高光谱成像快速检测壳聚糖涂膜草莓可溶性固形物
2019-11-08邵园园王永贤玄冠涛高宗梅
邵园园,王永贤,玄冠涛,3,高宗梅,刘 艺,韩 翔,高 冲
高光谱成像快速检测壳聚糖涂膜草莓可溶性固形物
邵园园1,2,王永贤1,玄冠涛1,3※,高宗梅4,刘 艺1,韩 翔1,高 冲1
(1. 山东农业大学机械与电子工程学院,泰安 271018;2. 农业部南京农业机械化研究所,南京 210014;3. 密苏里大学农业与食品工程学院,哥伦比亚 65211;4. 华盛顿州立大学精细与自动化农业研究中心,华盛顿 99350)
为了对壳聚糖涂膜草莓可溶性固形物含量(soluble solids content, SSC)进行快速检测,该文采用高光谱成像仪(400~1 000 nm)对0,0.5%,1% 浓度的壳聚糖(chitosan, CTS)涂膜草莓分别储藏1,2,4 d后进行成像,并测量样本SSC。通过分析SSC发现,0.5%和1%壳聚糖涂膜草莓,其SSC随着储藏天数的增加均高于0浓度壳聚糖涂膜草莓,说明了0.5% 和1% 壳聚糖涂层抑制了草莓中SSC的降低,能够延长草莓的新鲜口味。随后采用蒙特卡罗-偏最小二乘法(monte carlo-partial least squares, MCPLS)对异常样本进行剔除。对剔除异常样本后的光谱数据进行不同预处理,以确定最优的预处理方法。为提高运行速度和降低数据维数,采用竞争性自适应权重取样法(competitive adaptive reweighted sampling, CARS)和连续投影算法(successive projections algorithm, SPA)进行特征波段选择。最后,采用偏最小二乘回归(partial least square regression, PLSR)和支持向量回归(support vector regression, SVR)法建立回归模型。最终结果表明:SPA-SVR模型效果最佳,0浓度的壳聚糖涂膜的草莓,建模集精度2为0.865,预测集精度2为0.835;0.5%浓度的壳聚糖涂膜的草莓,建模集精度2为0.808,预测集精度2为0.799;1% 浓度的壳聚糖涂膜的草莓,建模集精度2为0.834,预测集精度2为0.875。对储藏第4天的部分样本图像进行主成分分析(principal component analysis, PCA),结果显示除第二主成分图像(PC2)中有部分噪声影响外,PC1和PC3均能完整反映草莓信息,且PC3图像明显呈现出不同浓度壳聚糖涂膜草莓的褐变程度,说明不同浓度的壳聚糖涂膜也会对草莓货架期产生不同影响。综上说明利用高光谱成像技术可以实现壳聚糖涂膜草莓SSC快速检测,有效指导草莓保鲜处理。
农产品;无损检测;高光谱;壳聚糖涂膜;草莓;SSC
0 引 言
草莓富含氨基酸、果糖、胡萝卜素和维生素等多种营养成分,被认为是生物活性化合物的重要来源,具有特有的外观和香甜的味道[1-3],一直以来深受广大消费者的喜爱。可溶性固形物含量(soluble solids content, SSC)是一种综合参数,主要由糖,酸,维生素,矿物质等成分组成,对果实品质的评价具有重要意义[4]。但草莓采摘时极易造成机械损伤,在贮藏过程中也容易出现腐烂,干瘪和生理性病变等现象[5],给草莓SSC造成影响的同时,也给市场营销带来一定的挑战。因此,在草莓储藏,配送和零售过程中,需要有效的方法来保持草莓的品质属性。
目前存在的研究[2,6]通过改变储藏环境的温度和相对湿度来提高草莓品质,然而草莓作为一种大多在常温下储藏和销售的水果,其温度控制和湿度控制策略受到一定的限制[7]。在过去的十几年中,人们对开发和使用生物基包装材料以延长保质期和提高新鲜、冷冻和配方食品质量的兴趣迅速增长[8]。其中,壳聚糖是一种从甲壳类动物外壳中提取的无毒高分子聚合物[9],由于壳聚糖涂层(chitosan, CTS)的可食用性和良好的成膜性[10],已经广泛应用在草莓保鲜和提高品质方面[11-13]。
虽然研究证实了壳聚糖或者壳聚糖中加入不同溶液涂膜可以提高草莓品质,但涂膜后都是通过物理或者化学方法对草莓SSC及其他理化性质进行测定。由于物理或者化学方法需要对样本进行大量处理,耗时费力,破坏性大,且对涂膜后的果实无法实现快速检测[14]。所以寻找一种无损、便捷、快速的检测方法是非常必要的。
高光谱成像技术作为一种无损,简便,准确的成像技术,融合了样本的空间和光谱信息[15],能够快速和无损的通过光谱信息获取水果的内部信息,已经广泛应用在水果品质测定等方面。ElMasry等[16]采用高光谱成像技术检测了草莓含水量、总可溶性固形物和酸度,基于全光谱建立的偏最小二乘模型,其预测相关系数分别达到0.9,0.8,0.87,基于特征波长建立的多元线性回归模型,其预测相关系数分别达到0.87,0.8,0.92。李瑞等[17]采用近红外光谱仪(900~1 700 nm)对蓝莓果实的糖度和酸度进行了无损检测,应用偏最小二乘回归法对整个果实的平均光谱建立硬度和糖度预测模型。结果表明硬度的校正集相关系数2和验证集相关系数2达到0.911和 0.871,糖度的为 0.891和0.774。王世芳等[4]利用JDSU便携式近红外光谱仪采集了西瓜样品瓜梗、瓜脐、赤道部位和整果的近红外反射光谱,采用SPXY算法对样品集进行划分,并且建立了西瓜各部位和SSC含量定量分析模型,结果表明赤道部位反射光谱和可溶性固形物含量相关性较高,经标准归一化预处理后,建立的偏最小二乘回归预测模型,预测集相关系数为0.864,预测集均方根误差为0.33%。高俊峰等[18]利用高光谱成像系统对3个品种240个甘蔗节进行了光谱信息采集。结果表明通过无信息变量消除(UVE)算法提取的特征波长与SSC含量建立的偏最小二乘回归模型预测效果最佳,其预测集的相关系数和均方根误差分别为0.813和0.810。Mo等[19]利用可见/近红外高光谱成像系统(400~1 000 nm)对苹果内部SSC进行了预测,采集了3种情况下的苹果切片的光谱信息与SSC建立了偏最小二乘回归模型,实验表明高光谱成像技术可以用于苹果内部SSC预测,并且绘制了SSC分布图,呈现了苹果内部SSC含量的分布情况。
因此,本研究的目的是探讨高光谱成像技术(400~1 000 nm)对不同浓度壳聚糖涂膜的草莓样本SSC快速和无损检测的可行性。
1 材料与方法
1.1 样本制备
试验研究的草莓品种为章姬,种植于山东省泰安市新绿蔬菜合作社果园,采摘于2019年3月22日上午,由有经验的果农随机选取成熟草莓进行采摘。草莓采摘后立即放入保鲜装置,运回实验室并进行壳聚糖涂膜。壳聚糖浓度不宜过高,否则溶液会变得黏稠,影响草莓外观,不利于货架期延长,一般选择0.5%、1%浓度壳聚糖溶液涂膜草莓[20-25]。涂膜后共得到360个形状大小均匀、无腐烂、无疤痕的草莓样本(图1)。

注:CTS为壳聚糖。 Note: CTS is chitosan.
乙酸和氢氧化钠(粒状)购买于天津市巴斯夫化工有限公司,壳聚糖(脱乙酰化≥90%)购买于上海蓝季科技发展有限公司,为了制备1L的0.5%和1%壳聚糖水溶液,把2组10 mL乙酸分别加入到2组900 mL蒸馏水中制成酸溶液,将5 g和10 g壳聚糖分别加入到酸溶液中。用1 mol/L的氢氧化钠溶液将溶液的pH值调整为6,体积调整为1 L。以pH值为6.0,0%壳聚糖的1 L水溶液作为对照[7]。
将挑选的360个草莓随机分成3组,每组120个样本。分别做以下处理:将3组草莓分别浸泡在0%,0.5%,1%壳聚糖水溶液1 min。将样本取出后在相对湿度50%,温度为20 ℃的环境中干燥3 h,随后将样本储藏在超市常用的聚对苯二甲酸类塑料(PET)保鲜盒中,储藏温度18±2 ℃,相对湿度50%。分别在储藏1,2,4 d随机选取3组中各40个草莓样本进行光谱数据采集。
1.2 高光谱成像系统
采用GaiaField 便携式高光谱系统(双利合谱,四川,中国)采集草莓高光谱信息,系统组成主要包括高光谱成像仪(GaiaField-V10E)成像镜头(HSIA-OL23)、专用光源(HSIA-LS-T-200W)、标准白板(HSIA-CT-150×150)、三脚架(HSIA-TP-S)及装有高光谱数据采集软件(SpecView)的专用计算机等。光谱范围为400~1 000 nm,光谱分辨率2.8 nm,入射狭缝宽30m,视场角22°,CCD像素1 394×1 040,光源对称分布,入射角度45°。
1.3 高光谱图像校正
为了获取清晰不失真的样本图像,数据采集系统参数设置为:相机曝光时间15 ms,镜头与样本间距离46 cm。为了消除相机暗电流、光照不均等对图像的影响,需要对高光谱成像系统进行黑白校正。通过遮盖镜头、扫描标准白板分别获得全黑标定图像I和全白图像I,根据公式(1)获得校正图像[26]。

式中0为校准后图像,I为原始高光谱图像。
整个草莓样本作为其感兴趣区域,利用软件ENVI4.6(Environment for Visualizing Images software, Research Systems Inc., Boulder, Co, USA)手工提取校正图像感兴趣区域(region of interest,ROI)的高光谱数据,并计算ROI内光谱反射率的平均值。
1.4 草莓样本SSC的测定
草莓样本高光谱图像采集完成后,用榨汁机获取每个样本的草莓汁,用吸管吸取草莓汁,将果汁滴于数显折射仪(PAL-1,Atago Co,Tokyo,Japan)镜面窗口,读取折射仪SSC并记录,每个样本重复进行3次试验,以SSC平均值作为单个样本的SSC真实值。
1.5 数据处理
1.5.1 蒙特卡罗偏最小二乘法剔除异常值
由于样本光谱数据采集和SSC测定时,环境或者仪器本身不稳定性等其他因素会产生数据误差,所以首先对异常值进行剔除。蒙特卡罗偏最小二乘法(monte carlo-partial least squares, MCPLS)以蒙特卡罗交互验证为基础,随机选择一定量的样本作为建模集和预测集以建立PLS模型,根据多次运行PLS模型预测结果的统计信息筛选异常样本[27],具有同时检测光谱值和理化值中异常值的优点。通过计算每一个样本在预测集中的预测残差平均值(Mean)和预测残差方差(standard deviation, STD),并且制作预测残差平均值和方差的散点图,具有较高预测残差平均值和方差的样本为异常样本[28]。
1.5.2 样本划分和光谱数据预处理
对剔除异常值之后的光谱数据和理化值采用光谱-理化值共生距离(samples set partitioning based on joint X-Y distances, SPXY)算法[4]对样本集进行划分,分别计算建模集、预测集样本的最大值、最小值、平均值和标准偏差,评估样本划分是否合理[29]。在获取光谱数据时,由于人为操作或环境等影响,易造成光谱曲线中包含大量噪声和其他干扰信息,所以对数据采用卷积平滑(Savitzky-Golay),基线校正(baseline correction),去趋势算法(de-trending),移动平滑(moving average smoothing, MA),多元散射校正(multiplicative scatter correction, MSC),变量标准化(standard normal variate, SNV)[30-33]进行预处理。采用留一交叉验证法(leave-one-out cross validation, LOOCV)进行内部交互验证,以交互验证的均方根误差(root mean square error of cross validation, RMSECV)和决定系数2选择预处理方法。
1.5.3 特征波长选取
由于高光谱波段之间高度相关的性质,导致了共线性和大量的冗余信息。为了提高运行速度,同时降低数据维数,本研究采用竞争性自适应权重取样(CARS)法和连续投影算法(SPA)进行特征波长选取。
竞争性自适应权重取样(competitive adaptive reweighted sampling, CARS)是一种基于适者生存和回归系数进行波长点选择的简单有效方法。利用回归系数绝对值的大小作为衡量波长重要性的指标,引入指数衰减函数对波长的保留率进行控制,根据交叉验证方法,选取交互验证最小均方根误差子集,其中包含的变量为最佳波长组合[34]。
连续投影算法(successive projections algorithm, SPA)是一种特征波段前向选择算法,通过比较波段投影向量大小,将最大投影量波段列为有效波段,并根据校正模型确定最佳的特征波段[35]。以建模集光谱数据为输入,均方根误差(root mean square error, RMSE)最小时,特征波段选取效果最优。
1.5.4 回归模型的建立与模型评价
偏最小二乘回归(partial least square regression, PLSR)法是一种多元数据分析方法,在光谱数据的建模中得到广泛应用,可解决变量之间多重相关性的问题。PLSR对光谱反射值矩阵和SSC矩阵同时进行分解,同时考虑光谱值信息和对应的理化性质信息,探究两者的对应关系,从而保证获得最佳的校正模型[36]。
支持向量回归(support vector regression, SVR)法是基于支持向量机的函数逼近回归问题的学习方法,主要思想是将原问题通过非线性变换转化为某个高维空间的线性问题,并在高维空间中进行线性求解。其优点是得到现有信息下的最优解,而不仅仅是样本趋于无穷大时的最优值[37]。
回归模型建立之后,通过以下参数衡量模型预测效果。以建模集精度2(determination coefficient of calibration set)和建模集均方根误差RMSEC(root mean square error of calibration)作为模型性能的辅助评价指标,以预测集精度2(determination coefficient of validation set)和预测集均方根误差 RMSEV(root mean square error of validation)作为主要评价指标[23]。2和2越大,RMSEC和RMSEV越小,模型的预测能力越强[38]。
2 结果与分析
2.1 草莓样本SSC分析
草莓样本的SSC随着储藏天数的增加,其变化趋势如图2所示。0%壳聚糖涂膜样本在3个储藏时间均保持较低SSC水平。随着天数的增加,1%壳聚糖涂膜样本SSC水平最高,储藏4 d时SSC仍然能达到8.57°Brix,这也说明了1%壳聚糖水溶液能够更加有效的抑制SSC的降低,保持草莓本身的新鲜口味。通过单因素方差分析(analysis of variance, ANOVA)发现,储藏1 d时,0与0.5% 以及0与1%壳聚糖涂膜样本间存在显著性差异(<0.05),储藏2 d时,3个浓度样本两两之间无显著性差异。而储藏4 d时,3个浓度涂膜样本两两之间有显著性差异(<0.05)。

注:每个值为平均值±标准偏差。
2.2 光谱分析
图3和图4分别为0,0.5%,1%壳聚糖涂膜后的草莓样本分别储藏1,2,4 d的全样本光谱反射曲线和平均光谱曲线,曲线总趋势基本一致,但第2天的平均光谱曲线相对反射率明显下降,结合SSC含量变化初步分析,0壳聚糖涂膜的样本平均光谱曲线相对反射率的下降与其SSC含量明显上升有关,而0.5%和1%浓度涂膜的样本平均光谱曲线相对反射率的下降,与壳聚糖涂层有密切的联系。进一步分析,400~490 nm为类胡萝卜素的强吸收带,所以在400~550 nm之间,平均光谱曲线反射率很低,形状也很平缓。550~800 nm平均光谱曲线快速增加,这与草莓表面为红色,对红光反射有关。在800和970 nm附近出现的光谱吸收峰或反射谷,分别为水的O-H三级和二级吸收倍频[37,39]。

图3 草霉样本全样本光谱反射曲线
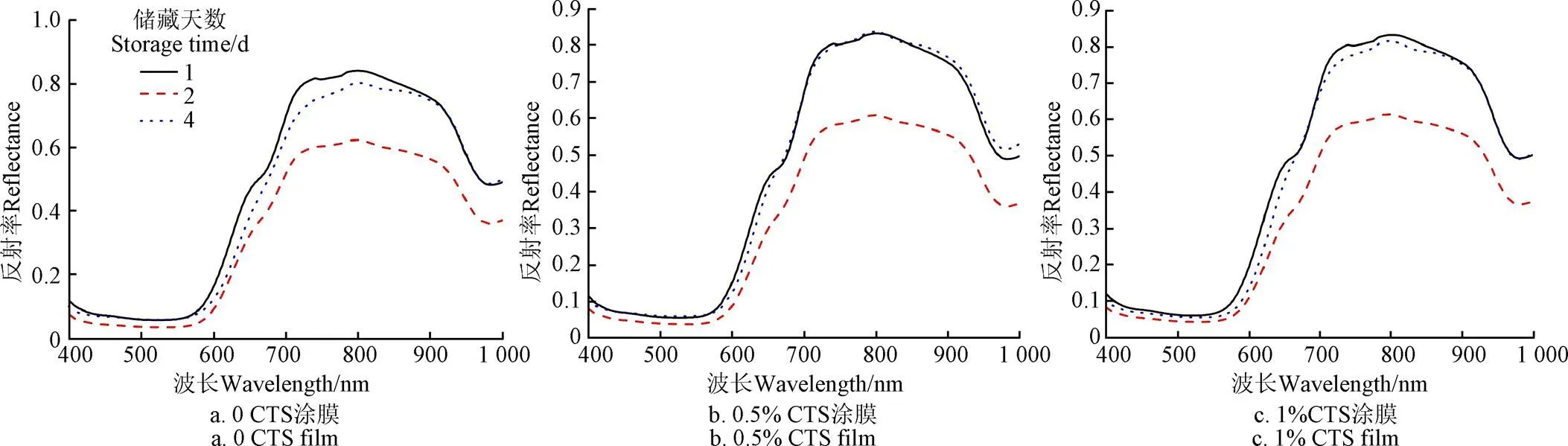
图4 草莓样本平均光谱曲线
2.3 异常样本剔除
试验样本处理过程中异常样本影响预处理过程的同时也会影响建模精度,所以对异常样本进行剔除来提高真实值与预测值的相关性[40]。采用MCPLS算法对异常样本进行剔除,随机选取样本中的75%为建模集,25%为预测集,设置重复次数=5000,尽量保证更多的样本有机会进入预测集。计算每个样本的均值和方差,以均值为横坐标,方差为纵坐标建立坐标系,均值和方差较大的为异常值。
蒙特卡洛检测结果如图5所示,图5a为0%壳聚糖涂膜样本检测结果,以Mean=0.82,STD=0.175为界限共剔除异常样本10个,分别为11,26,31,48,59,75,78,89,103,105号。图5b为0.5%壳聚糖涂膜样本检测结果,以Mean=0.64,STD=0.175为界限共剔除异常样本13个,分为别2,13,16,17,28,30,35,40,46,47,71,80,102号。图5c为1%壳聚糖涂膜样本检测结果,以Mean=0.9,STD=0.168为界限共剔除异常样本5个,分别为15,19,25,27,62号。因此,3组分别以110个,107个,115个样本用于SSC含量检测。
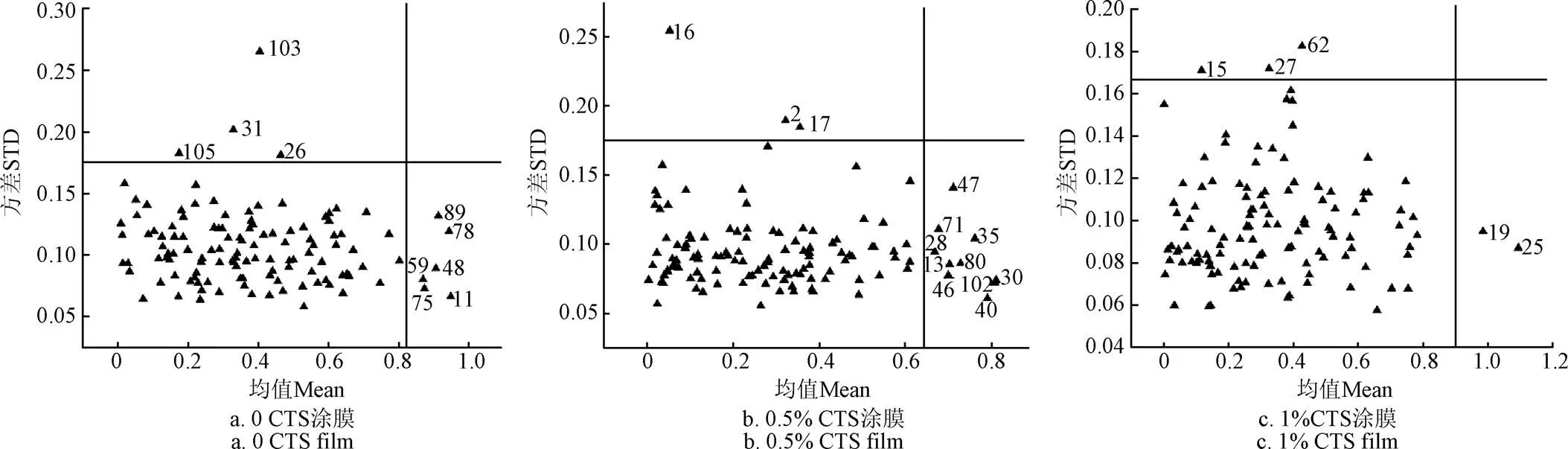
图5 剔除异常值的蒙特卡罗偏最小二乘法检测
2.4 样本集划分和光谱预处理
如表1所示,采用SPXY对剔除样本后的数据进行划分,3种浓度涂膜样本中的建模集均包含SSC最大值和最小值,且建模集和预测集包含较大范围的SSC值,因此,划分合理。为验证不同预处理方法的效果,分别建立不同预处理方法处理后的光谱与SSC值的PLSR模型,结果如表2所示。0浓度的壳聚糖涂膜的样本经MSC预处理的数据,0.5%和0.1浓度的壳聚糖涂膜的样本未经预处理的数据2最大,RMSECV最小,故分别使用其数据进行后续分析。
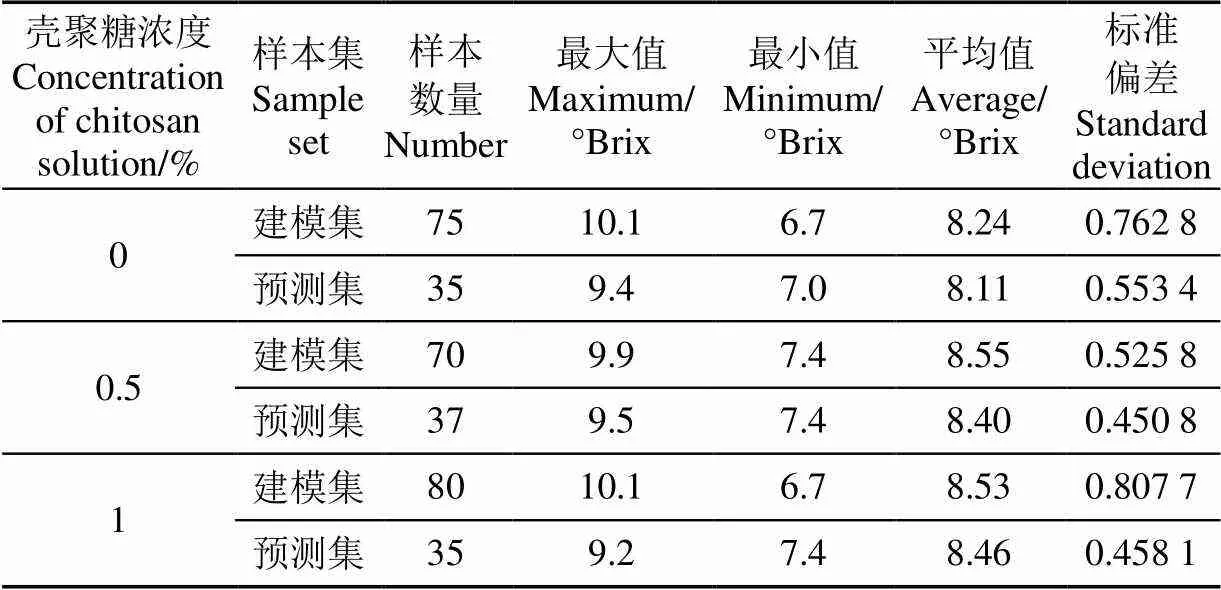
表1 建模集和预测集的SSC的统计分析
2.5 特征波段选取
全光谱模型建立共有256个光谱变量,大量的数据会降低运算速度,并且会导致信息冗余。为了提高速度和降低冗余,采用CARS和SPA挑选特征波长,CARS挑选特征波长过程如图6所示,SPA挑选特征波长如图7所示。图6中,3种浓度下的样本数据运行次数分别为155,151,165时,RMSECV最低,选取的特征波段数分别为32,30,20个,各占总波长变量的12.5%,11.7%,7.8%。图7中,3种浓度下的样本数据通过SPA挑选的特征波段分别为11,8,16个,各占总波长变量的4.3%,3.1%,6.3%。具体数值如表3所示。

表2 不同预处理方法的草莓SSC PLSR模型
注:‘2’表示校正集决定系数,‘RMSEC’表示校正集均方根误差,‘RMSECV’表示交互验证的校正集均方根误差,‘PCs’表示主成分数。
Note: ‘2’means determination coefficient of calibration set, ‘RMSEC’ means root mean square error of calibration set, ‘RMSECV’means root mean square error of cross validation, ‘PCs’means number of principal components.

图6 CARS挑选特征波长过程

图7 SPA挑选特征波长过程

表3 通过CARS,SPA挑选的特征波长
2.6 PLSR和SVR模型的建立
分别建立全光谱和特征波长的PLSR,SVR模型。其模型回归效果和模型参数如表4和表5所示。0壳聚糖涂膜的样本全光谱数据建立的PLSR模型2和2略高于SVR模型,效果较好。而挑选的特征波长建立的回归模型中,SPA-SVR效果最好,2和2值分别为0.865和0.835,RMSEC和RMSEV值分别为0.251和0.286。0.5%壳聚糖涂膜的样本全光谱数据建立的SVR模型2高于PLSR模型,而PLSR模型的2高于SVR模型。特征波长中,SPA-SVR的2和2值分别为0.808和0.799,RMSEC和RMSEV值分别为0.216和0.203,效果最佳。1%壳聚糖涂膜的样本全光谱数据建立的PLSR模型效果较好,特征波长中,SPA-SVR的2和2值分别为0.834和0.875,RMSEC和RMSEV值分别为0.334和0.170,预测效果最佳。每个浓度下的全光谱数据建立的PLSR和SVR模型2和2值均低于SPA-SVR模型,因此,SPA-SVR模型可以较好地预测0,0.5%,1%壳聚糖涂膜的草莓样本SSC含量。图8为3种浓度涂膜的草莓样本SPA-SVR模型建模与预测结果散点图。
2.7 草莓样本图像主成分分析
进一步分析壳聚糖涂膜草莓的形态变化,选取储藏第4天的部分样本进行主成分分析,如图9所示。从图中可以看出,除PC2中有部分噪声影响外,PC1和PC3均能完整反映草莓样本信息。其中,PC3图像反映草莓样本信息最明显,0壳聚糖涂膜样本白色区域为褐变区域,主要由果实组织中的酚类物质氧化成醌类物质导致。0.5%壳聚糖涂膜样本外表形态较完整,未出现严重的褐变现象。1%壳聚糖涂膜样本表明出现了较多的黑色变化区域,出现了部分褐变。这也说明了随着储藏天数的增加,0.5%浓度的壳聚糖涂层对于草莓样本表面形态保存比较完整,对草莓货架期的延长有较好的效果。

图8 SPA-SVR模型的预测结果

表4 不同特征波长下的SSC PLSR模型
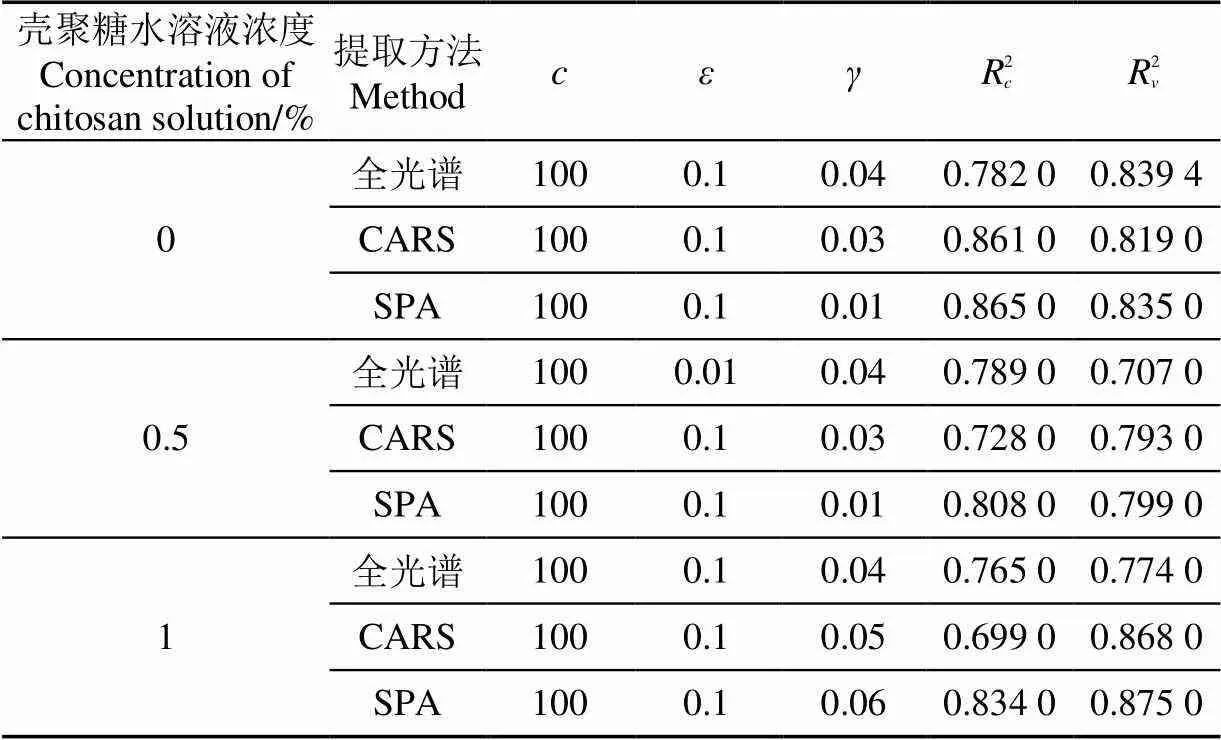
表5 不同特征波长下的SSC SVR模型
注:‘’表示惩罚系数,‘’表示不敏感损失系数,‘’表示宽度系数,‘2’表示建模集精度,‘2’表示预测集精度。
Note: ‘’ means Punishment coefficient, ‘’ means Insensitive loss coefficient, ‘’ means width coefficient, ‘2’means determination coefficient of calibration set, ‘2’ means determination coefficient of validation set.

图9 草莓图像主成分分析
3 结 论
采用高光谱成像仪(400~1 000 nm)对壳聚糖涂膜的草莓样本可溶性固形物含量(soluble solids content, SSC)进行预测,研究主要结论如下:
1)采用蒙特卡罗-偏最小二乘法算法对0,0.5%,1%浓度壳聚糖涂膜的草莓光谱数据和SSC值的异常值进行了剔除,剔除个数分别为10个,13个,5个。
2)分析比较了剔除异常样本后不同的光谱数据预处理,建模及特征波段提取方法,建立了光谱与草莓SSC值的回归模型。
3)对全光谱以及提取的特征波长建立偏最小二乘回归和支持向量回归模型。结果表明,SPA-SVR建立的SSC含量模型最优,0壳聚糖涂膜的样本建立的SPA-SVR模型2和2分别为0.865,0.835,RMSEC和RMSEV分别为0.251,0.286。0.5%壳聚糖涂膜的样本建立的SPA-SVR模型2和2分别为0.808, 0.799,RMSEC和RMSEV分别为0.216,0.203。1%壳聚糖涂膜的样本建立的SPA-SVR模型2和2分别为0.834,0.875,RMSEC和RMSEV分别为0.334,0.170。
4)通过比较涂膜样本的形态变化,选取储藏第4天的部分样本图像进行主成分分析,PC3中可以清晰的看到草莓样本表面形态的变化,0.5%浓度的壳聚糖涂层的保鲜效果更好,对延长草莓货架期具有较好的效果。
[1] Campaniello D, Bevilacqua A, Sinigaglia M, et al. Chitosan: Antimicrobial activity and potential applications for preserving minimally processed strawberries[J]. Food Microbiology, 2008, 25(8): 992-1000.
[2] Ktenioudaki A, O’Donnell C P, do Nascimento Nunes M C. Modelling the biochemical and sensory changes of strawberries during storage under diverse relative humidity conditions[J]. Postharvest Biology and Technology, 2019, 154: 148-158.
[3] Dhital R, Mora N B, Watson D G. Efficacy of limonene nano coatings on post-harvest shelf life of strawberries[J]. LWT 2018, 97: 124-134.
[4] 王世芳,韩平,崔广禄,等. SPXY算法的西瓜可溶性固形物近红外光谱检测[J]. 光谱学与光谱分析,2019, 39(3):738-742.
Wang Shifang, Han Ping, Cui Guanglu, et al. The NIR detection research of soluble solid in watermelon based on SPXY algorithm[J]. Spectroscopy and Spectral Analysis, 2019, 39(3): 738-742. (in Chinese with English abstract)
[5] Reddy M V B, Belkacemi K, Corcuff R, et al. Effect of pre-harvest chitosan sprays on post-harvest infection by Botrytis cinerea quality of strawberry fruit[J]. Postharvest Biology and Technology, 2000, 20(1): 39-51.
[6] Octavia L, Choo W S. Folate, ascorbic acid, anthocyanin and colour changes in strawberry () during refrigerated storage[J]. LWT-Food Science and Technology, 2017, 86: 652-659.
[7] Han C, Zuo J, Wang Q, et al. Effects of chitosan coating on postharvest quality and shelf life of sponge gourd () during storage[J]. Scientia Horticulturae, 2014, 166: 1-8.
[8] Diab T, Biliaderis C G, Gerasopoulos D. Physicochemical properties and application of pullulan edible films and coatings in fruit preservation[J]. Journal of the Science of Food and Agriculture, 2001, 81(10): 988-1000.
[9] Muzzarelli R A A, Boudrant J, Meyer D, et al. Current views on fungal chitin/chitosan, human chitinases, food preservation, glucans, pectins and inulin: A tribute to Henri Braconnot, precursor of the carbohydrate polymers science, on the chitin bicentennial[J]. Carbohydrate Polymers, 2012, 87(2): 995-1012.
[10] Gol N B, Patel P R, Rao T V R. Improvement of quality and shelf-life of strawberries with edible coatings enriched with chitosan[J]. Postharvest Biology and Technology, 2013, 85: 185-195.
[11] Almenar E, Hernández-menoz P, Gavara R. Evolution of selected volatiles in chitosan-coated strawberries () during refrigerated storage[J]. Journal of Agricultural and Food Chemistry, 2009, 57(3): 974-980.
[12] Perdones A L, Sánchez-González, Chiralt A, et al. Effect of chitosan–lemon essential oil coatings on storage-keeping quality of strawberry[J]. Postharvest Biology and Technology, 2012, 70: 32-41.
[13] Khalifa I, Barakat H, El-Mansy H A, et al. Enhancing the keeping quality of fresh strawberry using chitosan- incorporated olive processing wastes[J]. Food Bioscience, 2016, 13(1): 69-75.
[14] 赵芸,张初,刘飞,等. 采用可见/近红外光谱检测大麦叶片过氧化氢酶与过氧化物酶含量的研究[J]. 光谱学与光谱分析,2014,34(9):2382-2386.
Zhao Yun, Zhang Chu, Liu Fei, et al. Application of visible/near-infrared spectroscopy of catalase and peroxidase content in barley leaves[J]. Spectroscopy and Spectral Analysis, 2014, 34(9): 2382-2386. (in Chinese with English abstract)
[15] 李鸿强,孙红,李民赞. 基于可见/短波近红外光谱检测结球甘蓝维生素C含量[J]. 农业工程学报,2018,34(8):269-275.
Li Hongqiang, Sun Hong, Li Minzan, et al. Detection of vitamin C content in head cabbage based on visible/near-infrared spectroscopy [J]. Transactions of the Chinese Society of Agricultural Engineering (Transactions of the CSAE), 2018, 34(8): 269-275. (in Chinese with English abstract)
[16] ElMasry G, Wang Ning, ElSayed Adel, et al. Hyperspectral imaging for nondestructive determination of some quality attributes for strawberry[J]. Journal of Food Engineering,2007,81(1):98-107.
[17] 李瑞,傅隆生. 基于高光谱图像的蓝莓糖度和硬度无损测量[J]. 农业工程学报,2017,33(增刊1):362-366.
Li Rui, Fu Longsheng. Nondestructive measurement of firmness and sugar content of blueberries based on hyperspectral imaging[J]. Transactions of the Chinese Society of Agricultural Engineering (Transactions of the CSAE), 2017, 33(Supp.1): 362-366. (in Chinese with English abstract)
[18] 高俊峰,张初,谢传奇,等. 应用近红外高光谱成像技术预测甘蔗可溶性固形物含量[J]. 光谱学与光谱分析,2015,35(8):2154-2158.
Gao Junfeng, Zhang Chu, Xie Chuanqi, et al. Prediction the soluble solid content in sugarcanes by using near infrared hyperspectral imaging system[J]. Spectroscopy and Spectral Analysis, 2015, 35(8): 2154-2158. (in Chinese with English abstract)
[19] Mo C, Kim M S, Kim G , et al. Spatial assessment of soluble solid contents on apple slices using hyperspectral imaging[J]. Biosystems Engineering, 2017, 159: 10-21.
[20] Tezotto-Uliana J V, Fargoni G P, Geerdink G M, et al. Chitosan applications pre-or postharvest prolong raspberry shelf-life quality[J]. Postharvest Biology and Technology, 2014, 91: 72-77.
[21] Kerch G. Chitosan films and coatings prevent losses of fresh fruit nutritional quality: A review[J]. Trends in Food Science and Technology, 2015, 46(2): 159-166.
[22] Romanazzi G, Feliziani E. Use of chitosan to control postharvest decay of temperate fruit: Effectiveness and mechanisms of action[M]. Chitosan in the Preservation of Agricultural Commodities. Salt Lake City Academic Press, 2016: 155-177.
[23] Han C, Zuo J, Wang Q, et al. Effects of chitosan coating on postharvest quality and shelf life of sponge gourd (Luffa cylindrica) during storage[J]. Scientia horticulturae, 2014, 166: 1-8.
[24] 王哲,史红梅,任凤山,等.壳聚糖涂膜对‘红宝石无核’葡萄保鲜效果的影响[J]. 中外葡萄与葡萄酒,2019(3):25-28.
Wang Zhe, Shi Hongmei, Ren Fengshan, et al. Effect of chitosan coating on the preservation of ‘Ruby Seedless’ grape[J]. Chinese and Foreign Grapes and Wine, 2019(3): 25-28. (in Chinese with English abstract)
[25] 路志芳,陈现臣,袁超,等. 壳聚糖涂膜对鲜黄瓜的保鲜作用[J]. 江苏农业科学,2018,46(14):177-180.
Lu Zhifang, Chen Xianchen, Yuan Chao, et al. Fresh-keeping effect of chitosan coating on fresh cucumber[J]. Jiangsu Agricultural Science, 2018, 46(14): 177-180. (in Chinese with English abstract)
[26] 潘冉冉,骆一凡,王昌,等. 高光谱成像的油菜和杂草分类方法[J]. 光谱学与光谱分析,2017(11):252-257.
Pan Ranran, Luo Yifan, Wang Chang, et al. Classifications of oilseed rape and weeds based on hyperspectral imaging[J]. Spectroscopy and Spectral Analysis, 2017(11): 252-257. (in Chinese with English abstract)
[27] Guo W L, Du Y P, Zhou Y C, et al. At-line monitoring of key parameters of nisin fermentation by near infrared spectroscopy, chemometric modeling and model improvement[J]. World Journal of Microbiology and Biotechnology, 2012, 28(3): 993-1002.
[28] 何勇. 光谱及成像技术在农业中的应用[M]. 北京:科学出版社,2016.
[29] 李晓丽,魏玉震,徐劼,等. 基于高光谱成像的茶叶中EGCG分布可视化[J]. 农业工程学报,2018,34(7):180-186.
Li Xiaoli, Wei Yuzhen, Xu Jie, et al. EGCG distribution visualization in tea leaves based on hyperspectral imaging technology[J]. Transactions of the Chinese Society of Agricultural Engineering (Transactions of the CSAE), 2018, 34(7): 180-186. (in Chinese with English abstract)
[30] 褚小立. 近红外光谱分析技术实用手册[M]. 北京:机械工业出版社, 2016.
[31] Wu D, He Y, Nie P, et al. Hybrid variable selection in visible and near-infrared spectral analysis for non-invasive quality determination of grape juice[J]. Analytica Chimica Acta, 2010, 659(1/2): 229-237.
[32] Kong W, Zhao Y, Liu F, et al. Fast Analysis of Superoxide Dismutase (SOD) Activity in Barley Leaves Using Visible and Near Infrared Spectroscopy[J]. Sensors, 2012, 12(8): 10871-10880.
[33] Wang H, Peng J, Xie C, et al. Fruit quality evaluation using spectroscopy technology: A review[J]. Sensors, 2015, 15(5): 11889-11927.
[34] Li H, Liang Y, Xu Q, et al. Key wavelengths screening using competitive adaptive reweighted sampling method for multivariate calibration[J]. Analytica Chimica Acta, 2009, 648(1): 77-84.
[35] 高攀,张初,吕新,等.近红外高光谱成像的微破损棉种可视化识别含量[J]. 光谱学与光谱分析,2018,38(6):58-64.
Gao Pan, Zhang Chu, Lü Xin, et al. Visual identification of slight-damaged cotton seeds based on near infrared hyperspectral imaging[J]. Spectroscopy and Spectral Analysis, 2018, 38(6): 58-64. (in Chinese with English abstract)
[36] 孙红,郑涛,刘宁,等. 高光谱图像检测马铃薯植株叶绿素含量垂直分布[J]. 农业工程学报,2018,34(1):149-156.
Sun Hong, Zheng Tao, Liu Ning, et al. Vertical distribution of chlorophyll in potato plants based on hyperspectral imaging[J]. Transactions of the Chinese Society of Agricultural Engineering (Transactions of the CSAE), 2018, 34(1): 149-156. (in Chinese with English abstract)
[37] 褚小立. 化学计量学方法与分子光谱分析技术[M]. 北京:化学工业出版社, 2011.
[38] 单佳佳,吴建虎,陈菁菁,等. 基于高光谱成像的苹果多品质参数同时检测[J]. 光谱学与光谱分析,2010, 30(10):2729-2733.
Shan Jiajia, Wu Jianhu, Chen Jingjing, et al. Rapid nondestructive detection of apple quality attributes using hyperspectral scattering images[J]. Spectroscopy and Spectral Analysis, 2010, 30(10): 2729-2733. (in Chinese with English abstract)
[39] Zhang C, Guo C, Liu F, et al. Hyperspectral imaging analysis for ripeness evaluation of strawberry with support vector machine[J]. Journal of Food Engineering, 2016, 179:11-18.
[40] 小波,赵文杰. 农产品无损检测技术与数据分析方法[M]. 北京:中国轻工业出版社,2008.
Rapid detection of soluble solids content in strawberry coated with chitosan based on hyperspectral imaging
Shao Yuanyuan1,2, Wang Yongxian1, Xuan Guantao1,3※, Gao Zongmei4, Liu Yi1, Han Xiang1, Gao Chong1
(1.,271018,; 2.,210014,; 3.,65211; 4.)
Strawberries are popular fruit for their tender texture, juice and sweet taste. Prior on shelves, the harvesting and storage have always been the problems due to its fragility as well as susceptibility to rot. Chitosan coating has been widely used in fruit preservation, which can delay the storage time of fruits and has good preservation effect. The quality of chitosan-coated fruits is mostly detected by the typical conventional methods of physical or chemical testing. Since such methods need to deal with a large number of samples, which are time-consuming, laborious and destructive for detecting coated fruits. Therefore, in order to explore the possibility of detecting the soluble solids content (SSC) of strawberry coated with chitosan nondestructively and rapidly, hyperspectral imaging technology was employed to estimate the SSC of strawberry coated with chitosan in this study. Strawberry samples coated with 0, 0.5% and 1% chitosan acetic acid which were stored in 3 periods (1, 2 , 4 d). Outliers were eliminated by monte carlo-partial least squares (MCPLS) method, and the number of outliers was 10, 3 and 5 for the above respect treatments. Sample partitioning based on joint X-Y distance (SPXY) was used to split the data after eliminating outliers. After the partition of sample set, the modeling set contains the maximum and minimum SSC values in the three-concentration data, and the range of SSC values in the calibration set and validation set is large and the partition is reasonable. To find out the best model effect, Savitzky-Golay, baseline correction, De-trending, moving average smoothing (MA), multiplicative scatter correction (MSC) and standard normal variate (SNV) were used to pre-process the spectral data after eliminating the outliers. It was found that the strawberry sample data coated with 0 chitosan acetic acid solution pretreated by MSC had the best effect, while the strawberry sample data coated with 0.5% and 1% chitosan acetic acid solution without pretreatment had the best effect.2was the largest and RMSECV was the smallest. Competitive adaptive reweighted sampling (CARS) and successive projection algorithm (SPA) method were applied to select the effective wavelengths, which were helpful for enhancing computer velocity and reducing data dimension. The number of effective wavelengths selected by CARS and SPA for the three concentrations was 32, 30, 20 and 11, 8, 16, respectively. Finally, partial least square method (PLS) and support vector regression (SVR) were used to build regression models. The final results showed that the PLS regression model was less effective than the SVR model, while the full spectrum data and the data of characteristic bands selected by CARS are less effective in the SVR model, and the SPA-SVR model was the best. The value of2reached to 0.865 for strawberry samples coated with 0 chitosan acetic acid solution, and value of2reached to 0.835; for the strawberries coated with 0.5% chitosan acetic acid solution2was 0.808 and2was 0.799; and the2and2were 0.834 and 0.875 for strawberries coated with 1% chitosan acetic acid solution, respectively. These results validated the applicability of hyperspectral imaging technology on rapid detection of SSC in strawberry coated with chitosan.
agricultural products; non-destructive inspection; hyperspectral; coated with chitosan; strawberry; SSC
邵园园,王永贤,玄冠涛,高宗梅,刘 艺,韩 翔,高 冲. 高光谱成像快速检测壳聚糖涂膜草莓可溶性固形物[J]. 农业工程学报,2019,35(18):245-254.doi:10.11975/j.issn.1002-6819.2019.18.030 http://www.tcsae.org
Shao Yuanyuan, Wang Yongxian, Xuan Guantao, Gao Zongmei, Liu Yi, Han Xiang, Gao Chong. Rapid detection of soluble solids content in strawberry coated with chitosan based on hyperspectral imaging[J]. Transactions of the Chinese Society of Agricultural Engineering (Transactions of the CSAE), 2019, 35(18): 245-254. (in Chinese with English abstract) doi:10.11975/j.issn.1002-6819.2019.18.030 http://www.tcsae.org
2019-06-16
2019-07-19
国家自然科学基金(31701325,31671632)
邵园园,博士,副教授,主要从事高光谱农业应用及农业智能装备研究。Email:syy007@sdau.edu.cn
玄冠涛,博士,副教授,主要从事农业智能装备研究。Email:xuangt@sina.com
10.11975/j.issn.1002-6819.2019.18.030
TP391.4
A
1002-6819(2019)-18-0245-10
