碲钨酸盐磁性纳米复合材料选择性分离鸡蛋清中卵清蛋白
2019-10-09车峥岳诗雨吴南徐嘉蔚陈晴
车峥 岳诗雨 吴南 徐嘉蔚 陈晴


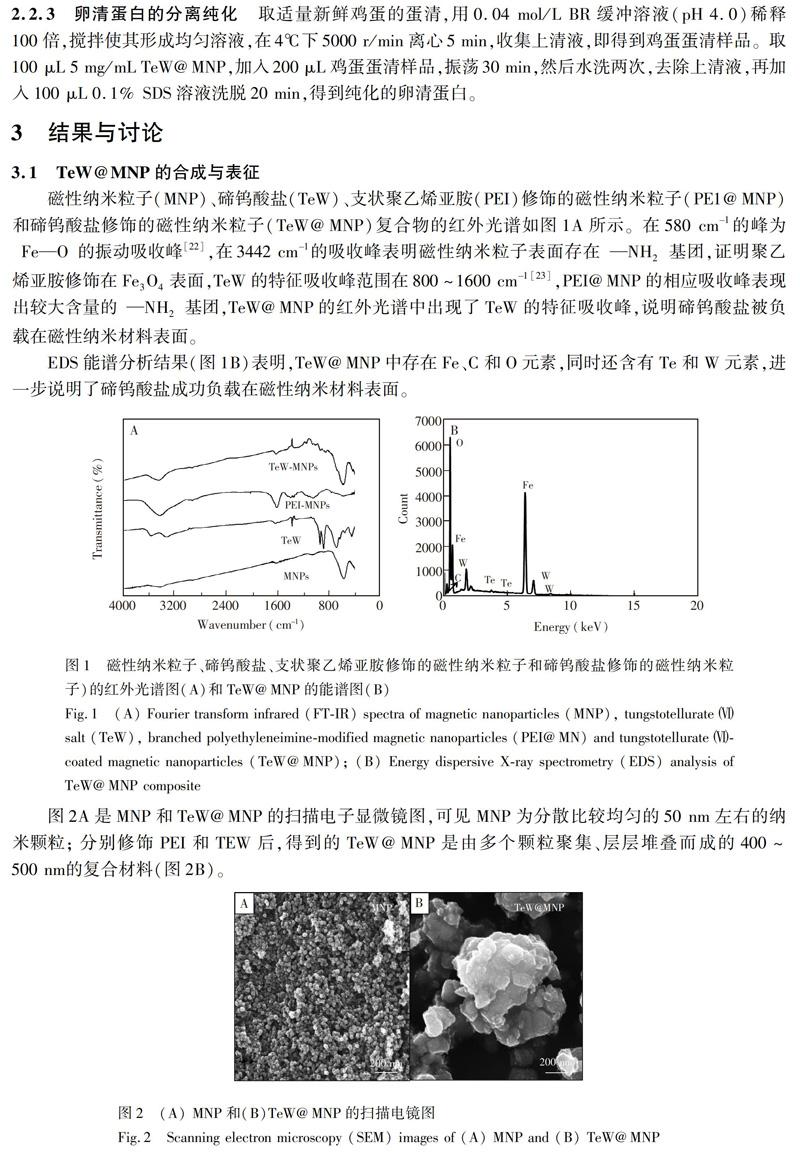

3.2 卵清蛋白的选择性吸附
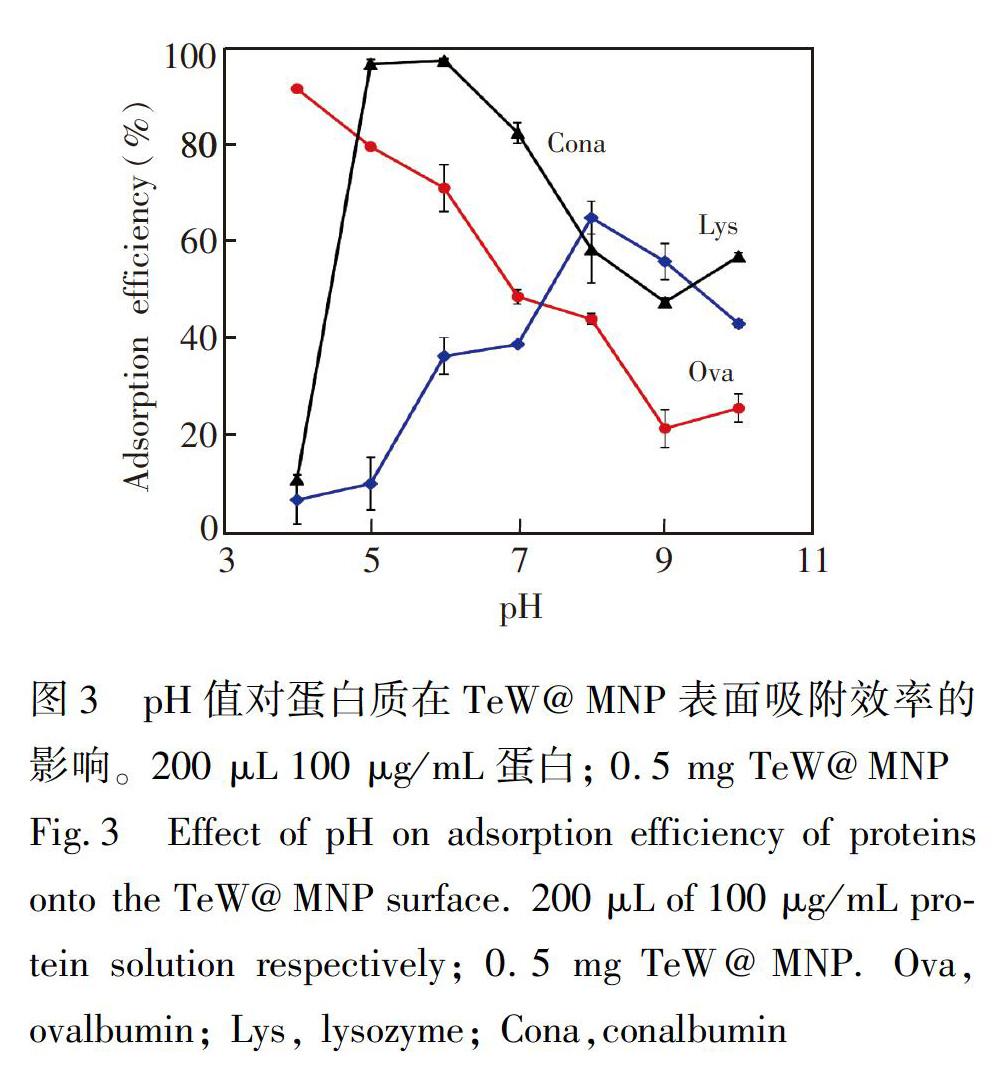
Ova、Cona和Lys是鸡蛋清中的蛋白质,等电点分别为4.5、6.5和9.8,其中Ova是鸡蛋清液中的主要蛋白质[24]。首先考察了pH值对这3种蛋白质吸附的影响。在pH 4.0~10.0的范围内,TeW@MNP对Ova、Cona和Lys的吸附效率如图3A所示,当pH=4.0时,Cona和Lys的吸附效率很低,而对Ova的吸附效率达到最大值91.6%。
Cona和Lys的吸附效率随着pH值增大先升高后降低,在pH=6.0时,对Cona的吸附效率达到97.4%,在pH=8.0时,Lys的吸附效率达到64.9%,而Ova的吸附效率随着pH值的增大而逐渐降低。因此,对于Ova和Cona吸附效率,在其等电点附近时达到最大,主要表现为蛋白质与TeW@MNP的疏水作用。Ova和Cona都是糖蛋白,在等电点附近时,其N糖苷键暴露[25],蛋白表面存在较多羟基,可与材料中TeW的氧原子形成氢键,从而使蛋白被吸附到材料上。随着pH值增大,材料中氧原子的质子化程度越来越弱,蛋白质暴露的疏水基团减少,氢键减弱,蛋白质的吸附效率随体系pH值的增加而降低。在pH=8.0时,溶菌酶的吸附效率达到最大,主要作用为其与TeW@MNP之间的静电作用[8]。综上,通过控制溶液的pH值,可实现在鸡蛋清液蛋白中选择性吸附Ova。
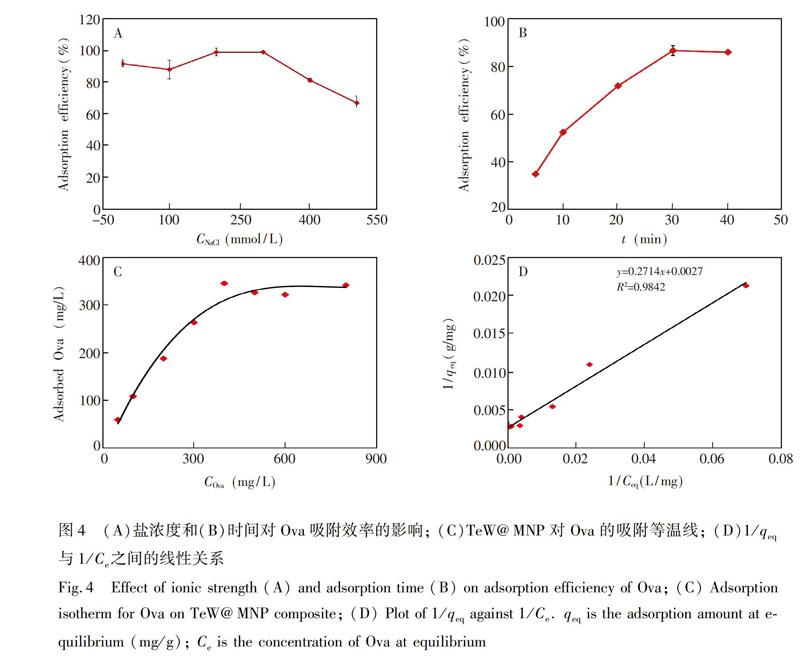
离子强度对于蛋白质分子与固相材料的相互作用有较大的影响。在pH=4.0条件下,分别配制了一系列含有不同浓度NaCl(0~500 mmol/L)的蛋白溶液,考察離子强度对Ova吸附的影响,结果如图4A所示。随着NaCl浓度增加,TeW@MNP对Ova的吸附效率先升高后降低, NaCl浓度为200 mmol/L时,吸附效率可达到98.9%,进一步验证了疏水作用为吸附的主要作用力[25]。由于实际样品中存在一定浓度的盐,所以本研究采用不加NaCl的0.04 mol/L BR溶液。
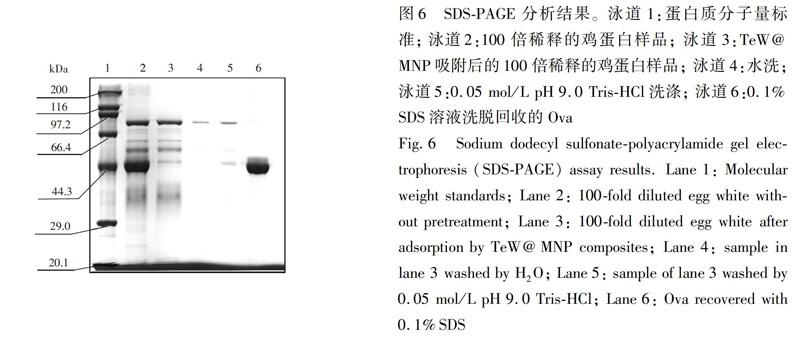
3.3 鸡蛋清中卵清蛋白的分离纯化
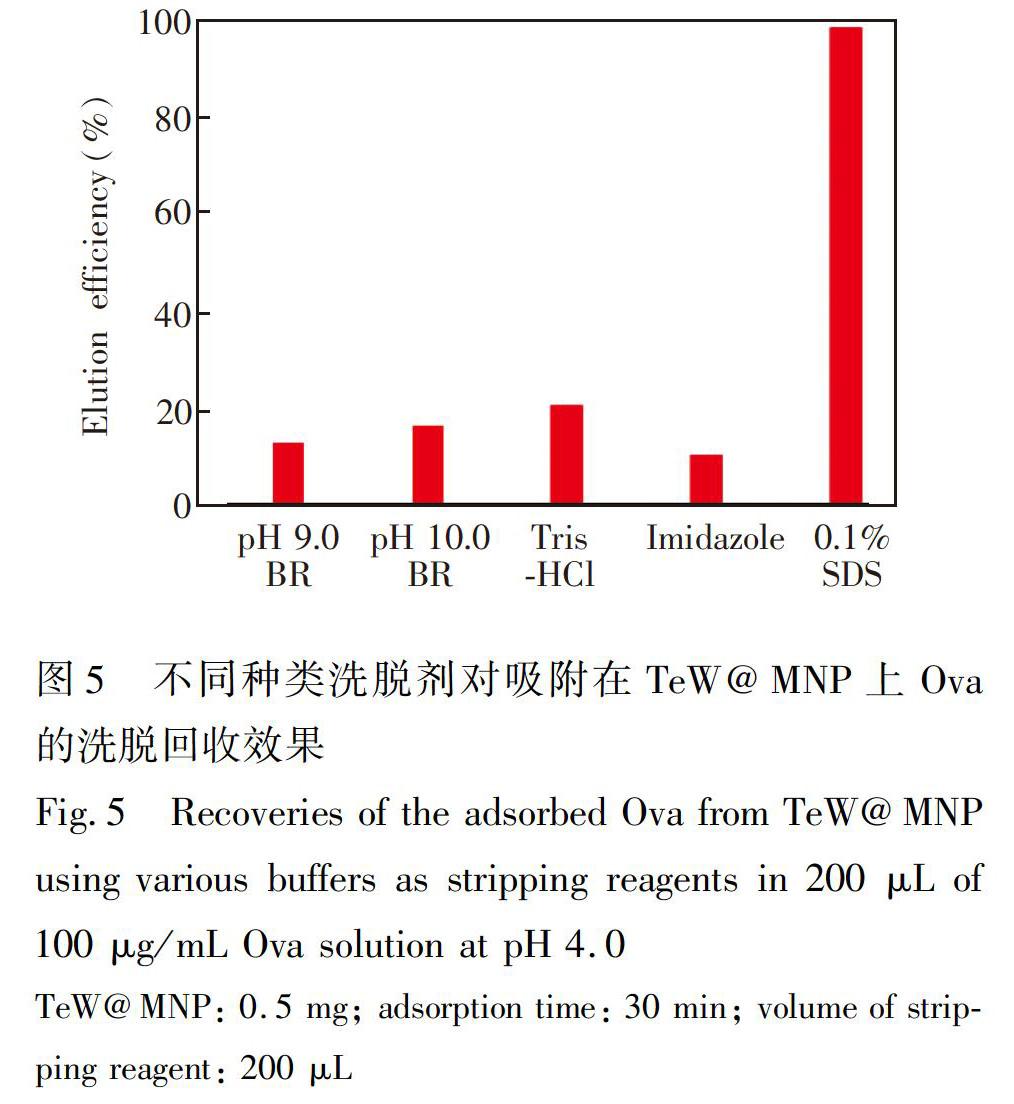
SDSPAGE分析结果如图6所示,泳道1是蛋白分质量标准(20.1~200 kDa); 泳道2是稀释100倍后的鸡蛋清蛋白样品,在29.0~116 kDa有3个主要的蛋白质条带,分别为伴清蛋白(77.9 kDa)、 卵清蛋白(44.5 kDa)和其它低分子量的蛋白; 泳道3是经TeW@MNP吸附后上清液的蛋白质条带,44.5 kDa 处Ova的条带基本消失,而其它条带没有明显变化; 泳道4和泳道5分别是水洗和0.05 mol/L pH 9.0 TrisHCl缓冲液洗脱的条带,分别洗去了非特异性吸附的杂蛋白; 泳道5是经0.1% SDS洗脱回收后得到的上清液,蛋白质条带出现在44.5 kDa,与Ova的条带相一致,说明以TeW@MNP为吸附剂,在鸡蛋清蛋白样品中分离纯化得到了卵清蛋白。
4 结 论
利用分子间静电作用制备了基于碲钨酸盐的磁性纳米复合材料TeW@MNP,采用红外光谱、能谱和扫描电镜等进行了表征。将制备的TeW@MNP应用于鸡蛋清中蛋白质的分离纯化,结果表明,碲钨酸盐在一定条件下对卵清蛋白具有选择性吸附性能,并有较高的吸附容量,以此建立了分离卵清蛋白的新方法,实现了鸡蛋蛋清中卵清蛋白的高纯度分离。本研究拓展了多金属氧簇在生命科学领域中的应用范围。
References
1 Yamase T, Pope M T. Polyoxometalate Chemistry for Nanocomposite Design. New York: Kluwer Academic/Plenum, 2002: 350
2 Long D, Tsunashima R, Cronin L. Angew. Chem. Int. Edit., 2010, 49(10): 1736-1758
3 Leng Y, Jiang Y, Peng H, Zhang Z, Liu M, Jie K, Zhang P, Dai S. Catal. Sci. Technol., 2019, 3: 1-7
4 Yang W, Gao L H, Wang K Z. Polyhedron, 2014, 82: 80-87
5 Liu D, Lu Y, Tan H Q, Wang T T, Wang E B. Cryst. Growth Des., 2015, 15(1): 103-114
6 Poppe K J, Warkentin U D E, Dierks T, Ermler U, Schneider K. J. Am. Chem. Soc., 2012, 134: 9768-9774
7 Zebisch M, Krauss M, Schfer P, Strter N. Acta Crystallogr. S D, 2014, 70(4): 1147-1154
8 Bijelic A, Molitor C, Mauracher S G, AlOweini R, Kortz U, Rompel A. Chembiochem. Eur. J. Chem. Biol., 2015, 16(2): 233-241
9 Wan H, Huang J, Liu Z, Li J, Zhang W, Zou H. Chem. Commun., 2015, 51: 9391-9394
10 Li Y, Zhang X, Deng C. Chem. Soc. Rev., 2013, 42(21): 8517-8539
11 Ma X, Zhang Y, Wang Z, Shen Y, Zhang M, Nie Q, Hou Y, Bai G. Mol. Nutr. Food Res., 2017, 61:1700332-1700339
12 Dong L, Feng S, Li S, Song P, Wang J. Anal. Chem., 2015, 87(5): 6849-6853
13 Cheng G, Wang Z G, Denagamage S, Zheng S Y. ACS Appl. Mater. Interfaces, 2016, 8(16): 10234-10242
14 Piovesana S, Capriotti A L, Cavaliere C, Ferraris F, Iglesias D, Marchesan S, Lagana A. Anal. Chem., 2016, 88(24): 12043-12050
15 Yang K, Liu J, Li S, Li Q, Wu Q, Zhou Y, Zhao Q, Deng N, Liang Z, Zhang L, Zhang Y. Chem. Commun., 2014, 50(67): 9521-9524
16 LIU QingYang, ZHANG TingTing. Chinese J. Anal. Chem., 2018, 46(4): 524-529
劉庆阳, 张婷婷. 分析化学, 2018, 46(4): 524-529
17 Meng H, Chen X W, Wang J H. J. Mater. Chem., 2011, 21: 14857-14863
18 WANG WeiWei, LIU SuQin, XUE Yun, WANG Yan, YAN Chao. Chinese Journal of Chromatography, 2017, 35(1): 99-104
王薇薇, 刘素琴, 薛 芸, 王 彦, 阎 超. 色谱, 2017, 35(1): 99-104
19 Chen X W, Chen S, Wang J H. Analyst, 2010, 135(7): 1736-1741
20 Liu J W, Zhang Y, Chen X W, Wang J H. ACS Appl. Mater. Interfaces, 2014, 6(13): 10196-10204
21 Liu J W, Yang T, Ma L Y, Chen X W, Wang J H. Nanotechnology, 2013, 24(50): 505704
22 Sun S, Ma M, Qiu N, Huang X, Cai Z, Huang Q, Hua X. Colloids Surf. B, 2011, 88: 246-253
23 Xu Z, Xi P, Chen F, Zeng Z. Transition. Met. Chem., 2008, 33: 237-241
24 Huntington J A, Stein P E. J. Chromatogr. B, 2001, 756: 189-198
25 de Groot J, Kosters H A, de Jongh H H. Biotechnol. Bioeng., 2006, 97(4): 735-741
Abstract Tungstotelluratecoated magnetic nanoparticles(MNP) were prepared by immobilization of tungstotellurate salt onto the surface of branched polyethyleneimine (PEI)modified magnetic nanoparticles via electrostatic interaction. The structure and composition of TeW@MNP were confirmed by characterization with FTIR, EDS and SEM. The obtained TeW@MNP composite had proven to be a promising adsorbent for the adsorption of ovalbumin in egg whites. Approximately 0.5 mg of TeW@MNP composite gave rise to a maximum adsorption efficiency of 91.6% for 100 μg/mL ovalbumin in 200 μL of sample solution at pH 4.0 (BR buffer). The retained ovalbumin was readily recovered by using 0.1% SDS as a stripping reagent, providing a recovery of 98.1%. The adsorption behavior of ovalbumin fitted Langmuir model, corresponding to a theoretical adsorption capacity of 373.4 mg/g. The TeW@MNP composite was practically applied to the selective isolation of ovalbumin from egg whites, and SDSPAGE assay results clearly indicated that the ovalbumin with high purity was obtained. The above experimental results illustrated the great application potentials of polyoxometalates for protein isolation and purification and extended the application scope of polyoxometalates in life science field.
Keywords Tungstotellurate; Magnetic nanoparticles; Ovalbumin; Solidphase extraction
