]目的 探讨大鼠脑损伤联合他克莫司促进坐骨神经再生的效果。方法 选取180只SD大鼠,随机分为4组,每组各45只,按照颅脑损伤模型(Feeney法)和坐骨神经损伤模型(Sunderland V型)造模,A1组:颅脑损伤合并坐骨神经损伤联合应用他克莫司;A2组:颅脑损伤合并坐骨神经损伤联合应用生理盐水;B1组:单纯坐骨神经损伤联合应用他克莫司;B2组:单纯坐骨神经损伤联合应用生理盐水。造模后第4、8、12周,测定坐骨神经指数(SFI)、腓肠肌恢复率,进行神经组织Masson染色;造模后第8、12周观察脊髓神经元辣根过氧化物(HRP)的逆行示踪标记。结果 造模后第4周,A1组与A2组、B1组与B2组的SFI比较,差异无统计学意义(P>0.05);造模后第4周,A1、A2组的SFI均优于B1、B2组,差异有统计学意义(P<0.01);造模后第8、12周,A2组与B1组的SFI比较,差异无统计学意义(P>0.05);造模后第8、12周,A1组的SFI优于A2、B1、B2组,A2、B1组的SFI均优于B2组,差异有统计学意义(P<0.01);4周后,各组大鼠的SFI逐渐升高(P<0.01)。造模后第4周,A2、B1、B2组的腓肠肌恢复率比较,差异无统计学意义(P>0.05);A1组造模后第4周的腓肠肌恢复率高于A2、B1、B2组,差异有统计学意义(P<0.01);造模后第8、12周,A1、A2、B1组的腓肠肌恢复率均明显高于B2组,差异有统计学意义(P<0.01);造模后第8、12周,A1组与A2组的腓肠肌恢复率比较,差异无统计学意义(P>0.05);A1、A2组造模后第8周的腓肠肌恢复率均高于B1组,差异有统计学意义(P<0.01);造模后第12周,A1组的腓肠肌恢复率高于B1组,差异有统计学意义(P<0.01);A2組与B1组造模后第12周的腓肠肌恢复率比较,差异无统计学意义(P>0.05);4周后,各组大鼠的腓肠肌恢复率逐渐升高(P<0.01)。Masson染色观察造模后第12周A1组胶原纤维均匀分布,并呈波浪形随神经纤维排列,A2、B1组可见绿染与红染分布均匀,B2组可见大量胶原纤维,少量再生的轴突。造模后第8周,A1组的HRP标记阳性率高于A2、B1、B2组,A2组的HRP标记阳性率高于B1、B2组,B1组的HRP标记阳性率高于B2组,差异有统计学意义(P<0.01);造模后第12周,A1组的HRP标记阳性率高于A2、B1、B2组,A2、B1组的HRP标记阳性率均高于B2组,差异有统计学意义(P<0.01);A2组造模后第12周的HRP标记阳性率与B1组比较,差异无统计学意义(P>0.05)。结论 脑损伤合并周围神经损伤联合应用他克莫司干预可促进神经损伤修复,颅脑损伤与他克莫司作用的机制不完全相同,脑损伤促进周围神经损伤修复的机制仍需进一步深入研究。
[关键词]颅脑损伤;周围神经;神经修复与再生;他克莫司
[中图分类号] R318 [文献标识码] A [文章编号] 1674-4721(2019)9(a)-0007-06
Effect of rat brain injury combined with Tacrolimus on promoting sciatic nerve regeneration
HE Xin-ze1 LIN Shu-qing1 YANG Tao1 WANG Cheng-gang1 LI Qin1 YU Li-zhi2 SUN Jin-zhan2 WANG Pei3
1. Department of Emergency, Binzhou Central Hospital, Shandong Province, Binzhou 251700, China; 2. Department of Trauma Orthopedics, Binzhou Central Hospital, Shandong Province, Binzhou 251700, China; 3. Department of Hand and Foot Surgery, Affiliated Hospital of Chengde Medical University, Hebei Province, Chengde 067000, China
[Abstract] Objective To investigate the effect of rat brain injury combined with Tacrolimus on promoting sciatic nerve regeneration. Methods A total of 180 SD rats were randomly divided into 4 groups with 45 rats in each group and modeled according to the craniocerebral injury model (Feeney method) and the sciatic nerve injury model (Sunderland V type). Group A1: cerebral injury combined with sciatic nerve injury and Tacrolimus; group A2: saline in cerebral injury combined with sciatic nerve injury; group B1: sciatic nerve injury combined with Tacrolimus; group B2: simple sciatic nerve injury combined saline. At the fourth, eighth, and twelfth weeks after modeling, the sciatic nerve function index (SFI) and the gastrocnemius recovery rate were measured, and the tissue was subjected to Masson staining. Retrograde tracer markers of spinal cord neurons horseradish peroxide (HRP) were observed at the eighth and twelfth weeks after modeling. Results At the fourth week after modeling, there was no significant difference in SFI between group A1 and group A2, group B1 and group B2 (P>0.05). At the fourth week after modeling, the SFI of the group A1 and A2 was better than that of the group B1 and B2, and the difference was statistically significant (P<0.01). At the eighth and twelfth weeks after modeling, there was no significant difference in SFI between group A2 and group B1 (P>0.05). At the eighth and twelfth weeks after modeling, the SFI of group A1 was better than that of group A2, B1 and B2, the SFI of group A2 and group B1 was better than that of group B2, and the differences were statistically significant (P<0.01). After 4 weeks, the SFI of each group of rats gradually increased (P<0.01). At the fourth week after modeling, the recovery rate of gastrocnemius muscle in group A2, B1 and B2 was not statistically significant (P>0.05). The recovery rate of gastrocnemius muscle at the fourth week after modeling in group A1 was higher than that in group A2, B1 and B2, and the differences were statistically significant (P<0.01). At the eighth and twelfth weeks after modeling, the recovery rate of gastrocnemius muscle in group A1, A2 and B1 was significantly higher than that in group B2, and the differences were statistically significant (P<0.01). At the eighth and twelfth weeks after modeling, there was no significant difference in the recovery rate of gastrocnemius between group A1 and group A2 (P>0.05). The recovery rate of gastrocnemius muscle in the group A1 and A2 was higher than that in the group B1 at the eighth week after modeling, and the differences were statistically significant (P<0.01). At the twelfth week after modeling, the recovery rate of gastrocnemius muscle in group A1 was higher than that in group B1, and the difference was statistically significant (P<0.01). There was no significant difference in the recovery rate of gastrocnemius muscle between the group A2 and the group B1 at the twelfth week after modeling (P>0.05). After 4 weeks, the recovery rate of gastrocnemius muscle in each group gradually increased (P<0.01). Masson staining showed that the collagen fibers at the twelfth week after modeling in the group A1 were evenly distributed, and they were arranged in a wave shape with nerve fibers, the green staining and red staining distribution were uniform in the group A2 and the group B1, and a large number of collagen fibers were observed in the group B2. At the eighth week after modeling, the positive rate of HRP label in group A1 was higher than that in group A2, B1 and B2, the positive rate of HRP label in group A2 was higher than that in group B1 and group B2, and the positive rate of HRP label in group B1 was higher than that in group B2, with statistically significant differences (P<0.01). At the twelfth week after modeling, the positive rate of HRP label in group A1 was higher than that in group A2, group B1 and group B2, and the positive rate of HRP label in group A2 and group B1 was higher than that in group B2, with statistically significant differences (P<0.01). There was no significant difference in the positive rate of HRP label at the twelfth week after modeling between group A2 and group B1 (P>0.05). Conclusion Brain injury combined with peripheral nerve injury and Tacrolimus intervention can promote the repair of nerve damage. The mechanism of brain injury and Tacrolimus is not exactly the same. The mechanism of brain damage promoting peripheral nerve injury repair needs further study.
[Key words] Craniocerebral injury; Peripheral nerve; Nerve repair and regeneration; Tacrolimus
临床工作中,颅脑损伤合并周围神经损伤的患者并不罕见,神经损伤后的恢复具有特殊性,功能恢复不全,致残率较高[1-5]。Wang等[2-9]研究发现,实验大鼠在颅脑损伤后合并周围神经损伤,损伤坐骨神经的修复速度加快,但修复机制尚不清楚。研究发现他克莫司可促进损伤神经修复再生[10-15]。大量的实验发现,他克莫司促进周围神经损伤后的再生,主要通过免疫抑制、神经营养来实现[16-22]。本研究拟通过他克莫司促進神经损伤恢复机制,探索颅脑损伤促进神经损伤恢复的机制。
1材料与方法
1.1造模动物与材料
本实验于2018年10月~2019年2月在滨州市中心医院完成。
实验动物:选用SPF级雄性SD大鼠[北京维通利华实验动物技术有限公司,许可证号:SCXK(京)2012-0001]180只,200~220 g。饲养在昼夜各12 h节律下,实验室温度维持在(23±1)℃。将大鼠完全随机分为4组,每组各45只。本研究经过滨州市中心医院动物伦理委员会批准,按照国际动物实验标准执行。
主要设备及试剂:手术显微镜(LZL-6A型,镇江中天公司);光学显微镜(BH-3型,Olympus公司);电子分析天平(ESJ200-4±0.0001 g);他克莫司(Tacrolimus)100 mg(Selleck);Masson三色染色试剂盒(标准型,江苏KeyGEN生物);Peroxidase来源于辣根100 mg(Sigma)。
1.2造模方法
1.2.1造模 禁食水4 h,术前30 min,肌肉注射头孢唑啉钠(鲁抗制药,20180120)10 mg/100 g预防感染,术区备皮剪毛。10%水合氯醛按照0.35 ml/100 g进行腹腔全麻;A1组和A2组:消毒,沿头部正中矢状切开,在颅骨冠状线后1.5 mm、中线偏左5 mm处开直径5 mm骨窗,撞击造成中度脑损伤;右侧臀部,显微镜下完全切断坐骨神经,9-0无损伤线缝合坐骨神经外膜4~6针[11]。B1组和B2组:大鼠仅于显微镜下完全切断右侧坐骨神经,缝合坐骨神经外膜。
1.2.2造模后干预 分笼饲养在同样环境中。他克莫司用0.9%氯化钠稀释,1 ml 0.9%氯化钠+1 mg他克莫司,现用现配制,4℃恒温保存;造模手术12 h后,A1组大鼠给予他克莫司,按1 mg/200 mg腹腔注射;A2组给予生理盐水,按 1 ml/200 mg腹腔注射;B1组大鼠给予他克莫司,按1 mg/200 mg腹腔注射;B2组大鼠给予生理盐水,按1 ml/200 mg腹腔注射。1次/d,连续2周。观察各组大鼠存活情况。
1.3观察指标
1.3.1坐骨神经指数(sciatic nerve function index,SFI)的测量 分别于造模后第4、8、12周,各组每次随机取10只大鼠。根据Schiaveto de Souza A的方法[8],实验侧足3个参数分别为:实验侧足印长度(SPL)、实验侧足趾宽度(STS)、实验侧中间足趾距离(SIT);正常侧足3个参数分别为正常侧足印长度(ZPL)、正常侧足趾宽度(ZTS)、正常侧中间足趾距离(ZIT)。SFI=-38.3×(SPL-ZPL)/ZPL+109.5×(STS-ZTS)/ZTS+13.2×(SIT-ZIT)/ZIT-8.8。以SFI值0时为正常,-100时为神经完全断离。
1.3.2腓肠肌恢复率 造模后第4、8、12周,各组随机取5只大鼠,取下完整的双侧腓肠肌,吸取肌肉周围的血液,双侧腓肠肌在分析天平上称重。计算腓肠肌的恢复率,推测运动功能恢复的情况。
1.3.3神经Masson染色 造模后第4、8、12周,各组随机取5只大鼠,切取吻合口远、近端0.5 cm坐骨神经,10%甲醛固定,梯度酒精脱水,石蜡包埋,切片厚度5 μm,染色、封片,光学显微镜下观察神经纤维、胶原纤维增生情况。
1.3.4辣根过氧化物酶(HRP)示踪 分别于造模后第8、12周,于坐骨神经断端以远0.5 cm处,注入30%HRP溶液5 μl。深麻醉下开胸,灌流固定,取坐骨神经相应脊髓节段,横断面连续振荡切片30 μm。光镜下统计脊髓前角运动神经元中被标记为蓝染颗粒的胞体数目。
1.4统计学方法
采用SPSS 19.0统计学软件进行数据分析,计量资料用均数±标准差(x±s)表示,两组间比较采用t检验,以P<0.05为差异有统计学意义。
2结果
2.1造模后各组大鼠基本生活状态的观察
造模后所有动物均存活,未发现切口感染情况。造模手术后5 d,B2组大鼠出现足部红肿明显,其他组红肿较轻;3周后,A2、B1、B2组大鼠足跟出现程度不同的溃疡面,A1组大鼠足部未见明显溃疡;12周时,各组大鼠足部溃疡基本痊愈,B2组大鼠出现足部的自食现象。
2.2各组大鼠造模后第4、8、12周SFI的比较
暗箱测试结果显示,造模后第4周,A1组与A2组、B1组与B2组的SFI比较,差异无统计学意义(P>0.05);造模后第4周,A1、A2组的SFI均优于B1、B2组,差异有统计学意义(P<0.01);造模后第8、12周,A2组与B1组的SFI比较,差异无统计学意义(P>0.05);造模后第8、12周,A1组的SFI优于A2、B1、B2组,A2、B1组的SFI均优于B2组,差异有统计学意义(P<0.01);4周后,各组大鼠的SFI逐渐升高(P<0.01)(表1)。
2.3各組大鼠造模后第4、8、12周腓肠肌恢复率的比较
4周后取材可见各组大鼠失神经支配的腓肠肌颜色均较健侧苍白,肌肉明显萎缩。
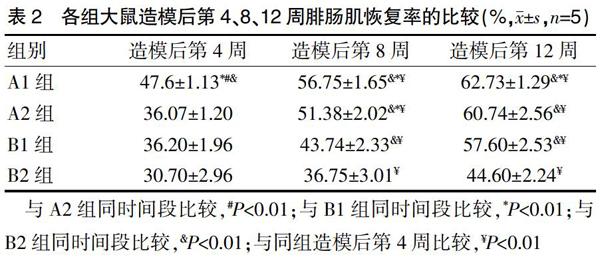
造模后第4周,A2、B1、B2组的腓肠肌恢复率比较,差异无统计学意义(P>0.05);A1组造模后第4周的腓肠肌恢复率高于A2、B1、B2组,差异有统计学意义(P<0.01);造模后第8、12周,A1、A2、B1组的腓肠肌恢复率均明显高于B2组,差异有统计学意义(P<0.01);造模后第8、12周,A1组与A2组的腓肠肌恢复率比较,差异无统计学意义(P>0.05);A1、A2组造模后第8周的腓肠肌恢复率均高于B1组,差异有统计学意义(P<0.01);造模后第12周,A1组的腓肠肌恢复率高于B1组,差异有统计学意义(P<0.01);A2组与B1组造模后第12周的腓肠肌恢复率比较,差异无统计学意义(P>0.05);4周后,各组大鼠的腓肠肌恢复率逐渐升高(P<0.01)(表2)。
2.4各组大鼠造模后第4、8、12周神经Masson三色法染色情况
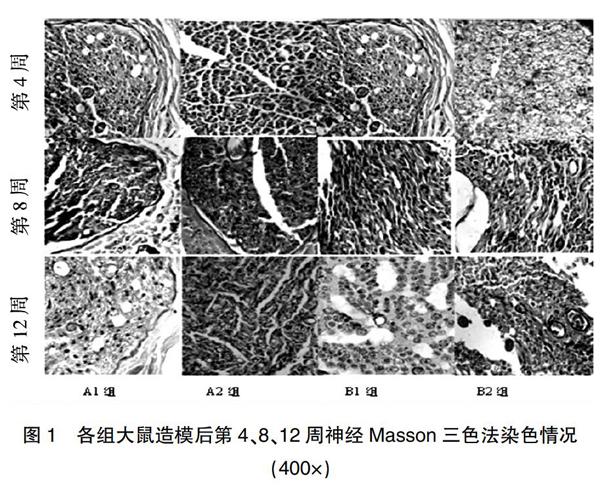
造模后第4周,A1、B1组神经纤维红染较多,绿色纤维较少,A2组绿染与红染均匀,B2组未见红染的神经纤维;造模后第8周,A1、A2、B1组可见绿染与红染均匀分布,B2组可见再生的轴突;造模后第12周,A1组胶原纤维均匀分布,并呈波浪形随神经纤维排列,A2、B1组可见绿染与红染分布均匀,B2组可见大量胶原纤维,少量再生的轴突(图1)。
2.5各组大鼠造模后第8、12周神经元HRP标记情况
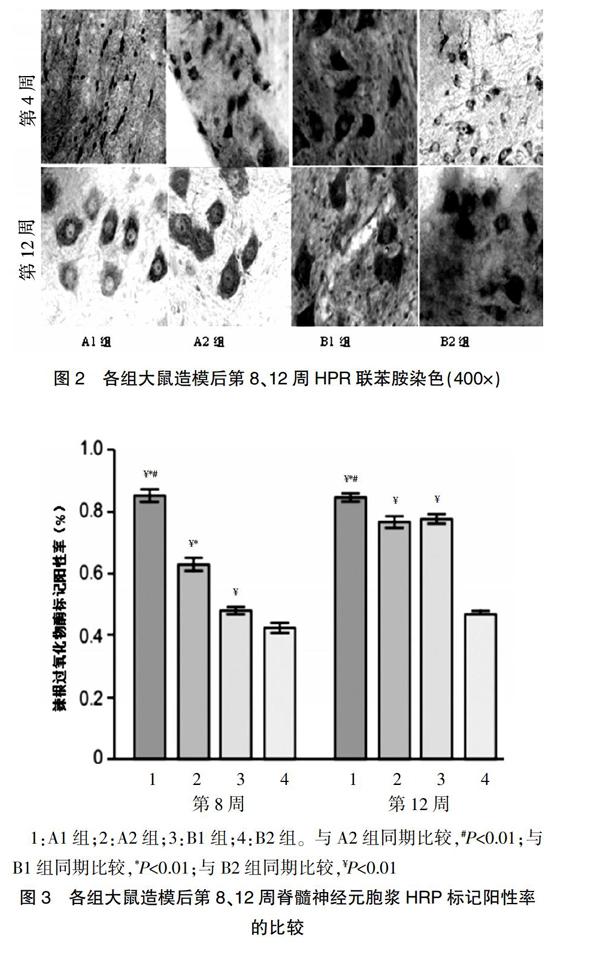
光镜下,相应脊髓节段的前角神经元细胞胞浆中发现标记成蓝黑色的HRP阳性颗粒细胞(图2)。
每高倍视野下进行计数,统计分析结果显示,造模后第8周,A1组的HRP标记阳性率高于A2、B1、B2组,A2组的HRP标记阳性率高于B1、B2组,B1组的HRP标记阳性率高于B2组,差异有统计学意义(P<0.01);造模后第12周,A1组的HRP标记阳性率高于A2、B1、B2组,A2、B1组的HRP标记阳性率均高于B2组,差异有统计学意义(P<0.01);A2组造模后第12周的HRP标记阳性率与B1组比较,差异无统计学意义(P>0.05)(图3)。
1:A1组;2:A2组;3:B1组;4:B2组。与A2组同期比较,#P<0.01;与B1组同期比较,*P<0.01;与B2组同期比较,¥P<0.01
3讨论
何新泽等[1,22-33]动物实验发现,大鼠脑损伤后对损伤的坐骨神经修复重建有明显促进作用,但具体修复机制尚不清楚。本研究通过动物实验比较颅脑损伤联合应用他克莫司的坐骨神经损伤大鼠,损伤后的坐骨神经损伤恢复的情况。在所有的实验观测项目中,颅脑损伤联合他克莫司(A1组)的坐骨神经修复效果优于其他组(P<0.05),提示颅脑损伤可以与他克莫司协同促进坐骨神经损伤后的修复,其作用机制与他克莫司不完全相同。
在Sunderland V型的外围神经损伤后,神经损伤切断了神经内分泌的养分,不能将其输送到靶子器官上,靶子器官将失去活性和营养的支持。根据目前的他克莫司促进神经机制,促进外围神经修复相对明确是两个功能领域,发挥神经营养功能,形成一个FKBP12的综合体,加入功能地区后表达GAP-43,一个超蛋白质神经生长,并促进形成和扩大神经的生长由神经脉冲生成的生物电能、靶器官,再生轴突的内径上升,厚厚的神经膜层,作用范围增加[22-23]。这证实了他克莫司有助于恢复外围神经的营养功能,萎缩身体的部分看起来更容易溃疡,颅脑损伤联合他克莫司组恢复较好,提示促进外形神经损伤的作用,结合功能或复杂的FKBP12地区来促进表达GAP-43。
在外围神经损伤后,轴突的退化立即发生,轴突碎片是由巨噬细胞吞噬清除,新的轴突、未退化的神经元延伸到由Schwann细胞组成的内膜神经的间隙,靶器官逐渐建立联系和营养[22-29]。Masson染色、HRP切片神经均显示,A1组在他克莫司的免疫抑制效果方面优于其他组(P<0.05),他克莫司结合FKBP12,形成钙复合物抑制碱(CAN),抑制T细胞的衰减,并抑制白介素-2(IL-2)、白介素-3(IL-3)的表达,以生产免疫抑制物被神经接收[6-11]。
周围神经的损伤伴随着血液神经屏障的破坏,刺激纤维增生和巨噬细胞的扩散,形成影响神经修复的瘢痕[24-25]。他克莫司可以抑制纤维素的扩散、迁移和生物活性,抑制纤维素的扩散,并通过免疫抑制作用促进神经损伤的重建和修复。Masson染色观察结果提示,A1组的瘢痕比A2、B1、B2组减少。神经内分泌系统的调节,创造微型环境的因素、Schwann细胞的信号有助于促进轴突再生[28-29]。神经交换的物质是轴浆的运输器官和再生轴芽器官通过内膜管再生作用于目标器官[25-29]。轴突内物质更好地恢复运输功能和靶器官的反馈是相互促进的[12-17]。HRP染色结果进一步提示,免疫神经内分泌系统在脑损伤期间有密切的关系[30],自主神经系统的中心结构被摧毁,导致免疫调节紊乱、改变或丧失,功能脑细胞的变性,导致caspase瀑布反应性的激活,导致神经凋亡。
综上所述,脑损伤伴随周围神经损伤动物早期应用他克莫司后,周围神经修复再生方面较好,脑损伤、他克莫司均可促进周围神经损伤的修复,并且协同促进神经损伤的修复效果更好,为脑损伤合并周围神经损伤的患者提供了新的治疗思路。但脑损伤促进周围神经损伤的具体机制仍需进一步深入研究。
[参考文献]
[1]何新泽,王维,马建军,等.颅脑损伤促进的坐骨神经再生[J].中国组织工程研究,2016,20(27):4061-4067.
[2]Wang W,Gao J,Na L,et al.Craniocerebral injury promotes the repair of peripheral nerve injury[J].Neural Regen Res,2014,9(18):1703-1708.
[3]Reeves TM,Trimmer PA,Colley BS,et al.Targeting Kv1.3 channels to reduce white matter pathology after traumatic brain injury[J].Expe Neurol,2016,283(PtA):188-203.
[4]Mohammadi R,Vahabzadeh B,Amini K.Sciatic nerve regeneration induced by transplantation of in vitro bone marrow stromal cells into an inside-out artery graft in rat[J].J Craniomaxillofac Surg,2014,42(7):1389-1396.
[5]Glaus SW,Johnson PJ,Mackinnon SE.Clinical strategies to enhance nerve regeneration in composite tissue allotransplantation[J].Hand Clin,2011,27(4):495-509.
[6]Mekaj AY,Morina AA,Manxhuka-Kerliu S,et al.Electrophysiological and functional evaluation of peroneal nerve regeneration in rabbit following topical hyaluronic acid or tacrolimus application after nerve repair[J].Niger Postgrad Med J,2015,22(3):179-184.
[7]Badavanis G,Pasmatzi E,Monastirli A,et al.Topical imiquimod is an effective and safe drug for molluscum contagiosum in children[J].Acta Dermatovenerol Croat,2017,25(2):164-166.
[8]Phillips BZ,Franco MJ,Yee A,et al.Direct radial to ulnar nerve transfer to restore intrinsic muscle function in combined proximal median and ulnar nerve injury:case report and surgical technique[J].J Hand Surg Am,2014,39(7):1358-1362.
[9]Zhao J,Zheng X,Fu C,et al.FK506-loaded chitosan conduit promotes the regeneration of injured sciatic nerves in the rat through the upregulation of brain-derived neurotrophic factor and TrkB[J].J Neurol Sci,2014,344(1-2):20-26.
[10]Gaudier-Diaz MM,Haines AH,Zhang N,et al.Social influences on microglial reactivity and neuronal damage after cardiac arrest/cardiopulmonary resuscitation[J].Physiol Behav,2018,194:437-449.
[11]Cheng XL,Wang P,Sun B,et al.The longitudinal epineural incision and complete nerve transection method for sciatic nerve injury[J].Neural Regen Res,2015,10(10):1663-1668.
[12]Dai Y,Wang C,Chiu LY,et al.Application of bioconjugation chemistry on biosensor fabrication for detection of TAR-DNA binding protein 43[J].Biosens Bioelectron,2018,117:60-67.
[13]何新澤,王成刚,于立志,等.他克莫司促进周围神经再生的研究进展[J].转化医学电子杂志,2018,5(12):102-104.
[14]He L,Yadgarov A,Sharif S,et al.Aging profoundly delays functional recovery from gustatory nerve injury[J].Neuroscience,2012,209:208-218.
[15]Huang CC,Yang W,Guo C,et al.Anatomical and functional dichotomy of ocular itch and pain[J].Nat Med,2018,24(8):1268-1276.
[16]何新泽,于立志,王成刚,等.跟腓韧带损伤的诊疗进展[J].足踝外科电子杂志,2018,5(2):59-61.
[17]Saeman MR,DeSpain K,Liu MM,et al.Effects of exercise on soleus in severe burn and muscle disuse atrophy[J].J Surg Res,2015,198(1):19-26.
[18]Shu B,Xie JL,Xu YB,et al.Effects of skin-derived precursors on wound healing of denervated skin in a nude mouse model[J].Int J Clin Exp Pathol,2015,8(3):2660-2669.
[19]Dong HY,Jiang XM,Niu CB,et al.Cerebrolysin improves sciatic nerve dysfunction in a mouse model of diabetic peripheral neuropathy[J].Neural Regen Res,2016,11(1):156-162.
[20]Khosravi A,Sharifi I,Tavakkoli H,et al.Embryonic toxico-pathological effects of meglumine antimoniate using a chick embryo model[J].PLoS One,2018,13(5):e0196424.
[21]Lin Q,Wesson RN,Maeda H,et al.Pharmacological mobilization of endogenous stem cells significantly promotes skin regeneration after full-thickness excision:the synergistic activity of AMD3100 and tacrolimus[J].J Invest Dermatol,2014,134(9):2458-2468.
[22]Florio F,Ferri C,Scapin C,et al.Sustained expression of negative regulators of myelination protects schwann cells from dysmyelination in a charcot-marie-tooth 1B mouse model[J].J Neurosci,2018,38(18):4275-4287.
[23]Croaker A,King GJ,Pyne JH,et al.Assessing the risk of epidemic dropsy from black salve use[J].J Appl Toxicol,2018, 38(10):1274-1281.
[24]Roque JS,Pomini KT,Buchaim RL,et al.Inside-out and standard vein grafts associated with platelet-rich plasma(PRP)in sciatic nerve repair.A histomorphometric study[J].Acta Cir Bras,2017,32(8):617-625.
[25]Ganguly S,Das P,Maity PP,et al.Green reduced graphene oxide toughened semi-IPN monolith hydrogel as dual responsive drug release system:rheological,physicomechanical,and electrical evaluations[J].J Phys Chem B,2018,122(29):7201-7218.
[26]Liu C,Yang H,Shi W,et al.MicroRNA-mediated regulation of Th17/Treg balance in autoimmune disease[J].Immunology,2018,155(4):427-434.
[27]Starr A,Sattler R.Synaptic dysfunction and altered excitability in C9ORF72 ALS/FTD[J].Brain Res,2018,1693(PtA):98-108.
[28]Quan Wang J,Di Yang M,Chen X,et al.Discovery of new chromen-4-one derivatives as telomerase inhibitors through regulating expression of dyskerin[J].J Enzyme Inhib Med Chem,2018,33(1):1199-1211.
[29]Liu S,Wu N,Miao J,et al.Protective effect of morin on myocardial ischemia reperfusion injury in rats[J].Int J Mol Med,2018,42(3):1379-1390.
[30]Kentner AC,Khan U,MacRae M,et al.The effect of antibiotics on social aversion following early life inflammation[J].Physiol Behav,2018,194:311-318.
[31]馬建军,何新泽,王浩琪,等.不同位置脑损伤模型大鼠坐骨神经的再生[J].中国组织工程研究,2017,21(36):5806-5811.
[32]He XZ,Ma JJ,Wang HQ,et al.Brain injury in combination with tacrolimus promotes the regeneration of injured peripheral nerves[J].Neural Regen Res,2017,12(6):987-994.
[33]何新泽,王维,呼铁民,等.周围神经损伤的修复:理论研究与技术应用[J].中国组织工程研究,2016,20(7):1044-1050.
(收稿日期:2019-03-29 本文编辑:任秀兰)
