Nuclear radiation shielding effectiveness and corrosion behavior of some steel alloys for nuclear reactor systems
2019-10-31SadawyElShazly
M.M.Sadawy ,R.M.El Shazly
a Mining and Petroleum Engineering Department,Al-Azhar University,Nasr City,Cairo,11371,Egypt
b Physics Department,Faculty of Science,Al-Azhar University,Nasr City,Cairo,11884,Egypt
Keywords:Steel alloys Macroscopic cross-section Mass attenuation coefficient Passive film Pitting
A B S T R A C T Different types of nuclear parameters and corrosion behavior were deduced for carbon steel(AISI 1018),austenitic(304 SS),and duplex(2507 SS)stainless steel alloys.Three types of neutron energies as well as nine gamma ray energy lines(121.78—1407.92 keV)were used to evaluate the macroscopic neutron cross-sections(∑,cm-1)and mass attenuation coefficients(σ,cm2⋅g-1)of gamma ray respectively.The corrosion behavior was investigated using different electrochemical techniques.The results showed that the stainless-steel alloys had a good attitude than that of carbon steel alloy for neutron and gamma ray parameters,especially the duplex stainless steel.The calculated values of mass attenuation coefficient using WinXcom computer program(Version 3.1),exhibited a very good agreement with the experimental values of that parameters.Moreover,the results indicated that duplex stainless-steel exhibited corrosion resistance higher than 304 SS and AISI 1018 steel alloys.
1. Introduction
Different types of nuclear reactors are considered to be the most probable and ideal solution for the problem of needing growth of electrical energy which the world faces nowadays.Stainless-steel alloys are the most commonly constructive and prospective materials for these types of nuclear reactors because of their excellent mechanical properties,weldability,corrosion resistance,irradiation damage resistance,lower induced radioactivity,availability,and easily fabricated by various methods[1—6].Moreover,these alloys exhibit a promising shielding property as they contain heavy metals such as Cr,Ni,Cu and Fe contributing radiation shielding[7].
For fusion reactors;materials,which be used,will be exposed to fusion neutrons with energy of 14 MeV.Radiation damage of these materials is characterized by synergistic effect of the cascade damage and nuclear transmutation products such as hydrogen and helium[1].The spectrum of gamma-ray energy,which produced in the fusion reactors,requires long lasting,adequate,cost effective shielding materials to optimize the radiation level and collective dose[8].Stainless steel is the most popular type of steel alloys applied where high corrosion resistance and levels of functionality over longer periods of time are required[9—11].It is well-known that the high corrosion resistance of stainless steels in aqueous environments arises from the existence of a thin oxide film on their surfaces.The stability of the oxide passive film and its susceptibility to breakdown depends on certain parameters such as temperature,pH,applied potentials and environments[12].
Steel alloys,in inner containment wall of double walled structure dome,were used for leak tightness under normal operation and accident conditions in modern reactor designs.The gamma rays emitted from reactors core are polychromatic, while the available data from the experimental results are little for gamma radiation[13—15].Moreover,the electrochemical properties and the corrosion product composition of the stainless and mild steels depend on the conditions(corrosive ions,temperature,etc.)in the secondary circuit of the nuclear power plant.Actually,it is impossible to determine the corrosion behavior for an actual case from theoretical data only.Therefore,the objective of the present study is to distinguish between three types of steel alloys by their nuclear properties and corrosion behavior to illustrate the possibility of their use in different places in nuclear reactors systems.
2. Materials
The present study was carried out by using sheets of carbon steel(AISI 1018),austenitic(304 SS),and duplex(2507 SS)stainlesssteel.The chemical compositions were presented in Table 1.Groups of samples were cut from each sheet and machined to 100 mm×100 mm×5 mm and 30 mm×10 mm×5 mm for nuclear and corrosion measurements respectively.The samples were mechanically polished by using grinding machine with SiC abrasive paper subsequently to 1200 grits and water as a lubricant.
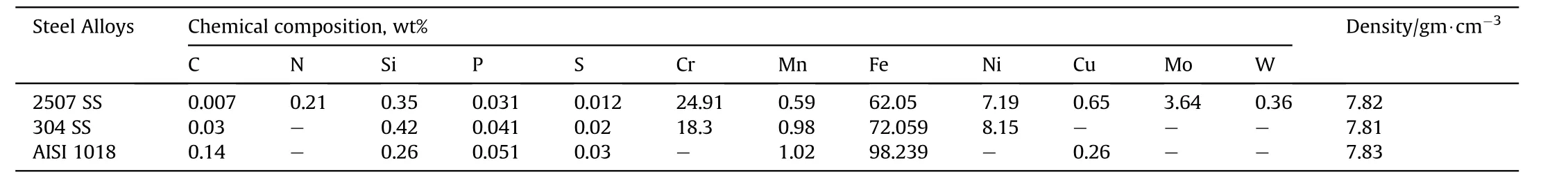
Table 1 Chemical composition and density of investigated steel Alloys.
3. Experimental measurements and theoretical calculations
3.1. Neutron and gamma ray measurements
The density of the investigated steel alloys was measured by using the standard Archimedes principle[14].The BF3neutron detector was used to detect the slow,total slow neutron,and neutron with energy greater than 10 keV fluxes emitted from241Am-Be neutron source with activity 100 mCi and neutron yield=(1.1—1.4)×107n⋅sec-1. To deduce the values of macroscopic neutron cross-section, the neutron transmitted fluxes were measured according to Eq.(1)[16,17]:

In slow neutrons measurements, the collimated beam was slowed down by 7 cm polyethylene block behind the sample,also the neutron of energy below 10 keV was cut off by a block of Born carbide B4C.The Schematic diagram of experimental setup was shown in Fig.1.
The gamma ray attenuation coefficients of the investigated steel alloys barriers were obtained for nine energy lines(121.78,344.27,661.64,778.9,964,1112.4,1173.23,1332.51,and 1407.92 keV)using collimated beam of gamma rays which emitted from 3.7 μCi Eu-152,9.5 μCi Cs-137,and 4.9 μCi Co-60 radioactive sources.The schematic diagram of experimental setup for gamma ray detection is shown in Fig.2.The 3′′×3′′NaI(Tl)scintillation detector was used to measure the gamma ray intensities for the studied energy lines.The Beer-Lambert's equation was used to evaluate the linear attenuation coefficients[18,19]:

where,Ioand I are the intensities of gamma rays before and after transmitted the sample respectively,μ(by cm-1)is the linear attenuation coefficient of the sample,and x is the thickness of the sample.Mass attenuation coefficient,σ(by cm2⋅g-1)is another important nuclear parameter which independent of density of the material,was evaluated from Eq.(3)[20,21]after considering superficial density of thickness.

The comparison between experimental and calculated data using the WinXCom computer program(Version 3.1)according to Eq.(4)was carried out[11,18,19]:

The maximum errors in ∑,σ,and half value layer(HVL)were evaluated using the following propagation of error formulas[19,22]:
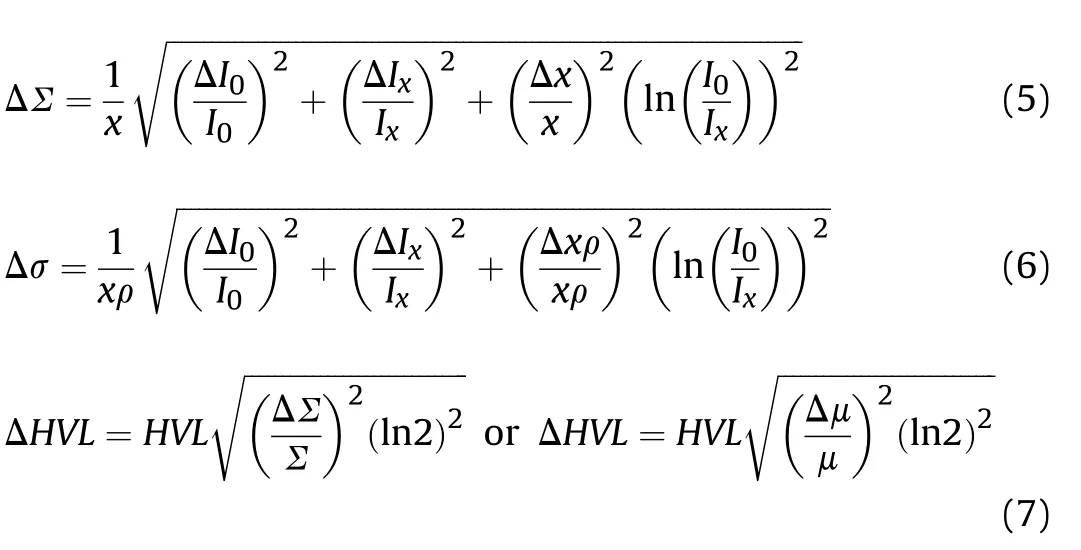
3.2. Corrosion measurements
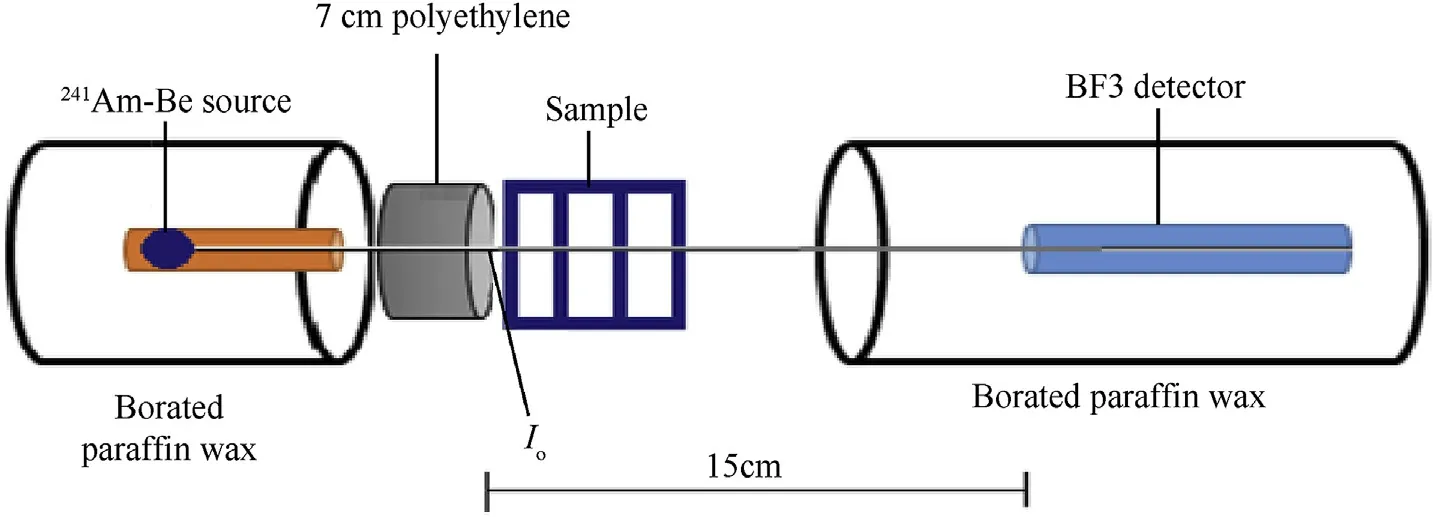
Fig.1.Schematic diagram of experimental setup for neutron detection.
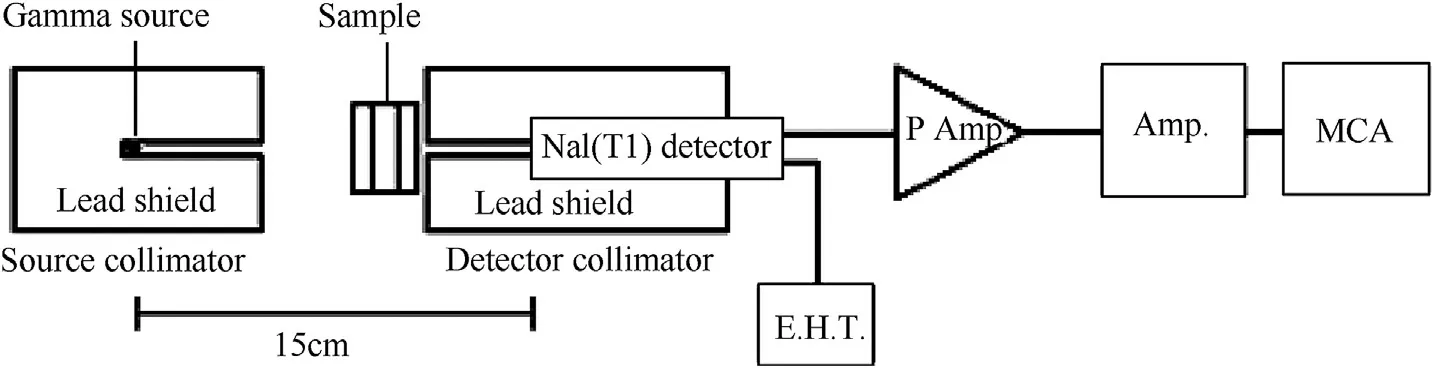
Fig.2.Schematic diagram of experimental setup for gamma ray detection.
The electrochemical corrosion tests were carried out using a three-electrode system.Ag/AgCl electrode was used as reference electrode,a platinum foil as counter-electrode and the investigated alloys with a surface area of 1.0 cm2as working electrode.The open-circuit potential(OCP)was recorded after immersion of the samples in the test solution for 15 min.The polarization tests were carried out at a scan rate of 0.5 mV⋅s-1.The PAR Calc Tafel Analysis software was used to fit the experimental data to the Stern-Geary model for a corroding system.Potentiostatic measurements were carried out at+500 mV(vs Ag/AgCl)for 120 s,where the anodic current was recorded as a function of time.All corrosion experiments were carried out in 3.5 wt%NaCl solution as electrolyte at different temperatures from 25 to 90°C.The solution was prepared from analytical grade and deionized water.The morphology of the surface after polarization was investigated by using optical microscope.
4. Results and discussions
4.1. Nuclear attenuation parameters
The values of cross-sections of slow,total slow neutrons(primary slow as well as slowdown in the studied steel alloys),and neutron with energy greater than 10 keV were deduced from the attenuation curves and presented in Fig. 3 and Table 2. It is observed that,the values of macroscopic cross-sections of slow neutrons in all types of steel alloys were the biggest values among the other neutron energies.This behavior of slow neutron may be attributed to radiative capture,(n,γ)reaction by Mn nuclei in all types of steel alloys and(n,n)reaction between slow neutrons and Fe nuclei.Moreover,the results show that the values of ∑for total slow neutron in duplex stainless-steel alloy are greater than that in other two types of steel alloys owing to(n,n)and(n,γ)reactions in slow down region by W nuclei.On the other hand,the results presented in Fig.3 and Table 2 show that there is insignificant change in macroscopic cross-section values of neutron with energy>10 keV for all alloys.This is due to(n,n)reactions,in this neutron energy range,with Fe,Mn,and Ni which are found in all types of samples.Also,this insignificant change may be attributed to the presence of W nuclei in duplex stainless steel which substitute the effect of low wt%of Fe in this type of alloy.
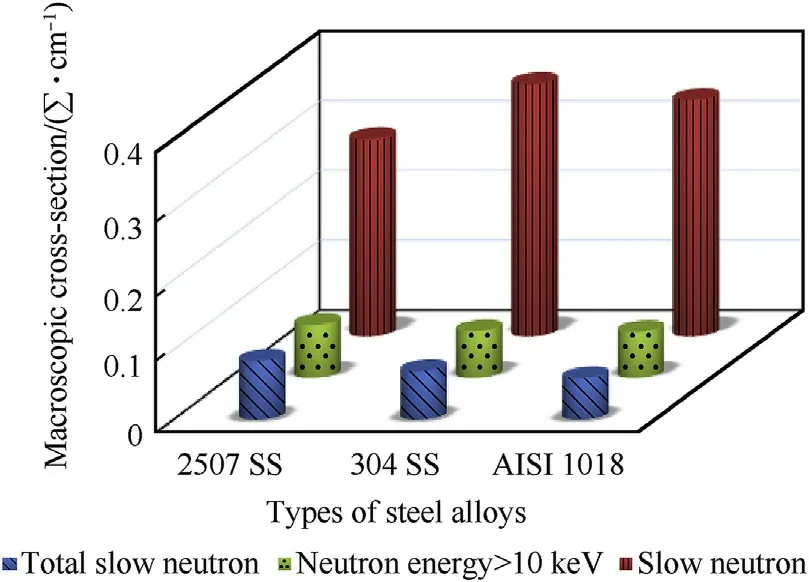
Fig.3.Macroscopic cross-sections of different types of steel alloys with different types of neutron energies.

Table 2 Macroscopic cross-sections of different types of steel alloys with different types of neutron energies.
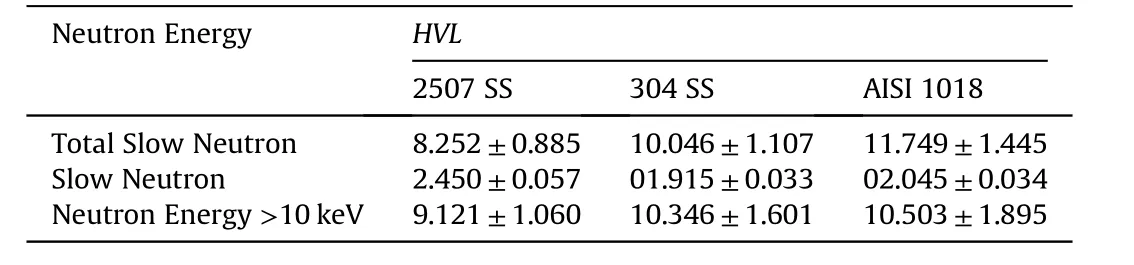
Table 3 Half value layer(HVL)of all investigated samples at different neutron energies.
The comparison of half value layers of the investigated alloys for different neutron energies is shown in Table 3.
The attenuation relations for the intensity of gamma rays,(in the energy range of 121—1407 keV)measured behind the investigated steel alloy barriers,are used to deduce the total linear attenuation coefficients(μ,cm-1)as a slope of these curves.
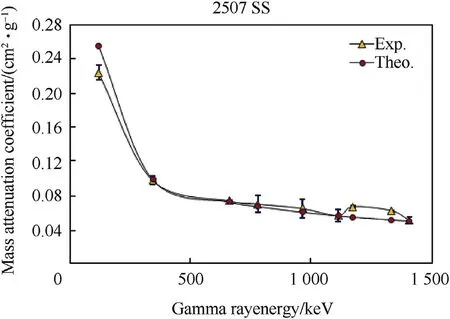
Fig.4.Mass attenuation coefficients of Duplex Stainless steel(2507 SS)samples as a function of gamma ray energy.
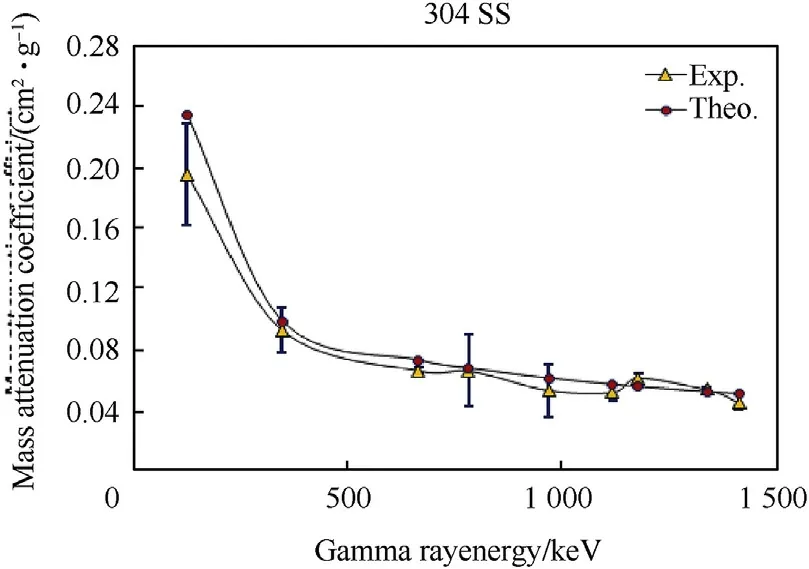
Fig.5.Mass attenuation coefficients of austenitic stainless steel(304 SS)samples as a function of gamma ray energy.
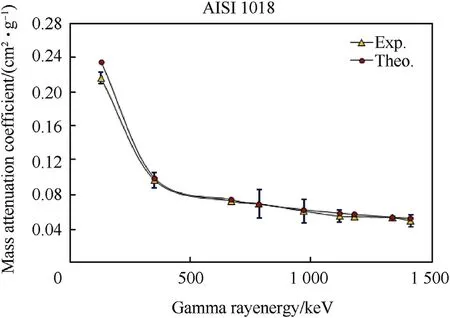
Fig.6.Mass attenuation coefficients of carbon steel(AISI 1018)samples as a function of gamma ray energy.
Fig.4-Fig.6 and Table 4 show the behavior of experimental and calculated mass attenuation coefficient(σ,cm2⋅g-1).On one side,the results show an excellent agreement between the experimental values of mass attenuation coefficients and that calculated by winXCom computer program(Version 3.1).On the other side,the behavior of all these curves can be divided into two regions.The first region from 121 to 400 keV was characterized by a sharp decrease in the values of mass attenuation coefficients with the increase of gamma ray energies.This behavior is due to the photoelectric reaction which predominates between the investigated alloy barriers and gamma rays.The second region from 400 to 1407 keV characterizes by a slight decrease in the values of mass attenuation coefficients with the increase of gamma ray energies.This is attributed to Compton scattering reaction which predominates in this stage.
Fig.7 shows the comparison between the average experimental values of mass attenuation coefficients of the investigated steel alloys at different gamma ray energies.The basic feature of this Figure is that,there is insignificant difference in the values of mass attenuation coefficients for all investigated alloys.This behavior is attributed to the values of densities of investigated steel alloys nearly the same.However,the duplex stainless-steel alloy has the highest value of mass attenuation coefficients for all used gamma ray energies owing to the presence of heavy nuclei such as Cr,Ni,Mo,and W nuclei as well as Fe nuclei.The comparison of half value layers of different types of investigated steel alloys for different investigated gamma ray energies is shown in Table 5.
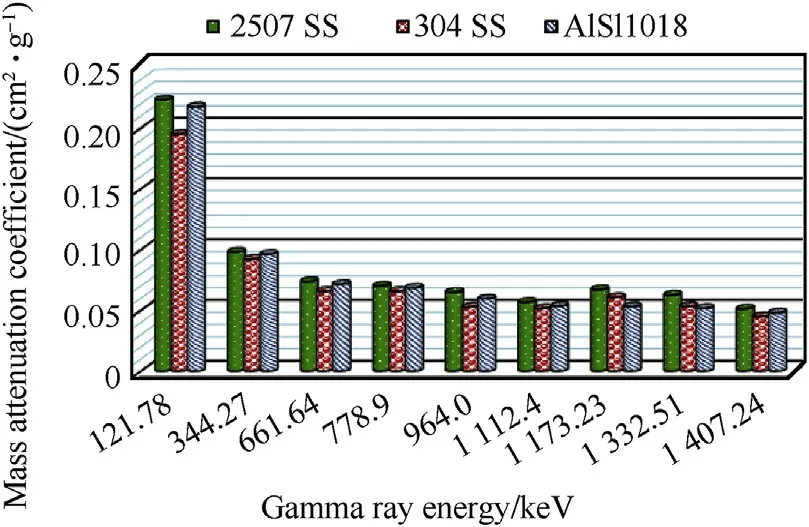
Fig.7.Comparison between mass attenuation coefficients of steel alloys samples with different gamma ray energies.
4.2. Corrosion behavior
4.2.1. Open circuit potential(OCP)
The variation of the free open circuit potential(OCP)with time for the investigated alloys in 3.5 wt%.NaCl solution at 25 and 90°C is shown in Fig.8 and Fig.9 respectively.It can be noted that the OCP as shown in Fig.8 tends from the moment of immersion towards more negative potentials for all alloys.This behavior represents the dissolution of steel alloys[4].After passing short time of immersion,the potentials of duplex and austenitic stainless steel shift towards more positive direction and stabilize while carbon steel shifts towards more negative potential with potential fluctuation.The positive shift in potential might be associated with thickening of the oxide film formed on the surface while the negative shift is attributed to the thinning and dissolution of the film.The OCP of the investigated alloy at 90°C is shown in Fig.9.It can be seen that increasing temperature to 90°C the OCP of all alloys shifts to more negative potentials with great potential fluctuation.However,duplex stainless steel still has more positive potentials comparing to the other alloys.This means that the protective ability of the passive film on duplex stainless steel is greater than the passive film of both austenitic stainless steel and carbon steel.
4.2.2. Potentiodynamic polarization
Potentiodynamic polarization curves of investigated alloys in 3.5 wt%.NaCl solution at different temperatures are shown in Fig.10-Fig.12.It can be observed from potentiodynamic polarization curves that the cathodic and anodic curves show a regularpattern for all alloys.The anodic current density continuously increases with increasing corrosion potential(Ecorr).This behavior shows that all investigated alloys have active dissolution behavior in 3.5 wt%.NaCl solution at different temperatures.Additionally,Fig.10-Fig.12 reveal that with increasing temperature,the Ecorrshifts to less noble potential and the corrosion current density(icorr)increases.The carbon steel has the highest current density while the duplex stainless steel has the lowest.On the other hand,the potentiodynamic polarization curves show that by increasing temperature the passive potential regions of duplex and austenitic stainless steel decreases.This behavior is attributed to the decrease in concentration of the dissolved oxygen and reduction of the pH values in some localized micro-regions,which prevent the formation of Cr6+[9—11].
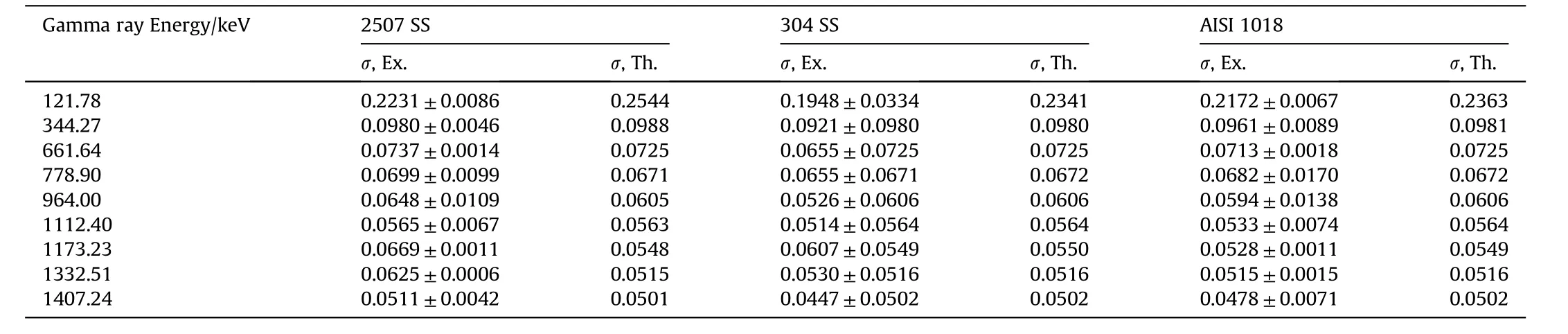
Table 4 Mass attenuation coefficients of the investigated alloys.
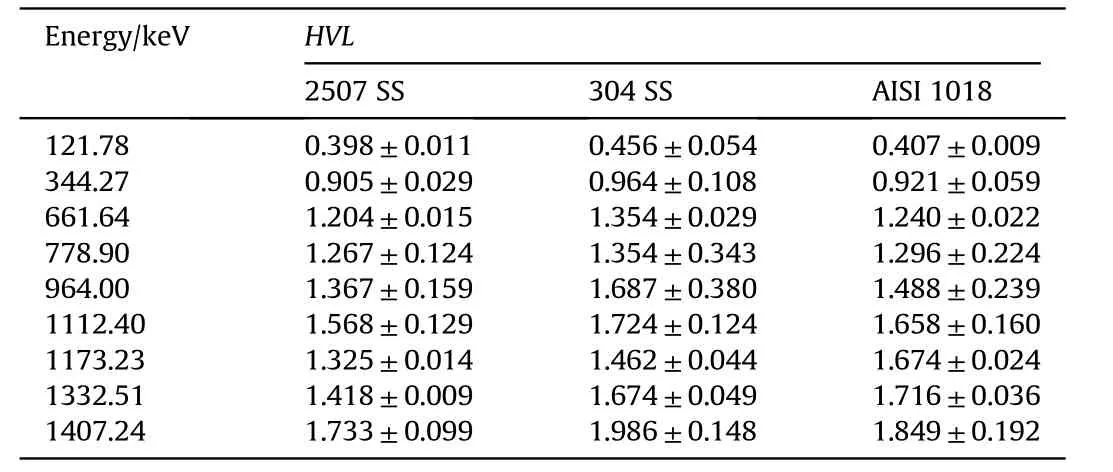
Table 5 Half value layer(HVL)of all investigated samples at different gamma ray energies.
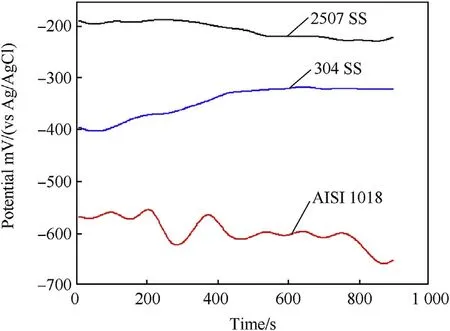
Fig.8.Potential-time curves of investigated steel alloys at 25°C in 3.5 wt%NaCl solution.
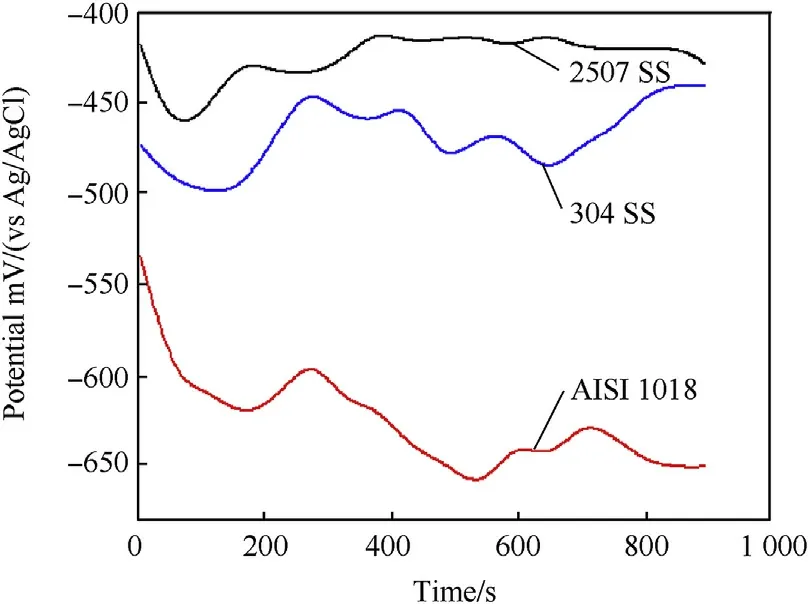
Fig.9.Potential-time curves of investigated steel alloys at 90°C in 3.5 wt%NaCl solution.
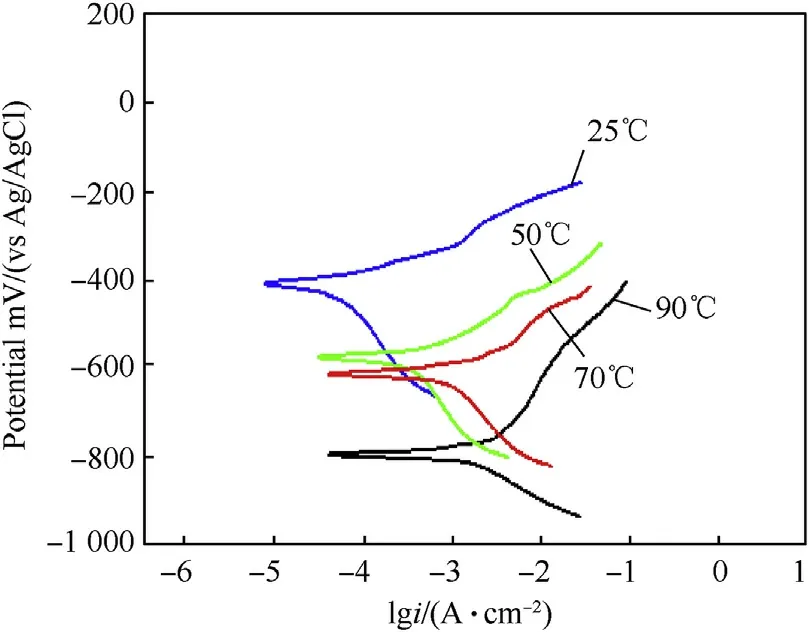
Fig.10.Potentiodynamic polarization curves of carbon steel(AISI 1018)in 3.5 wt%NaCl solution at different temperatures.
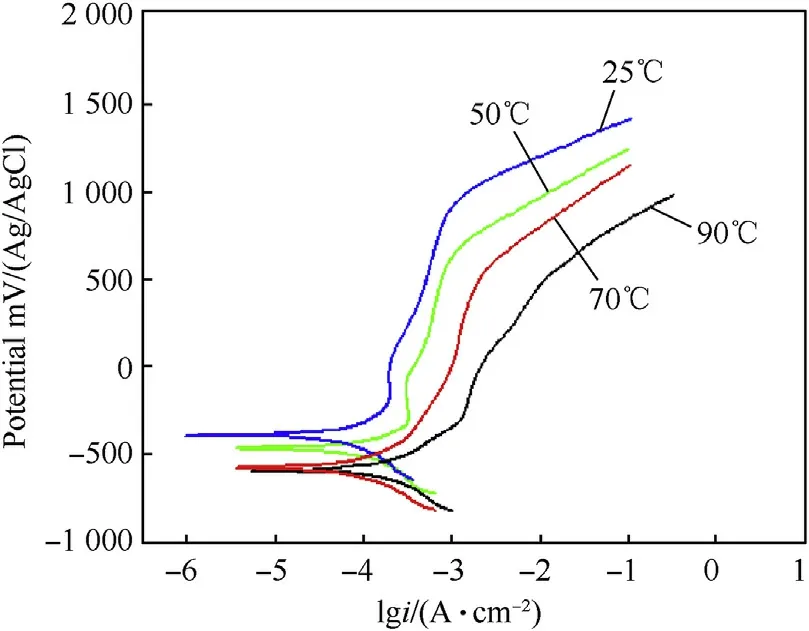
Fig.11.Potentiodynamic polarization curves of austenitic stainless steel(304 SS)in 3.5 wt%NaCl solution at different temperatures.
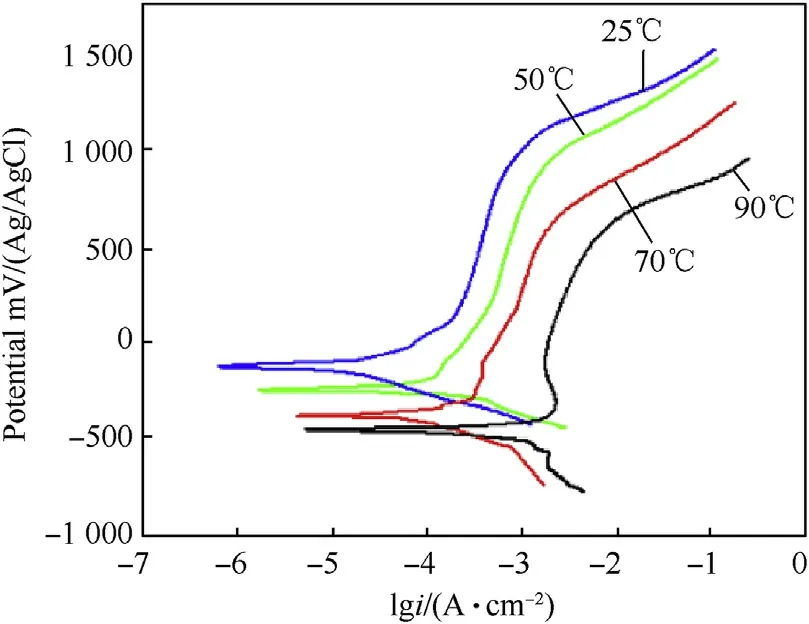
Fig.12.Potentiodynamic polarization curves of duplex stainless steel(2507 SS)in 3.5 wt%NaCl solution at different temperatures.
The electrochemical parameters extracted from potentiodynamic polarization curves are summarized in Table 6.It can be seen that the Ecorrfor duplex stainless steel is higher when compared with austenitic stainless steel and carbon steel at all different temperatures.In this case the rise in potential in noble direction with lower(icorr)for duplex stainless steel is due to thick protective film on the surface[5].
Fig.13 shows the breakdown potential(Eb)of the investigatedsteel alloys as a function of temperature.The Ebvalue decreases with increasing temperature due to the high mobility of aggressive chloride ions[11].However,the Ebof duplex stainless steel is higher when compared with the other steel alloys at different temperatures.This owes to the presence of molybdenum which promotes the passivation process and improves the pitting resistance.When molybdenum is incorporated into the passive film of stainless steel,it produces oxides with different oxidation states.However,the most common corrosion product incorporated into the passive layer is MoO42-,which is extremely stable and fixes this film[23,24].
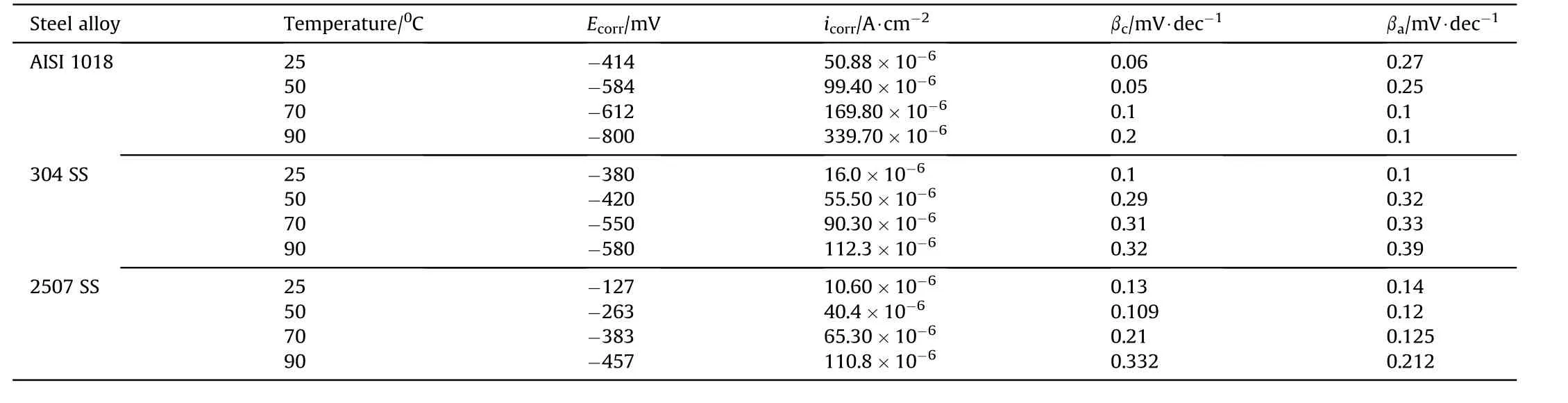
Table 6 Electrochemical parameters obtained from potentiodynamic polarization measurements of the investigated steel alloys at different temperatures in 3.5 wt%NaCl solution.
The activation energy(Ea)of the corrosion process of investigated steel alloys in 3.5 wt%.NaCl solution was calculated by using Arrhenius-equation:
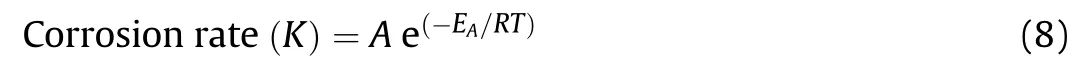
where,A is the Arrhenius constant,R is the universal gas constant and T is temperature.By plotting ln(K)versus(1/T),as shown in Fig.14 a straight line with a slope equals—Ea/R was obtained.The values of Eawere 25.79,27.32.and 32.06 kJ⋅mol-1for AISI 1018,304 SS and 2507 SS respectively.The Eavalue of duplex stainless steel >austenitic stainless steel >carbon steel.This means that the protective passive film of duplex stainless steel >austenitic stainless steel >carbon steel.
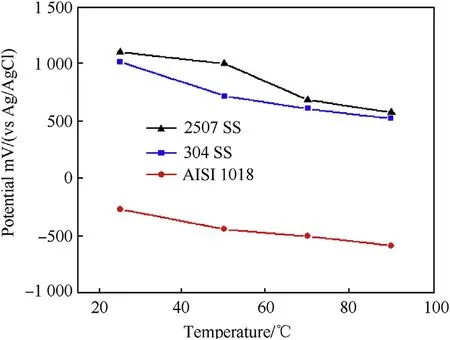
Fig.13.The breakdown potential(Eb)of the investigated steel alloys as a function of temperature in 3.5 wt%NaCl solution.
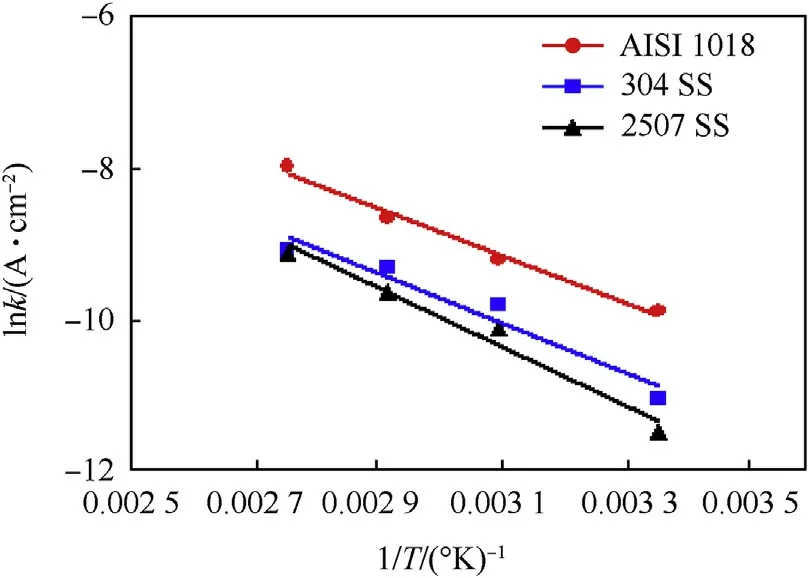
Fig.14.Arrhenius plots ln icorr vs.1/T for investigated steel alloys in 3.5%wt.NaCl solution.
On the other hand,Fig.15 and Fig.16 show the optical micrographs of the investigated steel alloys after potentiodynamic polarization tests at 25 and 90°C respectively.As shown in Fig.15(a)carbon steel alloy exhibits uniform dissolution due to absence of a protective oxide layer.While Fig.15(b)and Fig.15(c)indicate that the passive films of both austenitic and duplex stainless steels were dissolved in some selective sites due to defects in the passive layer and accompanied with some small pits.Increasing temperature to 90°C,the carbon steel alloy shows severely dissolution as shown in Fig.16(a)and size of pits increase obviously for both duplex and austenitic stainless steels (Fig.16(b) and Fig.15(c)). However,numbers and sizes of pits for duplex stainless are lower comparing with austenitic stainless steel.This means that the protective barrier layer of duplex stainless steel against corrosion attacks is stronger than the passive layer of austenitic stainless steel.
4.2.3. Potentiostatic current—time measurements
Potentiostatic measurements were carried out at 25 and 90°C to gain more information on the passivity of investigated steel alloys in 3.5 wt%NaCl solution.Fig.17 displays the current density variation with time at 25°C and+500 mV vs.(Ag/AgCl).The electrochemical behavior of carbon steel can be classified into two stages.In the first stage the current density increases in few seconds about 24 s and this represent the dissolution of alloy.In the second stage the current density fluctuates until the end of the test.This trend represented the accumulation of corrosion products of a porous γ-Fe2O3/Fe3O4film[25]and initiation of pits.On the other hand,Fig.17 shows that the current densities of austenitic stainless steel and duplex stainless steel keep constant.This behavior represents the dynamic equilibrium between the dissolution and growth of the passive film[10,11].Additionally,Fig.17 reveals that the passive current density of duplex stainless steel is lower than that of austenitic stainless steel.This means that the passive film of duplex stainless steel has low defects compared to austenitic stainless steel.Fig.18 shows the current density variation with time at 90°C and+500 mV vs.(Ag/AgCl).The results indicate that the current density of carbon steel fluctuated from the moment of immersion till the end of the experiment.This behavior was attributed to aggressive dissolution of surface and the corrosion product is very porous at 90°C which dose not have ability to passivate the surface.Furthermore,the current density of austenitic stainless steel and duplex stainless steel accompanied with the fluctuation of current density.This represents the formation of metastable pits on the surface[26].Presence of molybdenum in duplex stainless steel increases the incubation time of pits on the surface and decreases the current density compared with the other alloys.

Fig.15.Micrographs of surface morphology of investigated steel alloys after potentiodynamic measurements at 25°C.

Fig.16.Micrographs of surface morphology of investigated steel alloys after potentiodynamic measurements at 90°C.
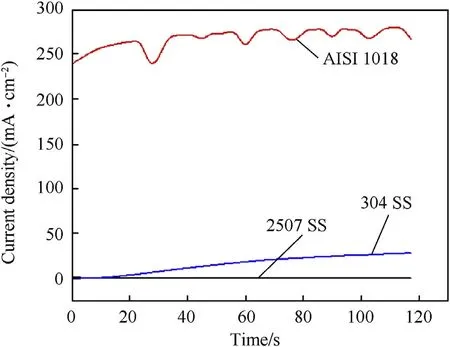
Fig.17.Potentiostatic current—time curves of investigated steel alloys at 25 °C and+500 mV in 3.5 wt%NaCl solution.
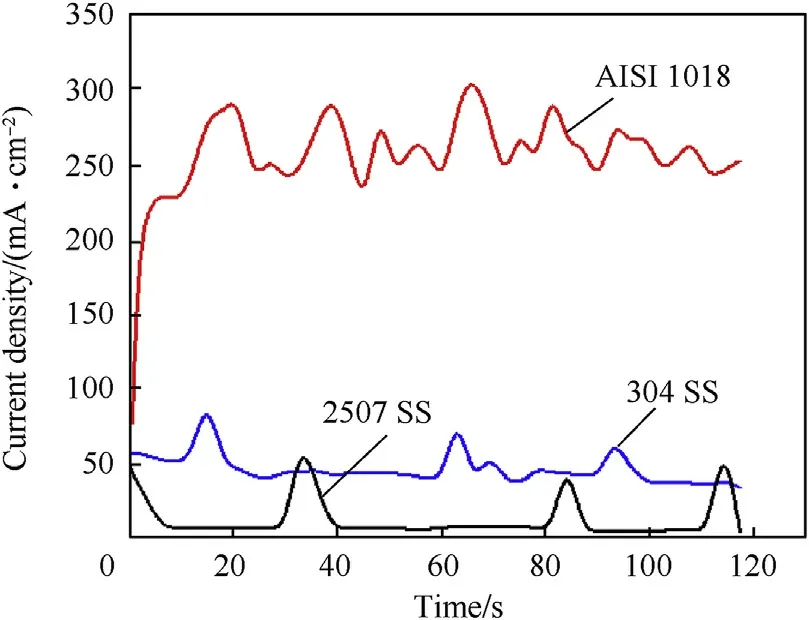
Fig.18.Potentiostatic current—time curves of investigated steel alloys at 90 °C and+500 mV in 3.5 wt%NaCl solution.
5. Conclusion
A comparison between three types of steel alloys was carried out from the point of view of nuclear and corrosion studies.From the obtained results,it can be conclude that,stainless steel alloys especially duplex 2507 SS,are a good candidate to be used in different position in nuclear reactor systems as reactor fuel cladding,pressure vessel,and piping inner layer,…etc.due to the following:
1)Duplex 2507 SS has better HVL than that of 304 SS and AISI 1018 for total slow neutron and neutron energy >10 keV.
2)Duplex stainless steel has the best values of mass attenuation coefficient of gamma ray energies and HVL.
3)The passive current density of duplex stainless steel is lower than that of austenitic stainless steel and carbon steel.
4)Pitting corrosion potential of investigated alloys decreased with increasing temperature.However,duplex stainless steel has more pitting resistance compared with other alloys at all different temperatures.
Acknowledgement
Authors appreciate their deeply thanks for Al Azhar University,Faculty of Science & Faculty of Engineering, Cairo, Egypt for providing us the necessary materials and measurements.
杂志排行
Defence Technology的其它文章
- Defence Technology
- Electrodynamic response study on railgun launcher based on electromechanical coupling model
- Effect of tongue clearance on hydraulic performance of double support vortex pump
- Electroless nickel fabrication on surface modified magnesium substrates
- The catalytic activity of transition metal oxide nanoparticles on thermal decomposition of ammonium perchlorate
- Surface modification of ammonium nitrate by coating with surfactant materials to reduce hygroscopicity
