Microwave-assisted catalytic oxidative desulfurization of gasoil fuel using synthesized CuO-ZnO nanocomposites
2019-10-10MustafaFadhilSaadAmmarMarwaAbdulJabbar
Mustafa H.Fadhil,Saad H.Ammar,Marwa F.Abdul Jabbar
(Chemical Engineering Department,Al-Nahrain University,Baghdad,Iraq)
Abstract:Recently,organosulfur removal from liquid petroleum fuels is very significant aspect of environment protecting and fuel cell requests.Therefore,improved approaches to remove sulfur are still essential.In the present work,a simple catalytic oxidative desulfurization (CODS)system for Iraqi gasoil fraction has been successfully developed using CuO-ZnO nanocomposites as catalysts,and H2O2 as oxidant under microwave irradiation.The main reaction parameters influencing sulfur conversion including microwave power,irradiation time,catalyst dosage and H2O2 to gasoil volume ratio have been investigated.The CuO-ZnO nanocomposites was synthesized with different weight ratios and characterized by XRD,FE-SEM,AFM and BET surface area methods.The results reveal that,high sulfur conversion (93%)has been achieved under suitable conditions of microwave CODS as follows:microwave power of 540 W,irradiation time of 15 min,catalyst dosage of 8 g/L (0.4 g),and H2O2∶gasoil volume ratio of 0.3.The catalyst reusability shows that the synthesized catalyst can be reused five times without an important loss in its activity.
Key words:microwave;oxidative desulfurization;nanocomposites;catalyst;gasoil
Currently,deep desulfurization processes become fully needed for liquid transportation fuels as sulfur consider incoming unfavorable for industrial units and its contamination role in the environment.Combustion of sulfur compounds emitted sulfur oxides in the form SOxemissions which contaminate air and form acid rains.Also,many metal catalysts may be poisoned during refining processing[1].Intensive efforts have been made to reduce sulfur content in transportation fuels.Many laws were enacted for this purpose to restrain sulfur content in the global environment that produced by fossil fuel burning.First regulation was set by European Union in 1998-2005,aiming to lower sulfur content until 5.0×10-5,then more attempts to change this level to 1.0×10-5in 2009.In the other hand,by 2006 United States reduced sulfur content in transportation fuel to 1.5×10-5.Other countries had a huge number of researches to achieve zero-sulfur content or less than 1.0×10-5in their fossil fuels[2].
For industrials intention,catalytic hydrodesulphurization (HDS)process is a widely used commercial technique,which required operation above 300 ℃ and hydrogen pressure greater than 2 MPa to convert sulfur compounds into hydrogen sulfide (H2S)[3].
The HDS technique has many disadvantages,including high cost of hydrogen,consuming high operating power causing low product,special vessels resist for high pressure.And the main problem that HDS process is inefficient to remove high molecular weight thiophenic-based sulfur compounds from fuels like benzothiophene (BT)and their derivatives.Wherefore,alternative or competitive technologies were considered to eliminate these complex compounds.Various types of deep desulfurization processes have been proposed and studied,such as extractive desulfurization (EDS),bio-desulfurization (BDS),oxidative desulfurization (ODS)and adsorptive desulfurization (ADS)[4].Among these proposed techniques,ODS achieved high sulfur elimination by converting sulfur compounds to sulfones then extraction via high polar solvent and attaining water soluble product.ODS method can be applied under normal condition at atmospheric pressure and room temperature[5,6],solid catalyst can be used also with ODS process[7,8].
Different solid catalyst and photocatalyst systems have been used extremely in catalytic oxidative desulfurization (CODS)for deep desulfurization process[9-11].Recently,the use of microwave irradiation power for deep removal of sulfur compounds from liquid fuels with or without of catalysts was reported.However,there are limited studys on the applying of microwave irradiation power in the oxidative desulfurization process in the presence of solid and recoverable (heterogonous)photocatalyst.
Mixed metal oxides (semiconductors)have an increased attention for many applications ranging from microelectronic circuits,fuel and solar cells and piezoelectric units to sensors and photocatalysts.Among several semiconductors,investigators concerned in ZnO-CuO mixed semiconductor system.Nontoxic,inexpensive,amply available ZnO is an-type semiconductor of 3.37 eV band gap,conductivity of around 10-7to 10-3s/cm,and has great excitation-binding energy (60 meV).ZnO has also high specific energy density,higher piezoelectric,electrical,and optoelectronic characteristics.Moreover,CuO is a naturalp-type semiconductor with a slight band gap (1.2 eV)and conductivity of about 10-4s/cm with applications in catalysis,photovoltaic,electrochemical and field emission[12,13].All these features of CuO and ZnO attracted us to choose ZnO-CuO hybrid nanocomposites as heterogonous catalyst for catalytic oxidative desulfurization of gasoil fuel under microwave irradiation.
Concerning the above observations,the present study focuses on eliminating sulfur content from Iraqi gasoil fraction using catalytic oxidative desulfurization process (CODS).For this purpose,CuO-ZnO nanocomposites in various ZnO to CuO weight ratios were synthesized and characterized,also ZnO and CuO nanoparticles were intended individually.Microwave power was applied to promote sulfur removal from gasoil fraction and tested in the presence of CuO-ZnO catalysts.The effects of several parameters such as microwave irradiation power and time,dose of CuO-ZnO catalyst,and volume ratio of oxidant to gasoil were investigated.Specific attention was paid to investigate effect of microwave power and irradiation time on desulfurization efficiency of gasoil fuel.
1 Materials and methods
1.1 Materials
The materials used in this study are zinc nitrate (Zn(NO3)2,99.9%),ammonium carbonate (NH4)2CO3,99.9%),copper chloride (CuCl2,99.7%),copper sulfate pentahydrate (CuSO4·5H2O,99.9%),and zinc chloride (ZnCl2,99.9%).All these chemicals are analytical grade supplied from Merck.Iraqi gasoil fraction (initial sulfur content = 1.01%)provided from Al-Dura refinery,Middle Oil Company,was used in this work,and its general characteristics of gasoil fraction are given in Table 1.

Table 1 General characteristics of light gasoil fraction
1.2 Catalysts preparation
1.2.1 Preparation of pure ZnO and CuO nanoparticles
Synthetic method for ZnO was based on Chen et al[14]using direct precipitation route.Briefly,5 g of Zn(NO3)2and 3.8 g of (NH4)2CO3was each dissolved in 8.5 mL of distilled water individually giving an mole ratio of [Zn(NO3)2∶(NH4)2CO3=1.5∶2.25].The (NH4)2CO3solution was added dropwise onto Zn(NO3)2solution for 60 min under high speed magnetic stirring until pH value proceeds maximum.The milky white precipitate was washed via distilled water and ethanol to pH value of 7 or less,then dried in thermal furnace at 80 ℃ for 16 h.The fine powder product was calcined at 550 ℃ for 2 h to obtain ZnO nanoparticles.
CuO nanoparticles was synthesized by simple precipitation according to procedure proposed by Phiwdanga et al[15].Firstly,CuCl2solution was prepared by dissolving 1.34 g of CuCl2in 100 mL distilled water.Then,0.1 mol/L NaOH solution was dropped slowly into CuCl2solution with stirring for about 60 min until pH value of 14.0.The obtained black solid precipitate was then washed by ethanol and deionized water for many recurring times until pH value became neutral,dried at 80 ℃ for 20 h and finally,calcined for 4 h at 500 ℃.
1.2.2 Preparation of CuO-ZnO nanocomposites
CuO-ZnO nanocomposites were prepared by co-precipitation route[16]with slight modification.1.351 g of ZnCl2(10 mmol)was dissolved in 50 mL distilled water under vigorous stirring and rising temperature to 40 ℃ to ensure complete dissolving to form pale white solution.On the other hand,1.248 g of CuSO4·5H2O (5 mmol)was dissolved in 50 mL distilled water.The tow solutions were mixed (giving weight ratio of 2∶1)under vigorous stirring and heated to 75 ℃.1.5 mol/L NaOH (prepared by dissolving 3 g NaOH in 50 mL distilled water)was then dropped gradually for about 73 min until pH value reached to 14.Final solution colored white blue was left to cool to room temperature.The suspension CuO-ZnO nanocomposite was sealed in a beaker.The upper liquid was withdrawn using syringe to prevent loss of nanoparticles.
The resulted CuO-ZnO nanocomposite was then washed with ethanol twice and several times with distilled water,dried in oven for 16 h at 70 ℃.Synthesis procedure of CuO-ZnO nanocomposites was repeated with the above procedure to prepare CuO-ZnO nanocomposites in the weight ratio of 1∶2 and 1∶1 by modification the weight of ZnCl2and CuSO4·5H2O.
1.3 Characterization techniques
The synthesized CuO-ZnO nanocomposites,as well as pure ZnO and CuO nanoparticles,was examined using X-Ray Diffraction (XRD)(Rigaku,D/Max-2000 diffractometer under CuKαradiation with a wavelength (λ)of 0.154 nm,tube voltage of 40 kV,tube current of 30 mA,and 2θrange ranging from 10° to 80°).BET surface area was analyzed for all prepared samples using Surface Area-Pore volume Analyzer (Quantachrome Autosorb-6iSA,USA).The surface morphology of all catalysts was investigated by scanning electron microscope,FE-SEM (TESCAN VEGA3,UK).
1.4 Microwave-assisted CODS procedure
The performance of synthesized CuO-ZnO nanocomposite as well as ZnO,CuO nanoparticles was assessed for catalytic oxidative desulfurization of Iraqi gasoil using microwave power.The microwave equipment used was a local domestic 900 W microwave oven (Media,China)with special modifications.The microwave oven was equipped with a reflux water condenser and electrical stirrer.A 100 mL reaction flask containing 50 mL of gasoil feed was used inside the microwave oven and the desired dosage of catalyst and H2O2oxidant were used.The reaction suspension was irradiated under microwaves using prespecified microwave power and irradiation time while keeping mixing at 400 r/min.Figure 1 shows the microwave reactor unit.In every predetermined contact time,2 mL of sample was drawn from the reaction flask,centrifuged and the clear oil in the upper layer was analyzed for sulfur content determined using X-ray fluorescence sulfur-analyzer (SLFA-2100,Horiba Ltd.,Japan).The sulfur conversion was calculated from the following equation:
(1)
Where,S0is the initial sulfur content (% or ×10-6)andStis sulfur content with time.
Several reaction parameters including microwave power (180,360,540 and 720 W),irradiation time (0.5 to 15 min),volume ratio (0,0.3,0.8 and 1)of H2O2∶gasoil and catalyst dosage (4,8,16 and 24 g/L)were investigated on sulfur conversion efficiency in the microwave catalytic oxidative desulfurization process.
As a preliminary study,all prepared catalysts were tested at the same experimental conditions in order to specify the best catalyst with higher sulfur conversion.The conditions included catalyst dosage of 8 g/L (0.4 g),H2O2∶gasoil volume ratio of 0.3,gasoil volume of 50 mL,microwave power of 540 W and irradiation time of 15 min.During each run,reaction temperature was measured for each level of power at different irradiation time (0.5,1.5,10 and 15 min).
The recyclability of the synthesized CuO-ZnO nanocomposite was studied using the above test conditions.After each reaction cycle,the catalyst was separated,washed with ethanol and water three times,dried at 50 ℃ for 9 h,and then reused in the next cycle.Five reaction cycles were conducted and for each cycle fresh H2O2was used.

Figure 1 Microwave reactor arrangement used for catalytic oxidative desulfurization
2 Results and discussion
2.1 Characterization of CuO-ZnO,ZnO and CuO nanoparticles
To identify crystalline structure of the prepared catalysts,analysis by X-ray powder diffraction (XRD)of ZnO-CuO nanocomposites as well as ZnO and CuO nanoparticles samples was conducted as revealed in Figure 2.The peaks resulted for ZnO nanoparticles (Figure 2a)at 2θ=31.8°,34.5°,36.2°,47.7°,56.7°,63.0°,66.3°,68.0°,69.1° and 72.5° are the lattice planes of (100),(002),(101),(102),(110),(200),(103),(112),(201)and (004)respectively with pure hexagonal wurtzite (B4)structure (JCPDS file No.79-0208).A most strong peak (101)is displayed at 2θ=36.2°.By using Debye-Scherrer equation at this dominant peak,the average particle size of ZnO nanoparticles was 28 nm.The intense and sharp ZnO diffraction peaks specify that the prepared ZnO nanoparticles are crystallized well.This is in agreement with many previous works[17-19].The characteristic diffraction peaks indicated that the prepared CuO nanoparticles (Figure 2b)were agreement with the standard patterns of monoclinic structure (JCPDS file no∶89-5899).For high ZnO content in the nanocomposites as shown in Figure 2c (with weight ratio of 2∶1),the diffraction peaks of CuO species have very low intensity.The XRD patterns of CuO-ZnO nanocomposites containing high CuO content (with weight ratio of (1∶1)and (1∶2))are shown in Figure 2d and 2e obviously indicate the appearance of CuO peaks and therefore,indicating to the formation of CuO-ZnO nanocomposite and no detectable peaks of impurities or other phases were observed.

Figure 2 X-ray powder diffraction patterns of catalystsa:ZnO;b:CuO;c:CuO-ZnO (2∶1);d:CuO-ZnO (1∶2);e:CuO-ZnO (1∶1)
The Field Emission Scanning Electron Microscopy (FE-SEM)images of ZnO and CuO as well as CuO-ZnO (2∶1)and (1∶2)nanocomposites are shown in Figure 3 (a),(b),(c)and (d)respectively.The FE-SEM image of ZnO nanoparticles (Figure 3(a))shows well distributed,spherical nanoparticle and has a regular crystalline structure with a diameter of about 45 nm.

Figure 3 FE-SEM images of catalysts (a):ZnO;(b):CuO;(c):CuO-ZnO (2∶1);(d):CuO-ZnO (1∶2)
The CuO image shows nanoparticles distributed randomly with a higher trend to form blocs and uniform polyhedron shapes.The SEM images of CuO-ZnO (2∶1)and (1∶2)nanocomposites (Figure 3 (c)and (d)shows uniformly dispersed short nanorods with an average diameter of about 45 nm and a length of 80-150 nm (length of 50-90 nm for CuO-ZnO (1∶2)nanocomposites).It is found that length of CuO-ZnO nanorods decreases with increasing CuO content (CuO-ZnO (1∶2)nanocomposites).Therefore,adding CuO species may influence morphology and size of the final ZnO-CuO nanocomposites.A similar observations have been stated recently[12,16,20].
The values of BET specific surface area (ABET),pore volume (vP)and average particle diameter (dp)of ZnO and CuO in addition to the CuO-ZnO (1∶2),CuO-ZnO (1∶1)and CuO-ZnO (2∶1)nanocomposites are listed in Table 2.The BET surface area of all ZnO-CuO samples is relatively higher than that of pure ZnO nanoparticles.The surface area of ZnO-CuO nanocomposites decreased with increasing CuO content in the resulted nanocomposites,showing considerable differences in the BET specific surface areas.This might be attributed to the introduction of excess crystalline CuO species onto the ZnO surface.A comparable result has been observed by Saravanan et al[12].

Table 2 Surface area values for prepared catalyst samples
2.2 Microwave CODS process
Effects of various experimental parameters including type of catalyst used (CuO-ZnO with different weight ratio,ZnO and CuO),catalyst dosage,H2O2∶gasoil volume ratio,microwave power and time of irradiation were examined.The catalyst recyclability was also evaluated.
2.2.1 Effect of microwave power and irradiation time
Before experiments,a preliminary study was carried out to test all prepared catalysts at specific conditions.Figure 4 shows the performance of each catalyst for microwave-assisted CODS.It is clear that CuO-ZnO (1∶2)nanocomposite sample shows higher sulfur conversion as compared with the other catalysts.This may attributed to the large surface area of CuO-ZnO (1∶2)nanocomposite as listed in Table 2.
Figure 5 displays effect of microwave power (from 180 to 720 W)and irradiation time on sulfur conversion percent using CuO-ZnO (1∶2)nanocomposite as catalyst.Experimental conditions include gasoil volume of 50 mL (initial sulfur content of 1.01%),catalyst dosage of 8 g/L (0.4 g),H2O2∶gasoil volume ratio of 0.3.Sulfur conversion increased clearly with increasing microwave power and irradiation time.
The highest sulfur conversion (94.22%)was obtained with power of 720 W after 15 min of irradiation period.This desulfurization efficiency was superior to that without microwave irradiation (9.7% conversion).It is noted that microwave energy is the important effect that participated to enhancement to catalytic oxidative desulfurization process.

Figure 4 Microwave-assisted CODS of gasoil fraction using prepared catalysts (CuO-ZnO with different weight ratio,ZnO and CuO)(experimental conditions:catalyst dosage=8 g/L (0.4 g),H2O2∶gasoil volume ratio=0.3,gasoil volume=50 mL,microwave power level=540 W and irradiation time=15 min)
The dipole and ionic characteristics are measures of how molecule is sensitive to microwave irradiation.Sulfur compounds have comparatively high dipole moments and dielectric constants,hence,they exhibit high obstruction to the microwave energy[21].
The process of heating by microwave irradiation is carried out through three proposed mechanisms:di-bipolar polarization,ionic conductivity and surface polarization[21,22].The first one assumes that heat generation occurs through collision reaction between polar compounds in the mixture under influence of oscillating electromagnetic field of the microwaves.In ionic conductivity,heat is generated by internal resistance of polar compounds resulting from internal electrical current,as a result of electromagnetic fluctuations of electrons or ions.The third one can be considered a combination of the above two mechanisms,so that radiation is absorbed by polar compounds and can pass easily through non-polar compounds.Additional polarity may be brought to the sulfur compounds by the microwave excitation,which probably leads to an increase in the total activation energy of the CODS process[23].Microwave irradiation has created two additional activation energies.The first was denoted to the fast oxidation and thermal decomposition of the catalyst using a strong oxidant,where precipitation desulfurization reaction could be accelerated.The second is attributed to transfer of thermal energy,under microwave radiation,from ultra-polar to non-polar sulfur compounds through which the whole system is heated and desulfurization efficiency is improved many times[24].
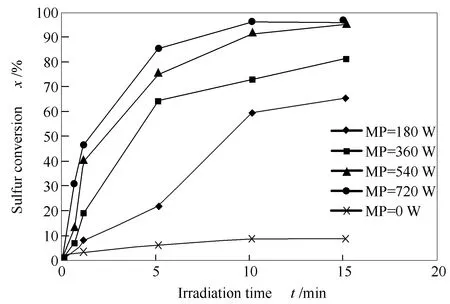
Figure 5 Effect of microwave power and irradiation time on sulfur conversion (experimental conditions:50 mL of gasoil volume,8 g/L of catalyst dosage and H2O2∶gasoil volume ratio of 0.3)
2.2.2 Effect of catalyst dosage
Different catalyst dosages were used for the CODS system containing H2O2∶gasoil volume ratio of 0.3.The reaction mixture was exposed to microwave irradiation power of 540 W and irradiation time of 15 min.The results are shown in Figure 6.It is clear that catalyst amount has an important effect on sulfur conversion.Low sulfur conversion (32.8%)was obtained without catalyst after microwave irradiation time of 15 min.The sulfur conversion increased obviously from 66.6% to 95.7% when the CuO-ZnO catalyst dosage increased from 4 g/L (0.2 g)to 16 g/L (0.8 g).Nevertheless,sulfur conversion could not clearly be improved when the catalyst dosage increased from 16 to 24 g/L.This perhaps is because of the catalyst agglomeration and therefore,low dispersion of the CuO-ZnO (1∶2)nanocomposite in the reaction mixture,consequently reducing availability to the required active sites of the catalyst.
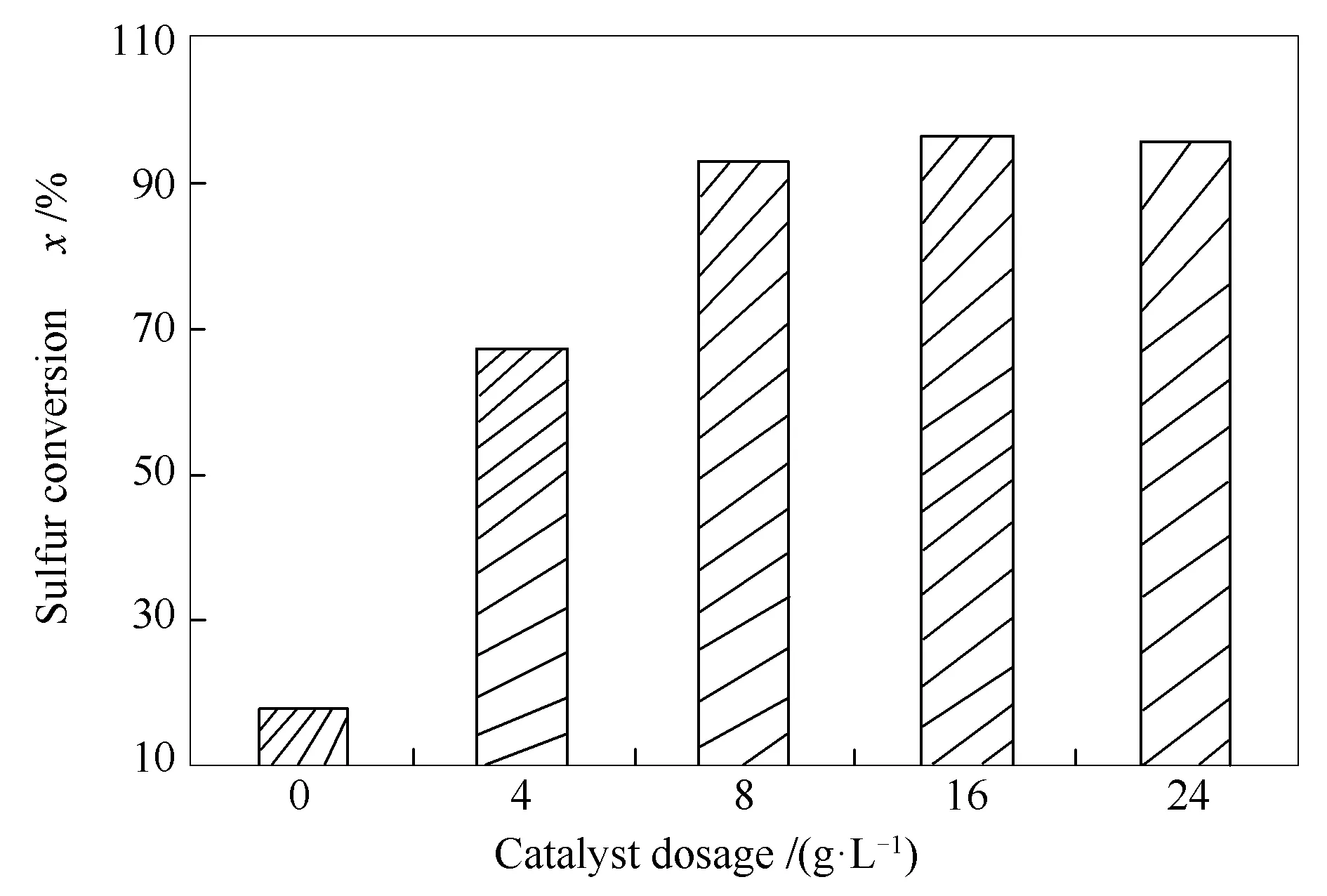
Figure 6 Effect of catalyst dosage on the sulfur conversion (experimental conditions:gasoil volume of 50 mL,microwave power of 540 W and H2O2∶gasoil volume ratio of 0.3)
2.2.3 Effect of H2O2∶gasoil volume ratio
Effect of different H2O2∶gasoil volume ratios were examined on the microwave CODS of gasoil using CuO-ZnO (1∶2)nanocomposite as catalyst.The results are illustrated in Figure 7.

Figure 7 Effect of H2O2:gasoil volume ratio on the sulfur conversion of gasoil (experimental conditions:gasoil volume of 50 mL,microwave power of 450 W,irradiation time of 15 min and catalyst dosage of 8 g/L)
Sulfur conversion initially increased as the ratio increased from 0.1 to 0.5 and then decreased.This is due to the increasing oxygen radicals necessary for oxidizing sulfur compounds and therefore the improved desulfurization rate.With increasing the ratio above 0.5,sulfur conversion decreased,properly because more H2O2oxidant may form a liquid film on catalyst surface,which unfavorably affects adsorption of sulfur compounds on the active sites of the catalyst surface.So,sulfur conversion was limited when using high amounts of H2O2oxidant.
2.2.4 Catalyst recyclability study
The catalyst recyclability of CuO-ZnO (1∶2)nanocomposite was studied in the microwave CODS of gasoil fraction.Catalyst from the first cycle was separated from the reaction mixture,washed with ethanol and water many times,dried and reused for the next run.For each reaction cycle,fresh H2O2oxidant was used with the regenerated catalyst.Figure 8 shows effect of catalyst recycling on sulfur conversion.

Figure 8 CuO-ZnO nanocomposite recyclability results (experimental conditions:gasoil volume of 50 mL,microwave power of 540 W,irradiation time of 15 min,catalyst dosage of 8 g/L and H2O2∶gasoil volume ratio of 0.3)
It is clear that the used CuO-ZnO nanocomposite as catalyst could be recycled five times before its activity decreased observably.Sulfur conversions were 93.3%,93%,92.02%,90.8%,and 89.1% for each cycle,respectively.The insignificant decrease in sulfur conversion after each reaction cycle may be attributed to accumulation of oxidation products in the catalyst.
3 Conclusions
In this work,efficient and regenerable catalysts compose of CuO-ZnO nanocomposites with different weight ratios,as well as the pure ZnO and CuO nanoparticles,have been synthesized,characterized,and used for catalytic oxidative desulfurization of gasoil fraction under microwave irradiation.Several reaction variables such as microwave power,irradiation time,catalyst dosage,H2O2∶gasoil volume ratio have been examined for sulfur conversion.The results reveal that,CuO-ZnO (with 1∶2 weight ratio)exhibits higher conversion (93%)under a specific conditions (microwave irradiation of 540 W,irradiation time of 15 min,catalyst dosage of 8 g/L and H2O2∶gasoil volume ratio of 0.3).The catalyst performance remains stable noticeably after five reaction cycles.Microwave CODS has great potential and displays notable performance in the process of desulfurization.The good sulfur conversion achieved with microwave support is due to the synergistic impact between microwave transferring energy and additional protonation,resulting in increased sulfones production pathways,and thus improving sulfur conversion efficiency.
