两个基于柔性双苯并咪唑配体的锌配位聚合物的合成、晶体结构和荧光性质
2019-10-09兰红红李小童楚文娟许春莺吉保明
兰红红 李小童 楚文娟 许春莺 吉保明*,
(1洛阳师范学院化学化工学院,河南功能导向多孔材料重点实验室,洛阳 471934)
(2国家知识产权局专利局专利审查协作河南中心,郑州 470018)
(3河南中烟工业有限责任公司技术中心,郑州 450000)
0 Introduction
Coordination polymers (CPs)have been extensively investigated in the past few decades.Because of their vast applications such as gas storage[1-2],catalysis[3-4],molecular magnetism[5-6]and luminescence[7-9],CPs play a significant role in the development of functional materials.The properties of CPs mainly depend on the organic ligands and the center metal ions because they are involved in the formation of the final CPs[10-11].This provides us with the opportunity to rationally select the precursors, which enables synthesis of CPs for specific structures and properties.
Aimed at the production of sensors and diodes,luminescent CPs materials have emerged as a rapidly growing field of research.The luminescence of CPs is commonly caused by the conjugated organic ligands and metal ions with closed shell electron configuration[12-14].CPs based on d10metal ions often display excellent luminescence behavior[15-18].Among these,ZnⅡCPs with organic linkers are one kind of the most interested luminescent CPs[19-21].
It is well known that polycarboxylate ligands and N-containing heterocyclic ligands are excellent candidates for the construction of CPs for their strong coordination abilities and diverse coordination modes[22-25].As a member of N-containing heterocyclic ligands,1,4-bis(benzimidazol-1-yl)-benzene(bbix)has been studied in the synthesis of CPs[26-27].It is noteworthy thatbbix based CPsexhibitsinteresting structures and luminescent properties.Our research has focused on its derivatives,1,4-bis(2-methylbenzimidazol-1-ylmethyl)benzene (bmb).The extra methyl of bmb will inevitably cause larger steric resistance.Does this affect its coordination abilities and consequently affect the structures and properties of the final CPs?Considering above mentioned points,we have synthesized some CPs based on bmb[28].As a continuation of our work,herein,two CPs,{[Zn(bmb)0.5(btec)0.5(H2O)].H2O}n(1)and{[Zn2(bmb)2(dcbp)].5H2O}n(2),were synthesized based on bmb and polycarboxylate ligands (H4btec=1,2,4,5-benzenetetracarboxylic acid,H4dcbp=4-(3,4-dicarboxybenzoyl)phthalic acid)(Scheme 1).The structures,thermal stabilities and luminescent properties of complexes 1 and 2 were studied in detail.
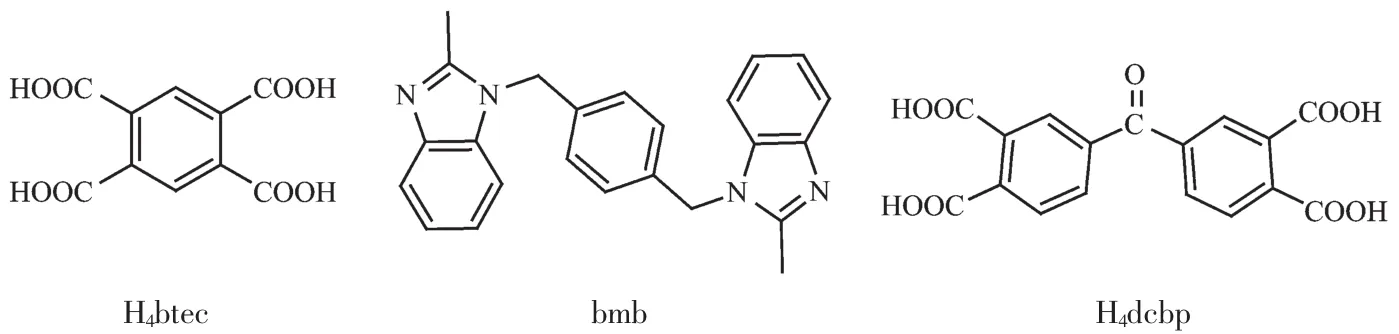
Scheme 1 Structures of the ligands
1 Experimental
1.1 Materials and measurement
All chemicals and reagents were commercially available and used withoutfurther purification.Ligands H4btec and H4dcbp were purchased from Alfa Aesar and bmb was synthesized according to the literature method[29].Elemental analyses(C,H and N)were performed on a ElementarVario EL Ⅲelemental analyzer.IR spectra were recorded on a Bruker EQUINOX55 spectrophotometer in the 4 000~400 cm-1region using KBr pellets.Thermogravimetric analyses(TGA)data were collected on a Perkin-Elmer TAG-7 instrument with a heating rate of 10℃.min-1.Powder X-ray diffraction (PXRD)measurements were carried out on a Bruker D8 Advance diffractometer with Cu Kα radiation(λ=0.154 18 nm)from 5°to 50°(2θ)at room temperature,using an operating voltage of 45 kV and an operating current of 40 mA.The luminescent spectra in solid states were performed on a Hitachi F-4500 fluorescence spectrophotometer at room temperature.
1.2 Synthesis of{[Zn(bmb)0.5(btec)0.5(H2O)].H2O}n(1)
A mixture of Zn(CH3COO)2.2H2O(44 mg,0.2 mmol),bmb (73.2 mg,0.2 mmol),H4btec(0.2 mmol,50.8 mg),and water(10 mL)was sealed in a Teflonlined stainless steel vessel(25 mL)and heated at 150℃for 5 days,and then cooled to room temperature naturally.The colorless block crystals of 1 were obtained with the yield of 41%based on Zn.Anal.Calcd.for C17H16N2O6Zn(%):C,49.83;H,3.94;N,6.84.Found(%):C,50.13;H,3.98;N,6.72.IR(KBr,cm-1):3 422(s),3 089(w),1 623(m),1 461(m),1 384(s),1 277(m),1 155(w),837(m),751(s),668(m),550(m),479(m).
1.3 Synthesis of{[Zn2(bmb)2(dcbp)].5H2O}n(2)
Complex 2 was prepared by Zn(CH3COO)2.2H2O(44 mg,0.2 mmol),bmb (73.2 mg,0.2 mmol),and H4dcbp(0.2 mmol,71.6 mg)using the same procedure for complex 1.Colorless block crystals of 2 were obtained with the yield of 33%based on Zn.Anal.Calcd.for C65H60N8O14Zn2(%):C,59.69;H,4.62;N,8.57.Found(%):C,59.54;H,4.71;N,8.43.IR(KBr,cm-1):3 422(s),1 712(w),1 648(m),1 596(m),1 510(s),1 476(m),1 412(s),1 363(m),1 291(w),1 138(m),1 013(w),868(m),762(s),672(m),613(m),509(m),475(m).
1.4 Structural determination
Single crystal X-ray diffraction data of the two complexes were collected on a Rigaku Saturn 724 CCD diffractomer(Mo Kα,λ=0.071 073 nm)at room temperature.Absorption corrections were applied by using multi-scan program.The structures were solved by direct methods and refined with a full-matrix leastsquares technique based on F2with the SHELXTL software[30]. All non-hydrogen atoms were refined anisotropically,the hydrogen atoms of water molecules were located from difference Fourier maps and the other hydrogen atoms were generated geometrically.Crystal data and structural refinement parameters for 1 and 2 are summarized in Table 1.The selected bond lengths and angles for 1 and 2 are listed in Table 2.
CCDC:1907377,1;1907378,2.
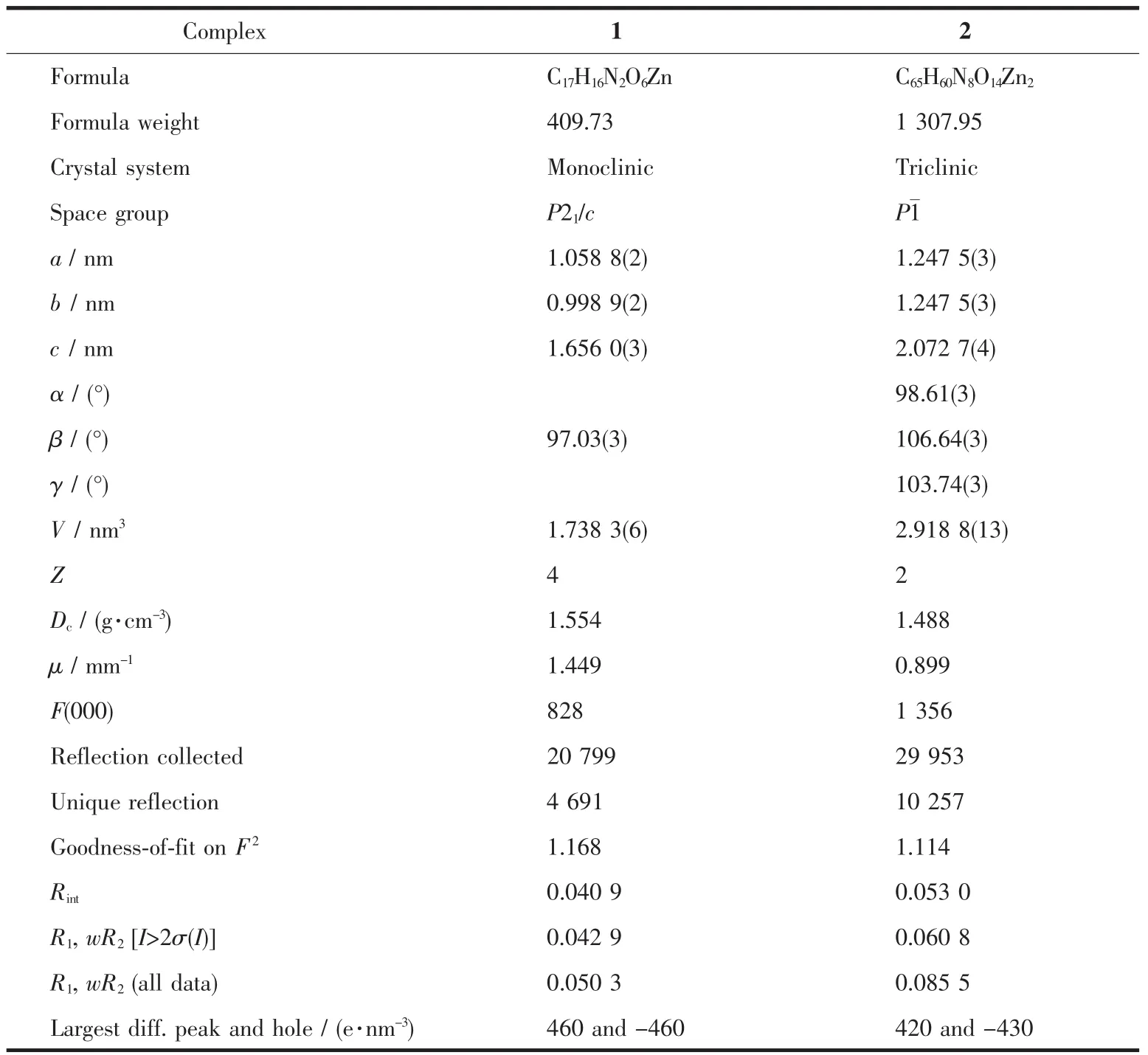
Table 1 Crystal data and structure refinement for 1 and 2
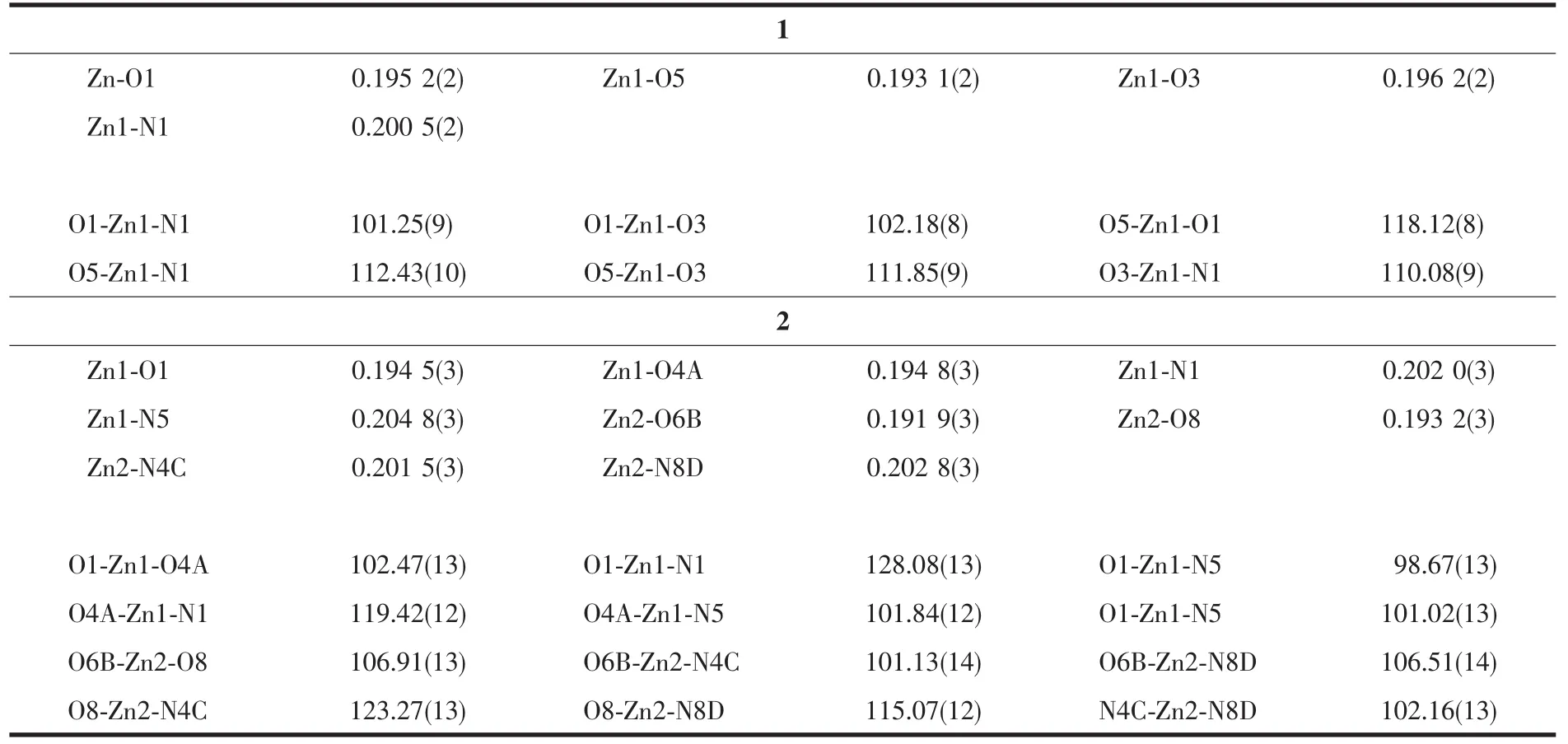
Table 2 Selected bond lengths(nm)and angles(°)for 1 and 2
2 Results and discussion
2.1 Crystal structure of{[Zn(bmb)0.5(1,2,4,5-btec)0.5(H2O)].H2O}n(1)
Single-crystal X-ray diffraction analysis reveals that polymer 1 crystallizes in monoclinic system with P21/c space group.The asymmetric unit of 1 consists of one ZnⅡion,a half bmb,a half 1,2,4,5-btec4-,one coordinated water molecule and one lattice water molecule.As shown in Fig.1a,Zn1 is surrounded by one nitrogen atom (N1)from one bmb ligand,two oxygen atoms(O1,O3)from two different 1,2,4,5-btec4-anions,and one oxygen atom (O5)from coordinated water molecule.The Zn-O bond lengths are in a range of 0.193 14(19)~0.196 22(17)nm,while the Zn-N ones is 0.200 5(2)nm.The coordination geometry of Zn1 can be described as a distorted tetrahedral geometry.

Fig.1 (a)Coordination environment of ZnⅡion in 1 with hydrogen atoms omitted for clarity;(b)2D sheet structure of 1;(c)3D framework of 1
In 1,the deprotonated 1,2,4,5-btec4-adopts a μ4-η1∶η1∶η1∶η1coordination mode.Four carboxylate groups coordinate with four ZnⅡ ions monodentately to generate a 2D sheet(Fig.1b).The adjacent sheets are further connected by the bmb ligand in μ2-η1∶η1mode to give a 3D framework (Fig.1c).The bridging bmb is extremely symmetric and displays trans-conformation.Two benzimidazole rings are coplanar and the dihedral angle between each benzimidazole and phenyl ring is 84.94°.The 3D framework is consolidated by intermolecular O-H…O hydrogen bonds(O5…O6 0.263 5(3)nm,O5…O2 0.263 2(3)nm,O6…O4 0.277 6(3)nm,O6…O3 0.276 4(3)nm).
2.2 Crystal structure of{[Zn2(bmb)2(dcbp)].5H2O}n(2)
Using a longer polycarboxylic acid ligand H4dcbp instead of 1,2,4,5-H4btec under the same reaction conditions affords complex 2,which belongs to the triclinic space group P1.There are two crystallographically independent ZnⅡions,two bmb ligands,one dcbp anion,and five lattice water molecules(Fig.2a)in the asymmetricunitof2.TheZn1/Zn2 are surrounded by two nitrogen atoms (N1,N5/N4,N8)from two different bmb ligands,two carboxyl oxygen atoms(O1,O4/O6,O8)from two dcbp ligands to give a distorted tetrahedron geometry,respectively.
In 2,two bmb ligands both show μ2-η1∶η1mode to coordinate with two ZnⅡions.In this manner,four bmb connect four adjacent ZnⅡions to form a Zn4(bmb)4secondary building units(SBUs)(Fig.2b).One bmb displays trans-conformation,and the dihedral angle between benzimidazole ring and benzene are 85.96(2)° and 77.16(2)°,and the dihedral angle between two benzimidazole rings is 19.55(1)°.The other bmb displays cis-conformation.The dihedral angle between benzimidazole ring and benzene are 84.38(2)° and 82.46(3)°,and the dihedral angle between two benzimidazole rings is 3.64(2)°.Then these SBUs are jointed by dcbp ligands in the μ4-η1∶η1∶η1∶η1mode to give a 3D framework (Fig.2c).The framework is also stabilized by intermolecular O-H…O hydrogen bonds(O11…O3 0.277 4(4)nm,O11…O9 0.277 7(4)nm,O12…O3 0.279 8(4)nm,O12…O14 0.285 1(5)nm,O14…O11 0.280 2(5)nm).
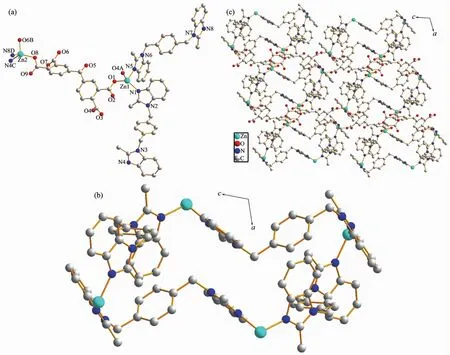
Fig.2 (a)Coordination environment of ZnⅡion in 2 with hydrogen atoms omitted for clarity;(b)Zn4(bmb)4SBUs in 2;(c)3D framework of 2
2.3 PXRD patterns and thermal analyses
Powder X-ray diffraction (PXRD)patterns of 1 and 2 weredetermined atroom temperatureto characterize their purity.As shown in Fig.3,the peak position of the measured patterns matched well with the simulated ones,indicating the purity of the samples.The thermal stability of 1 and 2 were investigated under nitrogen atmosphere by thermogravimetric analyses(TGA).As shown in Fig.4,polymer 1 lost its lattice water molecules at 123℃ (Obsd.4.25%,Calcd.4.43%).The release of coordinated water molecules(Obsd.4.48%,Calcd.4.43%)occurred in a temperature range of 213~318℃.Then the further weight losses are attributed to the decomposition of 1.Polymer 2 lost lattice water molecules from room temperature to 157℃ (Obsd.6.73%,Calcd.6.88%).The framework is stable up to 312℃,then the weight decreased quickly and continuously until 600℃.The main residueshould be ZnO (Obsd.15.48%,Calcd.15.67%).
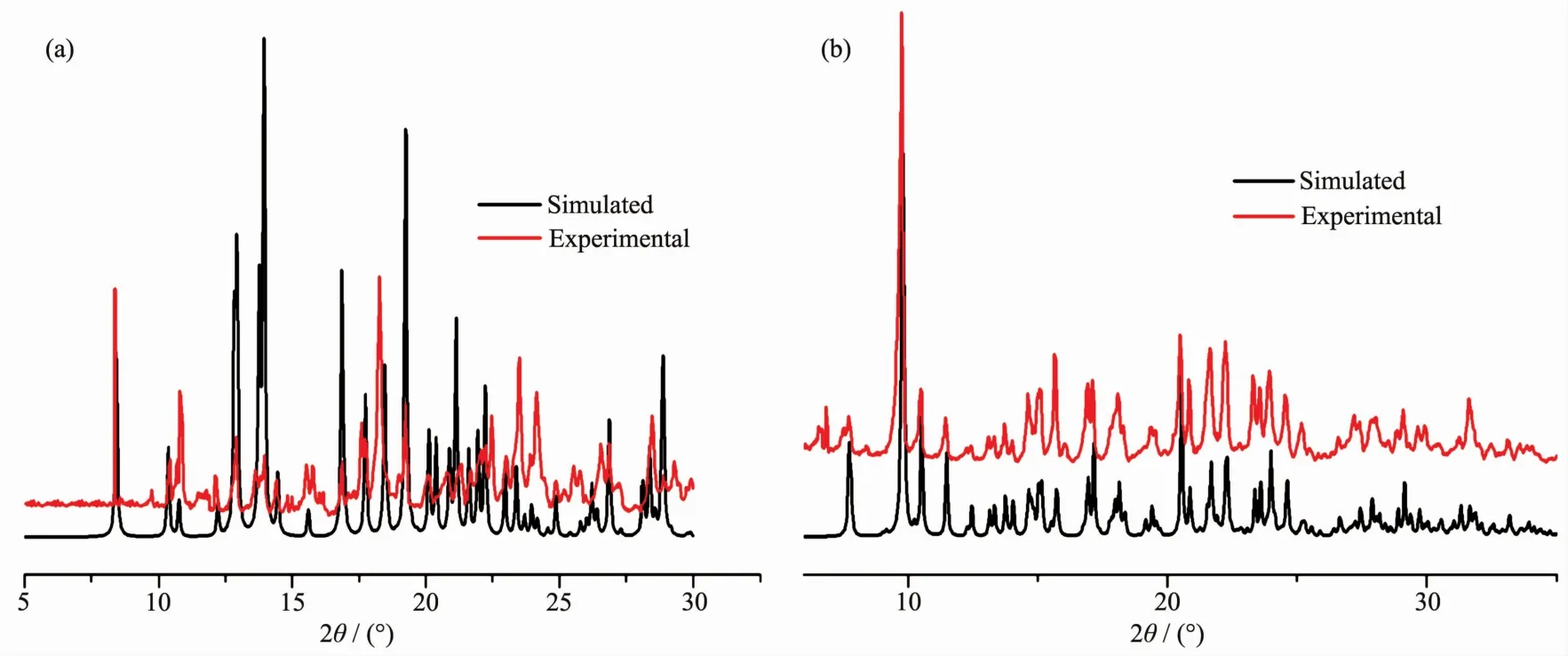
Fig.3 PXRD patterns of 1(a)and 2(b)
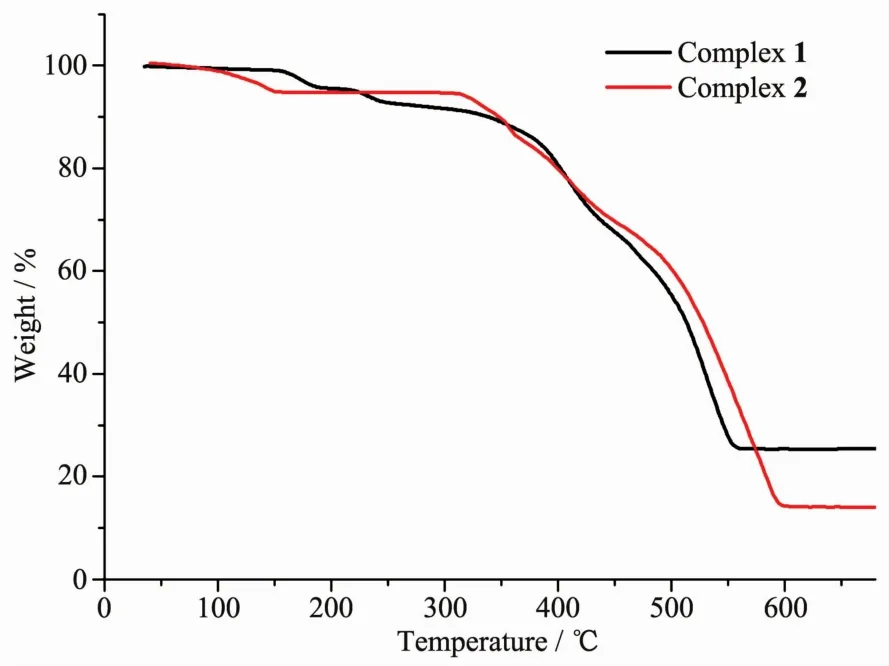
Fig.4 TGA curves of complexes 1 and 2
2.4 Photoluminescence properties
The solid state luminescence properties of complex 1 and 2 together with bmb,H4btec and H4dcbp were measured at room temperature(Fig.5).The free ligand bmb shows intense emission band at 309 nm upon excitation at 293 nm,which can be attributed to the π→π*transitions.The emission of 1,2,4,5-H4btec and H4dcbp is very weak compared to bmb and have no contribution to the luminescence of complexes 1 and 2.The maximum emission peaks of 1 and 2 are located at 451 nm(λex=391 nm)and 409 nm(λex=327 nm),respectively.The emissions of complexes 1 and 2 can probably be assigned to intraligand charge transitions of bmb[28].
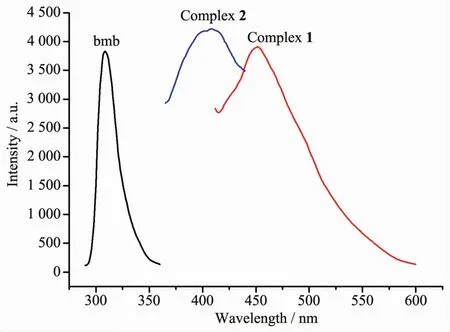
Fig.5 Solid-state photoluminescent spectra of bmb and complexes 1~2
3 Conclusions
In summary,two ZnⅡcoordination polymers with bmb and different carboxylic acid co-ligands have been synthesized and characterized.Complexes 1 and 2 all feature different 3D frameworks structures.The solid-state luminescent properties of 1 and 2 were investigated,and the emissions were assigned to intraligand charge transitions of bmb. The investigation should motivate further research of bmb as building block in the construction of coordination polymers.
