两种粒径纳米银对Nitrosomonas europaea的毒性效应
2019-10-23伍玲丽张晓雪舒昆慧司友斌
伍玲丽,张晓雪,舒昆慧,司友斌
两种粒径纳米银对的毒性效应
伍玲丽,张晓雪,舒昆慧,司友斌*
(安徽农业大学资源与环境学院,农田生态保育与污染防控安徽省重点实验室,安徽 合肥 230036)
为明晰不同粒径纳米银(AgNPs)对欧洲亚硝化毛杆菌()的毒性效应,采用室内培养方式,探究10nm和50nm的AgNPs对生长、氮转化能力、细胞结构、活性氧生成和功能基因表达的影响.结果表明,AgNPs暴露抑制生长,随着暴露时间的延长,细菌生长抑制率增加,在4h达到最大值;培养基中NH4+向NO2-转化速率减缓,的铵态氮转化能力降低;扫描电镜(SEM)图像显示AgNPs造成部分细菌表面塌陷且有孔洞,细胞膜受损严重;透射电镜(TEM)图像显示AgNPs造成细菌内部核物质消融,细胞质膜界限模糊;流式细胞仪(FCM)检测发现AgNPs增加细胞内活性氧的生成;qRT-PCR技术对AgNPs暴露后功能基因表达进行测定,发现AgNPs抑制功能基因的转录表达.综上所述,AgNPs通过与细胞膜相互作用和产生氧化应激损伤,抑制和的表达,进而影响铵态氮转化过程,且小粒径AgNPs的毒性强于大粒径.
纳米银;;氧化应激;、、基因丰度;氨氧化
纳米银(AgNPs)是尺寸范围为1~100nm的原子簇,由约20~15000个Ag原子组成,与固相Ag相比,具有电导率和热导率高、化学性质稳定、催化活性强等特性[1],广泛应用于电子器件、化妆品、生物医学和纺织业等方面[2].AgNPs的抗菌作用已被广泛描述,一些研究认为AgNPs释放的Ag+是其毒性的主要原因,自身的粒子特异性可以忽略不计[3-5],也有研究表示AgNPs的毒性主要取决于粒径大小[6].据报道,20~80nm AgNPs的毒性主要来源于Ag+的释放,而10nm AgNPs由于尺寸较小,更易穿透细菌的细胞膜,毒性主要归于自身的尺寸效应[7-8].AgNPs可以与细胞膜上蛋白质结合,与含有氧、磷、氮或硫原子的电子供体形成络合物,导致膜结合酶等蛋白质失活,也可干扰呼吸链并阻碍能量的产生[9-10].此外,AgNPs会造成细胞膜脂肪酸组成改变,改变膜的流动性[11],导致细胞内容物的泄漏[12].AgNPs还可以与细菌的遗传物质结合,阻断翻译和转录[13-14],也有研究表明AgNPs进入细胞内造成活性氧物质(ROS)累积导致膜损伤和基因毒性[15].
环境中的氨氧化菌群主要负责驱动NH4+-N向NO2--N的转化,由于其独特的生理特性对环境因子变化较为敏感[16].欧洲亚硝化毛杆菌()为氨氧化模式菌株,一直以来都作为重点研究对象[17].AgNPs已经被证明对和其他氨氧化细菌具有毒性效应[18-19].Alito等[20]在一项短期实验中发现AgNPs能毒害氨氧化微生物,使活性污泥中的氨氧化速率降低30%左右.Wu等[21]发现在ZnONPs暴露下膜完整性受到破坏,细胞生长和硝化速率受到抑制.Yuan等[22]将暴露在不同粒径AgNPs中,发现细胞壁遭到破坏,且与生物合成、能量产生和与氨氧化过程有关的重要蛋白质的表达受到抑制.Choi等[23]发现AgNPs对生长和氨氧化速率的抑制可能与AgNPs诱导造成细胞体内ROS累积有关.目前,已有大量研究表明AgNPs对氨氧化过程具有负面影响,但AgNPs对氨氧化微生物毒性机制仍不清楚[24].本研究以为对象,不仅从细胞水平探究两种粒径AgNPs对生长、细胞膜损伤、ROS生成的影响,更是从分子水平解析AgNPs对功能基因表达的影响,以期揭示AgNPs对可能的毒性机制.
1 材料与方法
1.1 实验材料
1.1.1 供试材料 10nm的AgNPs(Ag10)表面由聚乙烯吡咯烷酮(PVP)包被,购于南京先丰纳米有限公司,平均粒径为10±5nm,形态如图1A所示.50nm的AgNPs(Ag50)表面无包被,购于南京埃瑞普纳米材料有限公司,平均粒径为50±5nm,形态如图1B所示.醋酸银(CH3COOAg,又名乙酸银),纯度³99.5%,密度3.26g/cm3,分子量166.91,CAS号563-63-3,购自西亚试剂.
1.1.2 菌种与培养基(ATCC 19718)由中国科学院城市环境研究所于昌平教授课题组提供.培养基配方:1.32g/L (NH4)2SO4,0.20g/L MgSO4∙7H2O,0.02g/L CaCl2∙2H2O,0.087g/LK2HPO4, 2.52g/L N-2-羟乙基哌嗪-Ni3-丙磺酸(EPPS),1mg/L C10H12FeN2NaO8∙3H2O,0.1mg/L Na2MoO4∙ 2H2O和ZnSO4∙7H2O,0.172mg/L MnSO4,0.04mg/L CoCl2∙6H2O, 2.5mg/L酚红,0.25mg/L CuSO4∙5H2O.KHCO3溶液调节pH值至6.9~7.5.
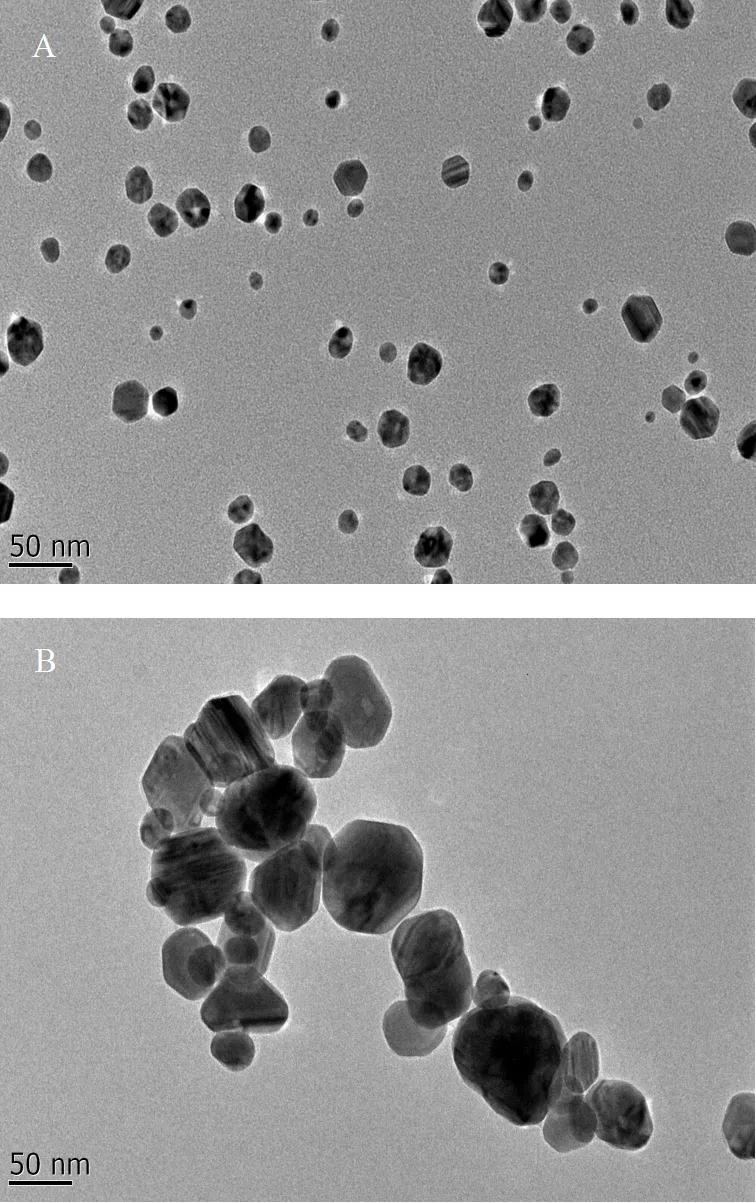
图1 两种粒径纳米银TEM图(A:10nm; B:50nm)
1.2 菌悬液制备
将培养至对数生长期后4000r/min离心收集菌体,KH2PO4缓冲液洗涤残余培养基,菌体重悬于缓冲液中,4℃避光保存备用.
1.3 N. europaea生长及NH4+、NO2-含量测定
将两种粒径AgNPs和菌悬液加入高温灭菌后的培养基中,使AgNPs最终浓度为5,10,20mg/L,同时控制初始OD600为0.07,于30℃、150r/min恒温避光培养,按时取样,每个处理三个重复.纳氏试剂比色法测定培养基中NH4+含量,盐酸N-(1-萘基)-乙二胺比色法测定NO2-含量.同时用紫外分光光度计(UV-2550,菁华,中国)测定OD600,生长抑制率(%)=1-处理组OD600/空白组OD600´100%.
1.4 培养基中AgNPs的Ag+释放量
将AgNPs(10nm终浓度为5,10mg/L;50nm终浓度为10,20mg/L)暴露在培养基中,培养不同时间后装入超滤离心管(Millipore 3kDa,Amicon,美国)中,4500r/min离心过滤,电感耦合等离子发射光谱仪(ICP-MS 7500CX, Agilent,美国)测定滤液中Ag+含量,每组三个重复.
1.5 N. europaea细胞形态观察
Ag10(10mg/L)和Ag50(20mg/L)处理12h后离心(10000r/min,4℃)收集菌体,0.1mol/L磷酸盐缓冲液(PBS)漂洗两次,2.5%戊二醛固定4h,PBS再漂洗3次,乙醇梯度脱水,丙酮替代20min后干燥过夜,前处理结束后样品镀膜进行扫描电镜SEM(S-4800,日立,日本)观察.透射电镜样品用戊二醛固定后再用1%锇酸固定1~2h,然后脱水、浸透、包埋、烘干、切片和染色后在透射电镜TEM(H-7650,日立,日本)下观察,同时设置空白对照组.
1.6 N. europaea细胞内ROS检测
乙酰半胱氨酸(N-acetyl-L-cysteine,NAC)作为抗氧化剂可以清除细胞内多余的ROS,降低AgNPs产生的氧化损伤[25],本实验添加NAC验证细胞内部ROS的产生.将预培养1h后10000r/min离心收集菌体,PBS洗涤后加入DCFH-DA探针,按照活性氧检测试剂盒(碧云天S0033,上海)的说明,于30℃孵育30min后利用流式细胞仪(FCM, FACSCalibur, BD,美国)于488nm为激发波长、525nm为发射波长的条件下检测荧光强度.试验共5个处理:空白对照(CK)、Ag10(10mg/L)、Ag50(20mg/L)、Ag10(10mg/L)+5mmol/L NAC、Ag50(20mg/L)+ 5mmol/L NAC.
1.7 功能基因amoA、hao、merA表达分析
1.7.1 RNA提取与反转录 取约108个菌体,加入溶菌酶消化10min,4℃和12000r/min下离心2min,使用UNIQ-10柱式Ⅰ总RNA抽提试剂盒(生工,上海)提取总RNA,按照TransScript All-in-One First-Stand cDNA Synthesis SupriMix for qPCR(TransGen, 北京)试剂盒进行反转录.RT体系与条件:10μmol/L Random Primer 1μL,RNA 6μL,RNase free dH2O 5μL,混合后70℃热激3min,冰浴5min,然后继续加入dNTP Mixture 2μL,RNase free dH2O 0.5μL,M-MLV 1μL,5´M-MLV Buffer 4μL,RNase Inhibitor 0.5μL,总体积共20μL.将各组分混匀后置于42℃水浴1h,得到第一链cDNA.
1.7.2 Real-Time PCR 本实验根据Ultra SYBR Mixture(康为世纪, 北京)试剂盒说明,采用SYBR Green I法实时荧光定量PCR(相对定量)进行mRNA表达量测定.20μL反应体系为:10μL 2×SYBR Premix EX-Taq Mi,0.5μL 10μmol/L PCR Forward Primer和PCR Reverse Primer,0.4μL ROX (50×),2μL Template cDNA,6.6μL RNase Free dH2O.、、的所用引物如表1所示,每个样品3次重复.
Real-time PCR扩增条件: 95℃预变性10min,40个循环( 95℃变性15s, 61℃退火35s,97℃延伸10s) 最后从65℃升至97℃获得熔解曲线,采用2-△△Ct法进行相对表达量计算.

表1 Real-time PCR实验所用引物
1.8 数据处理与分析
试验数据采用SPSS 18.0进行统计分析,并对不同处理间的数据用单因素方差分析. (ANOVA)和Duncan多重比较进行显著性差异(<0.05)检验,再用Origin 9.0绘图.
2 结果与分析
2.1 AgNPs对N. europaea生长抑制
暴露在两种粒径的AgNPs中,细菌生长受到显著抑制(图2).生长与AgNPs剂量呈负相关,且在同等剂量下,10nm AgNPs对生长的抑制作用比50nm强.生长抑制率随着AgNPs暴露时间的延长而增加,在4h达到最大值.4h后抑制率没有显著变化,甚至10nm(5mg/L)和50nm(5、10mg/L)AgNPs处理组抑制率出现降低趋势,说明开始适应AgNPs存在.
2.2 AgNPs对N. europaea铵态氮转化的影响
随着培养时间延长,无银对照组中铵态氮由于向硝酸盐氮转化,其含量逐渐降低.不同粒径、不同剂量AgNPs暴露下铵态氮转化能力受到抑制(图3).AgNPs刺激8h后,对照组中NH4+含量减少了0.10mg/mL.Ag10(5,10,20mg/L)处理组NH4+浓度分别降低0.09,0.07,0.06mg/mL,而Ag50(5,10, 20mg/L)处理组NH4+浓度分别减低0.10,0.09, 0.07mg/mL.由于NH4+的转化,培养液中NO2-含量逐渐增加,与对照组相比,AgNPs暴露组中NO2-含量明显降低.其中,Ag10(10,20mg/L),Ag50(20mg/L)处理下,培养液中NO2-含量几乎不变,而Ag10(5mg/L)和Ag50(5,10mg/L)处理组NO2-含量略有增加,但显著低于对照组.
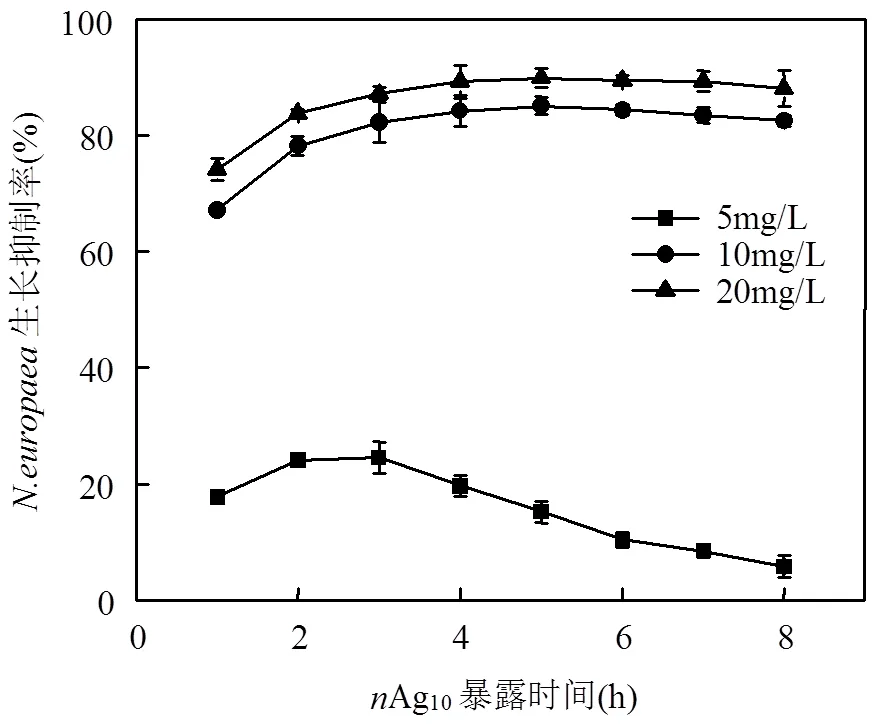
图2 两种粒径纳米银暴露下N. europaea生长抑制率变化
2.3 AgNPs的Ag+ 释放
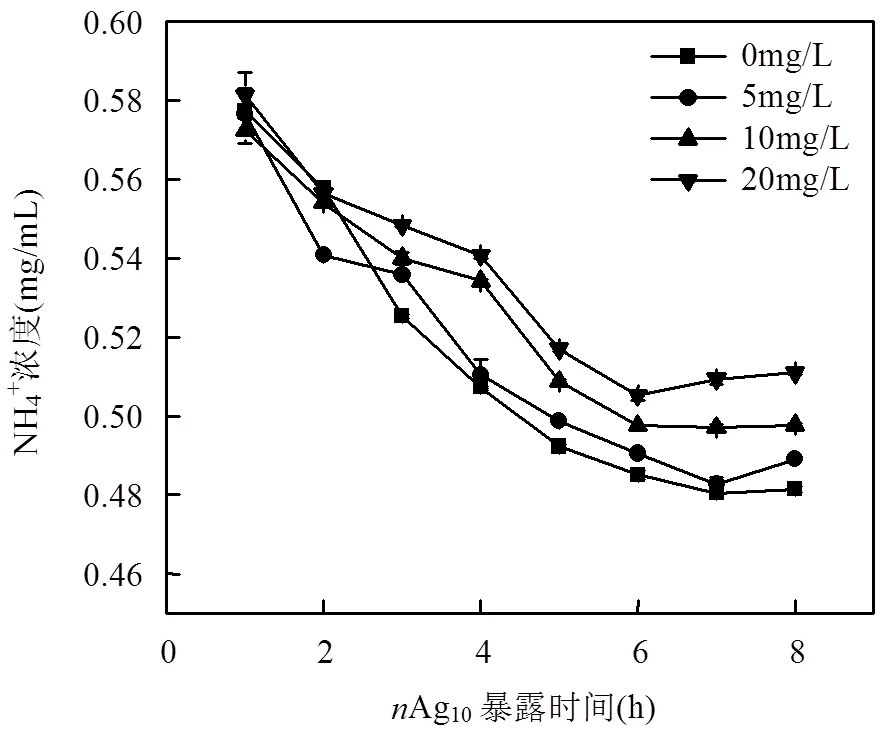
暴露在环境中的AgNPs容易游离出Ag+,本研究选取10nm(5,10mg/L)和50nm(10,20mg/L)的AgNPs进行Ag+释放量的测定(图4).前6h内培养基中Ag+含量随着培养时间的延长而增多,6h后趋于稳定.暴露12h后,Ag10(5,10mg/L)Ag+释放量占1.09%、0.75%;Ag50(10、20mg/L)的Ag+释放量高达5.41%、4.56%.粒径为50nm的AgNPs释放的Ag+量显著高于10nm的AgNPs,且50nm的AgNPs初始游离出的Ag+较高.

图4 培养基中AgNPs的Ag+释放量
2.4 AgNPs对N.europaea细胞形态及结构的影响
通过SEM和TEM观察AgNPs处理12h后细胞形态与内部结构的变化,其中图A、C、E放大40000倍,图B、F放大12000倍,图D放大15000倍.空白组(图5A、B)中细胞饱满且表面光滑,结构清晰完整,细胞内部物质分布均匀.扫描电镜图像(图5C、E)显示AgNPs处理造成细胞膜表面出现塌陷,且有大小不一的孔洞,细胞内容物流出.透射电镜图像(图5D、F)显示AgNPs处理造成细胞内核解体且中央出现大片空白区域,细胞器聚集于细胞边缘,细胞质壁界限模糊.
A、B:空白对照; C、D:10mg/LAg10; E、F:20mg/LAg50
2.5 AgNPs对N.europaea的氧化应激作用
暴露在两种粒径AgNPs中细胞内ROS含量显著上升(图6).空白组中细胞内平均荧光强度为6.7,Ag10(10mg/L)和Ag50(20mg/L)处理组中细胞内平均荧光强度升高到35.2和28.1,添加NAC后荧光强度又显著降低为12.7和12.5.说明AgNPs能刺激内部ROS的产生和累积,AgNPs氧化应激作用可能是对产生毒性效应的原因之一.
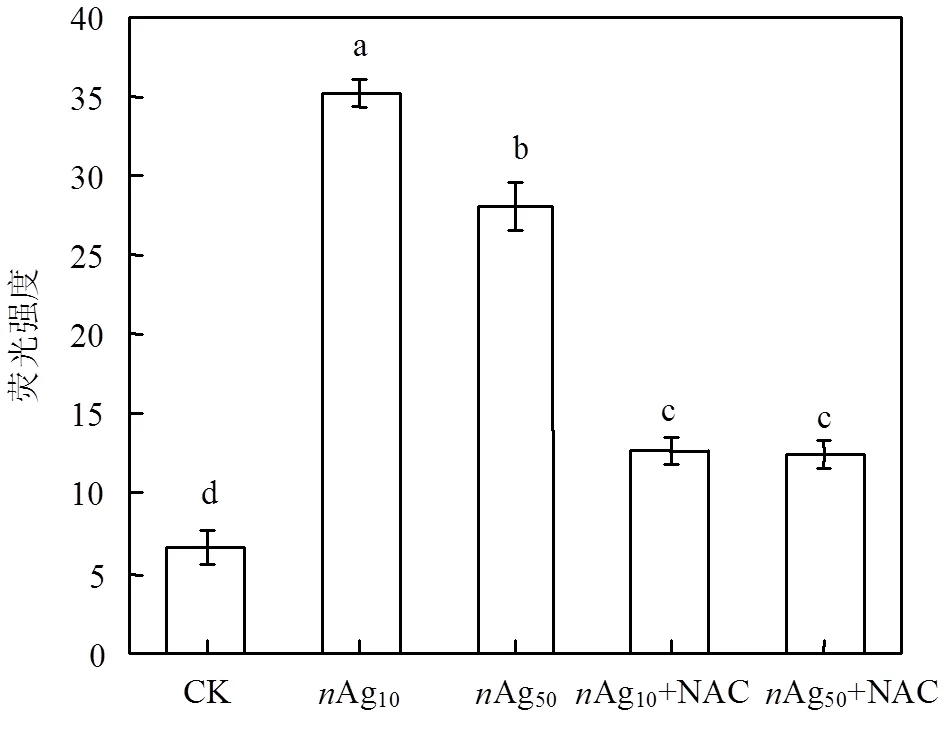
图6 两种粒径纳米银暴露后N. europaea细胞内ROS含量变化
Ag10为10mg/L;Ag50为20mg/L;NAC为5mmol/L
图中标有不同小写字母者为Duncan检测下差异显著(<0.05),下同
2.6 AgNPs对N. europaea功能基因表达的影响
负责编码氨氧加氧酶,主要调控NH4+到NH2OH的转化,负责编码羟氨氧化还原酶,主要调控NH2OH到NO2-转化过程[26],merA调控重金属应激[27].本研究以的16S RNA为参照基因,以无银处理为对照组,AgNPs和Ag+暴露12h后功能基因、、表达如图7所示.基因表达下调为负性调控(抑制表达),表达上调则为正性调控(促进表达).Ag+处理组中、、表达与对照相比分别下调23.26、1.96、12.82倍;Ag10(10mg/L)处理组、表达分别下调13.33、8.06倍,表达上调1.46倍;Ag50(20mg/L)处理组、表达分别下调1.37、2.79倍,则上调了1.90倍.说明Ag+能显著抑制、、表达,AgNPs处理下、表达下调,轻微上调.
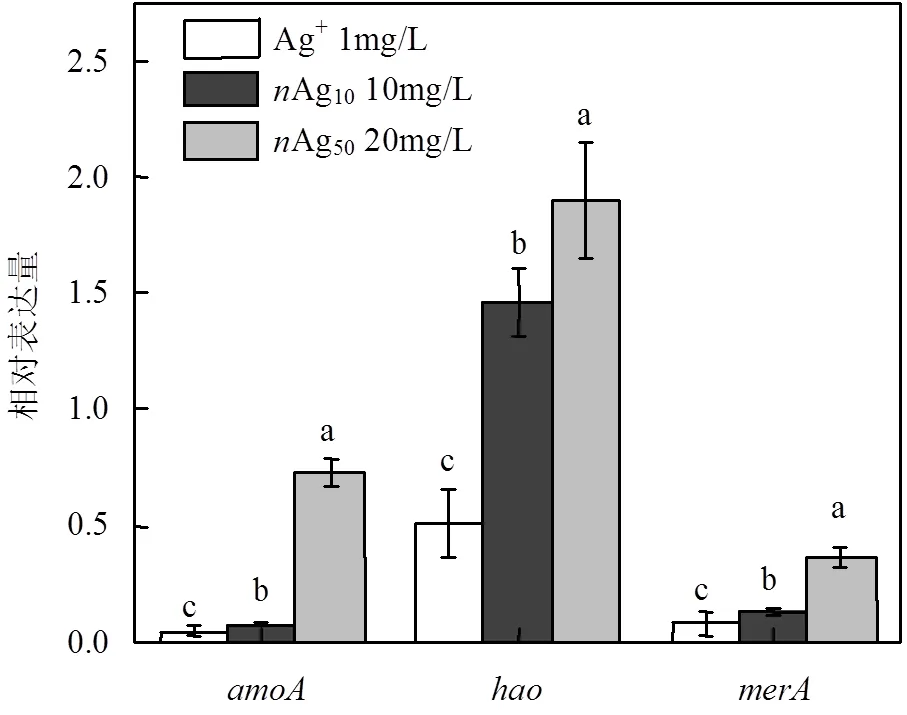
图7 纳米银和银离子胁迫下N. europaea基因表达量变化
3 讨论
3.1 AgNPs对N. europaea生长抑制与其释放的Ag+量相关
.对环境因子的变化较为敏感.本研究发现AgNPs抑制生长,培养基中NH4+向NO2-转化过程受阻,氨氧化过程减缓,且10nm比50nm的AgNPs影响更大. Radniecki等[28]得出类似结论,并发现对小粒径AgNPs的敏感性增加是由于其表面积与体积比较大,AgNPs的毒性与其颗粒大小有关.纳米材料与细胞直接接触被广泛认为是其生物毒性来源之一[29],也有大量文献证实AgNPs对氨氧化微生物的毒性与释放的Ag+密切相关[30-31].本研究中50nm的AgNPs释放的Ag+高于10nm的AgNPs,12h后50nm(20mg/L)的AgNPs释放的Ag+高达1mg/L,而10nm的AgNPs Ag+释放量只有几十μg/L,可能是因为10nm的AgNPs表面由PVP包被,在液体环境中较为稳定,而50nm的AgNPs表面无包被,更易释放Ag+,这与Arnaout和Gunsch[32]的研究结果相近.
3.2 AgNPs造成N. europaea的细胞结构损伤及细胞内活性氧的累积
扫描电镜(SEM)和透射电镜(TEM)图像显示,AgNPs造成细胞表面塌陷且有孔洞,细菌内部核物质消融且质膜界限不明显,AgNPs与细菌表面直接作用可能是AgNPs导致死亡的原因之一.通过染色进行电镜观察已成为一种普遍方法,本研究得出在银胁迫下细胞膜遭到破坏与他人在文献中描述的一样[9].AgNPs不仅可以直接破坏细胞结构损伤细胞,还可以诱导细胞体内ROS的累积,对造成氧化损伤.ROS主要包括羟基自由基(·OH)、超氧阴离子(·O2-)、单线态氧(1O2)、过氧化氢(H2O2)等具有杀菌作用的自由基[33].电子自旋共振光谱(EPR)被认为是证明ROS存在的有效工具,我们前期工作已检测到AgNPs产生的自由基为·OH[34].当微生物体内ROS含量超过自身抗氧化防御能力会导致生物体谷胱甘肽(GSH)的耗竭以及多种抗氧化酶活性的改变,是AgNPs毒性作用的可能机制之一[35].AgNPs暴露下内部ROS含量测定结果显示两种粒径AgNPs暴露后细胞内ROS含量均明显增高,NAC处理后细胞内ROS显著降低,进一步印证了AgNPs的氧化应激作用.氧化应激是AgNPs毒性机制中报道最多的,ROS的产生会导致细胞壁损伤、细胞膜破坏、蛋白质损伤和电子传递中断[36].
3.3 AgNPs对N. europaea功能基因amoA、hao和merA表达的影响
qRT-PCR测定结果显示两种粒径AgNPs均抑制和表达,轻微上调.其中负责调控氨氧化过程第一步的基因受AgNPs影响最大,这可能是AgNPs影响铵态氮转化的主要原因,与Michels等[26]研究结果类似.也有文献报道AgNPs虽然抑制硝化作用,的表达却没有改变,AgNPs不影响的转录[32].用于NADPH还原,在重金属存在时倾向于上调[37],本研究发现AgNPs暴露下表达受抑制,说明AgNPs对的影响与一般重金属应激机制不同.10nm的AgNPs对功能基因表达的抑制效果比50nm的AgNPs强,可能是小粒径AgNPs更易进入细胞内部,与细胞内物质(包括核酸)结合,破坏细胞内部结构[38].Choi等[39]同样发现小粒径AgNPs对氨氧化细菌基因的损伤较严重.根据nAg50(20mg/L)12h释放的Ag+量,选择1mg/L Ag+暴露下对功能基因表达的影响,发现Ag+对、、表达抑制较明显.10nm的由PVP包被的AgNPs释放的Ag+较少但对基因的影响依旧很大,其中PVP作为一种涂层材料是无毒的[40],说明AgNPs对基因的表达与粒径大小有关.50nm的AgNPs的毒性可能部分来自释放的Ag+,部分由于本身的特异抗菌性,10nm AgNPs的毒性主要是自身作用的结果[41].AgNPs的毒性大小与其浓度及粒径有关.
虽然本文利用两种不同粒径的AgNPs探讨了对的毒性作用,并发现AgNPs通过直接破坏细胞结构、产生氧化应激和影响和基因表达进而影响铵态氮的转化,但是关于AgNPs对毒性作用及其影响机理还有待进一步的研究.
4 结论
4.1 AgNPs抑制了生长,导致铵态氮转化过程延缓,且粒径越小抑制作用越强.
4.2 AgNPs对的毒性作用主要有两条途径:AgNPs能直接作用于表面,破坏细胞膜,导致胞内物质流出,核物质消融且质膜界限不明显;刺激细菌体内ROS生成,ROS累积造成氧化损伤.
4.3 实时荧光定量PCR结果显示,AgNPs抑制和表达,对的表达影响较小.
[1] Reidy B, Haase A, Luch A, et al. Mechanisms of silver nanoparticle release, transformation and toxicity: A critical review of current knowledge and recommendations for future studies and applications [J]. Materials, 2013,6(6):2295-2350.
[2] Vance M E , Todd K , Vejerano E P , et al. Nanotechnology in the real world: redeveloping the nanomaterial consumer products inventory [J]. Beilstein Journal of Nanotechnology, 2015,6:1769-1780.
[3] Franci G, Falanga A, Galdiero S, et al. Silver nanoparticles as potential antibacterial agents [J]. Molecules, 2015,20(5):8856-8874.
[4] Ivask A, Elbadawy A, Kaweeteerawat C, et al. Toxicity mechanisms invary for silver nanoparticles and differ from ionic silver [J]. ACS Nano, 2014,8(1):374-386.
[5] Palza H. Antimicrobial polymers with metal nanoparticles [J]. International Journal of Molecular Sciences, 2015,16(1):2099-2116.
[6] Wu D, Fan W, Kishen A, et al. Evaluation of the antibacterial efficacy of silver nanoparticles against, biofilm [J]. Journal of Endodontics, 2014,40(2):285-290.
[7] Hsiao I L, Hsieh Y K, Wang C F, et al. Trojan-horse mechanism in the cellular uptake of silver nanoparticles verified by direct intra- and extracellular silver speciation analysis [J]. Environmental Science & Technology, 2015,49(6):3813-3821.
[8] Liu J, Sonshine D A, Shervani S, et al. Controlled release of biologically active silver from nanosilver surfaces [J]. ACS Nano, 2010,4(11):6903-6913.
[9] Morones J R, Elechiguerra J L, Camacho A, et al. The bactericidal effect of silver nanoparticles [J]. Nanotechnology, 2005,16(10):2346- 2353.
[10] Tian X, Jiang X M, Welch C, et al. Bactericidal effects of silver nanoparticles onand the underlying mechanism [J]. ACS Applied Materials & Interfaces, 2018,10(10):8443-8450.
[11] Yan M L, Li Gang H, Xue T Y, et al. Surface ligand controls silver ion release of nanosilver and its antibacterial activity against[J]. International Journal of Nanomedicine, 2017,12:3193-3206.
[12] Anas A, Jiya J, Rameez M J, et al. Sequential interactions of silver-silica nanocomposite (Ag-SiO2NC) with cell wall, metabolism and genetic stability of, a multiple antibiotic-resistant bacterium [J]. Letters in Applied Microbiology, 2013,56(1):57-62.
[13] Zheng X, Wang J, Chen Y, et al. Comprehensive analysis of transcriptional and proteomic profiling reveals silver nanoparticles- induced toxicity to bacterial denitrification [J]. Journal of Hazardous Materials, 2017,344:291-298.
[14] Rajesh S, Dharanishanthi V, Kanna A V. Antibacterial mechanism of biogenic silver nanoparticles of[J]. Journal of Experimental Nanoscience, 2014,10(15):1-10.
[15] Samarajeewa A D, Velicogna J R, Princz J I, et al. Effect of silver nanoparticles on soil microbial growth, activity and community diversity in a sandy loam soil [J]. Environmental Pollution, 2017,220: 504-513.
[16] Vipindas P V, Anas A, Jasmin C, et al. Bacterial domination over archaea in ammonia oxidation in a monsoon-driven tropical estuary [J]. Microbial Ecology, 2015,69(3):544-553.
[17] Giao N T, Limpiyakorn T, Kunapongkiti P, et al. Influence of silver nanoparticles and liberated silver ions on nitrifying sludge: ammonia oxidation inhibitory kinetics and mechanism [J]. Environmental Science and Pollution Research, 2017,24(10):9229-9240.
[18] Yang Y, Wang J, Xiu Z, et al. Impacts of silver nanoparticles on cellular and transcriptional activity of nitrogen-cycling bacteria [J]. Environmental Toxicology & Chemistry, 2013,32(7):1488-1494.
[19] Yang Y, Li M, Michels C, et al. Differential sensitivity of nitrifying bacteria to silver nanoparticles in activated sludge [J]. Environmental Toxicology and Chemistry, 2014,33(10):2234-2239.
[20] Alito C L, Gunsch C K. Assessingthe effects of silver nanoparticles on biological nutrient removal in bench-scale activated sludge sequencing batch reactors [J]. Environmental Science & Technology, 2014,48(2):970-976.
[21] Wu J, Zhan M, Chang Y, et al. Adaption and recovery ofto chronic TiO2nanoparticle exposure [J]. Water Research, 2018,147:429-439.
[22] Yuan Z H, Li J W, Cui L, et al. Interaction of silver nanoparticles with pure nitrifying bacteria [J]. Chemosphere, 2013,90(4):1404-1411.
[23] Choi O K, Hu Z Q. Nitrification inhibition by silver nanoparticles [J]. Water Science & Technology, 2009,59(9):1769-1780.
[24] 刘美婷,余 冉,陈良辉,等.典型纳米金属氧化物对氨氧化菌的生物胁迫影响 [J]. 中国环境科学, 2015, 35(1):190-195. Liu M T, Yu R, Chen L H, et al. Biological effects of typical metal oxide nanoparticles on[J]. China Environmental Science, 2015,35(1):190-195.
[25] Jiang X, Miclaus T, Wang L, et al. Fast intracellular dissolution and persistent cellular uptake of silver nanoparticles in CHO-K1cells: implication for cytotoxicity [J]. Nanotoxicology, 2015,9(2):181-189.
[26] Michels C, Yang Y, Moreira S H, et al. Silver nanoparticles temporarily retard NO2production without significantly affecting N2O release by[J]. Environmental Toxicology & Chemistry, 2015,34(10):2231-2235.
[27] Park S, Ely R L. Candidate stress genes offor monitoring inhibition of nitrification by heavy metals [J]. Applied and Environmental Microbiology, 2008,74(17):5475-5482.
[28] Radniecki T S, Stankus D P, Neigh A, et al. Influence of liberated silver from silver nanoparticles on nitrification inhibition of[J]. Chemosphere, 2011,85(1):43-49.
[29] Yu R, Wu J, Liu M, et al. Physiological and transcriptional responses ofto TiO2and ZnO nanoparticles and their mixtures [J]. Environmental Science & Pollution Research International, 2016,23(13):13023-13034.
[30] Mijnendonckx K, Leys N, Mahillon J, et al. Antimicrobial silver: uses, toxicity and potential for resistance [J]. Biometals, 2013,26(4):609- 621.
[31] Barker L K, Giska J R, Radniecki T S, et al. Effects of short and long-term exposure of silver nanoparticles and silver ions to, biofilms and planktonic cells [J]. Chemosphere, 2018,206:606-614.
[32] Arnaout C L, Gunsch C K. Impacts of silver nanoparticle coating on the nitrification potential of[J]. Environmental Science & Technology, 2012,46(10):5387-5395.
[33] Hou J, You G X, Xu Y, et al. Antioxidant enzyme activities as biomarkers of fluvial biofilm to ZnONPs ecotoxicity and the integrated biomarker responses (IBR) assessment [J]. Ecotoxicology and Environmental Safety, 2016,133:10-17.
[34] Zhang L, Wu L L, Si Y B, et al.Size-dependent cytotoxicity of silver nanoparticles toGrowth inhibition, cell injury, oxidative stress and internalization [J]. PLOSE One, 2018,13(12): e0209020.
[35] Jeong E, Chae S R, Kang S T, et al. Effects of silver nanoparticles on biological nitrogen removal processes [J]. Water Science & Technology, 2012,65(7):1298-1303.
[36] Manke A, Wang L, Rojanasakul Y. Mechanisms of nanoparticle induced oxidative stress and toxicity [J]. BioMed Research International, 2013,2013:1-15.
[37] Rosen B P. Bacterial resistance to heavy metals and metalloids [J]. Journal of Biological Inorganic Chemistry, 1996,1(4):273-277.
[38] Marie S L, Kathryn J, Millstone J E, et al. Emerging investigator series: It’s not all about the ion: support for particle specific contributions to silver nanoparticle antimicrobial activity [J]. Environmental Science Nano, 2018,5(9):2047-2068.
[39] Choi O, Hu Z. Size dependent and reactive oxygen species related nanosilver toxicity to nitrifying bacteria [J]. Environmental Science & Technology, 2008,42(12):4583-4588.
[40] Ellegaard-Jensen L, Jensen K A, Johansen A. Nano-silver induces dose-response effects on the nematode[J]. Ecotoxicology and Environmental Safety. 2012,80:216-23.
[41] Wang J, Shu K H, Zhang L, et al. Effects of silver nanoparticles on soil microbial communities and bacterial nitrification in suburban vegetable soils [J]. Pedosphere, 2017,27(3):482-490.
Toxicity of two sizes of silver nanoparticles to.
WU Ling-li, ZHANG Xiao-xue, SHU Kun-hui, SI You-bin*
(Anhui Province Key Laboratory of Farmland Ecological Conservation and Pollution Prevention, School of Resources and Environment, Anhui Agricultural University, Hefei 230036, China)., 2019,39(10):4401~4408
The laboratory incubation experiments were conducted to study the toxicity of silver nanoparticles with different particle sizes on, and the effects of two sizes of nanosilver (10nm and 50nm) on the bacterial growth, nitrogen transformation, cellular structure, reactive oxygen generation and gene expression were investigated. The results showed that nanosilver inhibited the growth of. With the extension of exposure time, the inhibition rate of bacterial growth activity increased and reached to maximum at 4h. In the medium, the transformation rate of NH4+to NO2-was slowed down, and the nitrogen transformation ability bywas reduced. Scanning electron microscopy (SEM) images showed that nanosilver heavily damaged the cell membrane by causing holes on the surface of bacteria. Transmission electron microscope (TEM) images showed that the nuclear material inside the bacteria was disappeared and the boundary of the cytoplasmic membrane was blurred. Flow cytometry (FCM) was employed to detect that nanosilver could generate intracellular reactive oxygen (ROS) in the cells. qRT-PCR technology was used to determine the expression ofandofafter the exposure to nanosilver, and it was found that nanosilver inhibited the expression of the functional genes. In conclusion, nanosilver could interact with cell membrane, generated oxidative stress damage and inhibited the expression of functional genesandof, which further affected the transformation process of ammonium nitrogen. In addition, the toxicity of nanosilver with small particle size was stronger than that of large particle size.
nanosilver;;oxidative stress;gene expression of,, and;ammoxidation
X171.5
A
1000-6923(2019)10-4401-08
伍玲丽(1994-),女,安徽芜湖人,安徽农业大学硕士研究生,主要研究方向为微生物毒理.
2019-03-11
国家自然科学基金重点项目(41430752);安徽农业大学研究生创新基金(2019ysj-67)
* 责任作者, 教授, youbinsi@ahau.edu.cn
