蜂窝状Ag纳米颗粒膜制备及应用于表面增强拉曼散射基底
2019-10-09刘元君张俊豪袁爱华
刘元君 叶 芬 王 威 张俊豪 晏 超 袁爱华
(1江苏科技大学环境与化学工程学院,镇江 202018)
(2江苏科技大学海洋装备研究院,镇江 212003)
(3江苏科技大学材料科学与工程学院,镇江 212003)
0 Introduction
Surface enhanced Raman scattering (SERS)technique has been extensively applied to various fields in the past few decades including environment,health care and biology due to the ultrasensitivity,excellent selectivity,rapid detection capabilityaswellas nondestructive testing[1-2].The enhancement of Raman signature strongly depends on the active substrate,most of which are composed by noble metal units,including Ag or Au with various micro-/nanostructures[3-5].The reported enhancement factor (EF)of these SERS substrates can reach up to 106~1014.However,high SERS enhancement is often accompanied by low uniformity and reproducibility.
Suitable SERS substrates should possess not only a large surface area to adsorb amounts of analyte molecules but also abundant and uniform"hot spots".It was confirmed that substrates with periodic ordered microstructures exhibit superior SERS performance over disordered ones.Periodic nanoparticle arrays possess uniform and high density of hot spots and so good reproducibility[6-8].Up to date,several strategies have been employed to prepare ordered SERS substrates including electron beam lithography techniques,nanosphere lithography,and so on[7,9-11].Although these strategies are quite effective for the fabrication of ordered SERS substrates,the involved complicated and expensive devices highly limit their wide application.Thus,it is highly expected to fabricate ordered patterns with noble metal nanoparticles by a more easy operation way for highly sensitive SERS substrates[12-13].
Recently,breath figure method was known as a simple and efficient technique for the fabrication of honeycomb patterned polymer films[14-15].In this work,the amphiphilic diblock copolymer,polystyrene-blockpoly (4-vinylpyridine)(PS-b-P4VP),was adopted to induce a patterned film with honeycomb structure by a static breath figure method.After UV-light treatment,the top PS layer of the as-prepared PS-b-P4VP pattern film can be easily peeled off with an adhesive tape,fully exposing the P4VP domain in the film.On this treated honeycomb polymer pattern,silver ions can be adsorbed on and were in situ reduced by UV-assisted reduction method,forming Ag nanoparticles array film.R6G was then used as the probe molecular to investigate the SERS performance of this silver particle array substrate.The results indicate that the Ag nanoparticles array on honeycomb-patterned film shows an enhancement factor as high as 1.31X109,limit of detection(LOD)low to 10-10mol.L-1.The collected SERS signals on 120 positions show small relative standard deviation(only~12%),suggesting the favorable reliability and good reproducibility of the prepared Ag nanoparticles array film SERS active substrate.
1 Experimental
1.1 Chemicals
Amphiphilic diblock copolymer polystyrene-b-poly(4-vinylpyridine)(PS-b-P4VP,Mn=122 000 kg.mol-1for PS and 22 000 kg.mol-1for P4VP)was purchased from Polymer Source.Trichloromethane (CHCl3)and AgNO3were purchased from Sinopharm Chemical Reagent Co.,Ltd.R6G was purchased from J&K Scientific,Ltd.ITO glasses were provided by Huanan Scientificand TechnicalCorporation.Allofthe glasses were cut into 1 cmX1 cm square shape.Water used in all experiments was deionized and ultrafiltrated with a Lab Water system.
1.2 Preparation of honeycomb-like PS-b-P4VP films
All the experiments were carried out at room temperature.ITO glasses(1 cmX1 cm)were firstly immersed in an acidic mixture of 98%H2SO4and 30%H2O2(7∶3;V/V)with ultrasonic cleaning for 30 min and were then strongly rinsed by deionized water for several times.The cleaned wafers were then dried under nitrogen flow.
Typically,a saturated relative humidity environment was firstly made by adding 5 mL of distilled water in 30 mL of glass bottle,which was sealed by plastic wrap for 2 h.A piece of ITO glass was placed on the top of a glass upholder in the glass bottle to make the ITO glass 5 mm higher than the water level.Then,themicro-patterned film wasprepared by dropping a 50 μL of 30 mg.mL-1of PS-b-P4VP trichloromethane solution onto the surface of ITO glass.After 30 min,the thus prepared pattern film was put under the irradiation of UV-light that was generated by a commercial 5 W of Hg lamp.The Hg lamp provides light irradiation with a wavelength range of 200~320 nm (the strongest line is 254 nm).The distance between the pattern film and the Hg lamp is fixed to be 20 cm.After UV-light irradiation of 2 h,the top PS layer of the honeycomb-patterned film was then peeled off with a commercially available adhesive tape,leaving the P4VP layer.
1.3 In situ generation of Ag nanoparticles array on the honeycomb-patterned films
0.05 mol.L-1AgNO3solution was prepared by dissolving AgNO3in a mixture solution of deionized water and ethanol(1∶1,V/V).The processed honeycombpatterned film was put into a petri dish.Then 3 mL of the AgNO3solution was dropped into the petri dish to immerse the film.Finally,to expose the petri dish under UV light irradiation for 3~5 h induces the formation of Ag nanoparticles onto the honeycombpatterned film.For comparison,Ag nanoparticles array films were also obtained with 0.02 mol.L-1or 0.08 mol.L-1AgNO3and UV light irradiation of 3 h.In addition,Ag nanoparticles loaded on pristine ITO glass were also prepared with 3 mL 0.05 mol.L-1AgNO3and UV light irradiation of 3 h.
1.4 Surface-enhanced Raman scattering analysis
The obtained Ag nanoparticles array on honeycomb-patterned film was used as SERS substrate.The substrates were soaked into 2 mL of R6G~aqueous solution and allowed to be adsorbed for 8 h,and then took out and dried under ambient condition before measurements.Raman spectra were measured by a laser confocal Raman microspectroscope with an accumulation time of 2 s.A 532 nm laser with power of 50 mW was used as the excitation source.The light spot of the laser on the substrate has a diameter of about 2 μm.
1.5 Characterizations
The surface morphology of the samples was detected by using a field emission scanning electron microscope (FE-SEM,3 kV,S-4800,Hitachi).Silver composition was analyzed by X-ray photoelectron spectrometer(XPS)(ESCALAB 250Xi,Thermo Fisher Scientific Inc.).Surface-enhanced Raman scattering analysis was measured with a Raman spectrometer(Renishaw in Via,England).
2 Results and discussion
Ag nanoparticle array film was prepared with the assistance of PS-b-P4VP honeycomb-patterned film,which was prepared by static breath figure method(Fig.1).With the evaporation of CHCl3,the temperature of the solution quickly decreases.Water droplets are then condensed and self-assembled onto the surface.After the complete evaporation of the solvent and water droplets,honeycomb-patterned film is formed on ITO glass[16].The honeycomb-patterned film was treated under UV-irradiation for 2 h to peel off the top layer,fully exposing the bottom P4VP domain.

Fig.1 Schematic illustration for the preparation of Ag nanoparticles array
The processed honeycomb-patterned film was then immersed intoAgNO3solution.Duringthe immersing process,P4VP region will adsorb Ag+due to its electron-rich pyridine ring.When exposed to UV light,the pyridine ring will be excited and electronhole pairswillbe formed.The photo-generated electrons have strong reducing ability and can reduce Ag+,causing the in situ generation of Ag nanoparticles on the surface of the honeycomb-patterned porous films[17-19]. Compared with other methods such as chemical reducing agent route and electrochemical deposition method[20-22],this irradiation assisted reduction strategy is simple and green.
As shown in Fig.2a,the obtained PS-b-P4VP polymerfilm showsporousfeature with regular arrangement, demonstrating the formation of honeycomb-like structure.The prepared porous film shows homogeneous pore size.75.6%of the pores have sizes in the range of 2.8~3.0 μm.After peeling off the top layer of the honeycomb-like PS-b-P4VP film byan adhesive tape,the "dark domain"(corresponding to P4VP part)(Fig.2b)becomes easily accessible[14].Fig.2c and d show SEM images of the formed Ag nanoparticles array film.The corresponding particle size distribution in Fig.2e indicates that the Ag nanoparticle size is in the range of 20~50 nm.The presence of silver on the film is further confirmed by energy dispersive spectrum(EDS,Fig.2f).It should be noted that if the top layer was not peeled off,no Ag nanoparticles can be formed on the film by this method.

Fig.2 SEM images of(a)the formed PS-b-P4VP film;(b)the film after being peeled off the top layer;(c)the film loaded with Ag nanoparticles and(d)Ag nanoparticles in one pore;(e)size distribution of Ag nanoparticles;(f)EDS spectrum
X-ray photoelectron spectrum (XPS)illustrates elements of Ag,C,N,and O(Fig.3a).The elements of C and N come from the PS-b-P4VP film,while O wouldcomefrom theadsorbedH2O.Thehighresolution XPS spectrum of Ag region contains two bands with binding energies of 368.4 eV and 374.4 eV,exactly corresponding to Ag03d5/2and Ag03d3/2,respectively (Fig.3b)[23-24].This indicates that metallic Ag nanoparticles rather than other silver compounds exist on the honeycomb-patterned film.
It was proposed that most SERS-active system must possess metal particles with size in the range of 20~70 nm[17].This value is well consistent with that of our prepared Ag nanoparticle array film (20~50 nm),which was then investigated as SERS substrate.R6G was employed as a probe compound to test the SERS performance.Fig.3c shows the obtained Raman spectra of R6G solution(10-7mol.L-1)with our prepared Ag nanoparticle array substrates.The spectrum is well consistent with previously reported data[16].All these peak intensities are much higher than those obtained with bare ITO substrate and even a much higher concentration of 0.1 mol.L-1.
In order to quantitatively demonstrate the SERS performance,the EF was calculated according to the following formula[25].

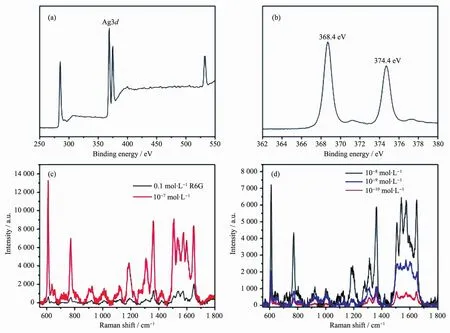
Fig.3 (a)XPS spectrum of Ag nanoparticles array;(b)High resolution XPS spectrum for Ag3d;Raman scattering spectra of(c)0.1 mol.L-1R6G measured on bare ITO substrate and 10-7mol.L-1R6G measured on Ag nanoparticles array film and(d)10-8~10-10mol.L-1of R6G solution on Ag nanoparticle array film
Where N0and I0are the number of molecules and intensity of regular Raman measurement with 0.1 mol.L-1R6G aqueous solution,NSERSand ISERSare those with our prepared SERS substrate measurement under the same solution[25].From this formula,EF value of 2.18X107is calculated based on the peak at 610 cm-1.In addition,the practical limit of detection,as shown in Fig.3d,can reach to 10-10mol.L-1.The corresponding EF values for 10-8,10-9,and 10-10mol.L-1of R6G aqueous solution are 1.26X108,3.67X108,and 1.31X109,respectively.These results indicate that the as-prepared Ag nanoparticle array substrates would be used as active SERS substrate.Fig.4 shows the approximate linear range of 10-10~10-8mol.L-1for the detection of R6G based on the peak intensity at 610 cm-1.

Fig.4 Linear range for the detection of R6G based on the Raman peak intensity at 610 cm-1
For comparison,the SERS performance of contrast substrates prepared with 0.02 mol.L-1or 0.08 mol.L-1AgNO3by keeping the UV irradiation reduction time of 3 h was also investigated,which provide relatively lower EF values of 6.97X106and 3.15X106toward 10-7mol.L-1of R6G,respectively.This indicates that there is an optimal density or size of Ag nanoparticles on the polymer film for SERS performance since the concentration of AgNO3solution would influence Ag nanoparticles density or size on the film.It was also found that the Ag nanoparticles array film prepared with longer UV light irradiation time of 5 h also show relatively lower EF of 6.85X105.Additionally,Ag nanoparticles loaded on pristine ITO glasswere also prepared atthe same reaction condition for comparison.The SERS measurement on this contrast flat SERS substrate shows EF value of about 4.19X106.This value is much smaller than that of our prepared honeycomb SERS substrate (2.18X 107),suggesting the honeycomb microstructure on the SERS substrate is highly favorable for the SERS performance.
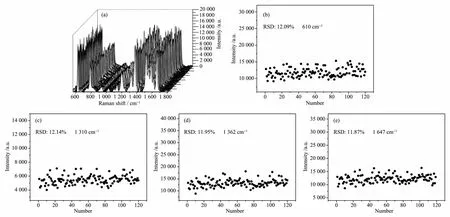
Fig.5 (a)Raman scattering spectra of 10-7mol.L-1R6G measured by 120 points over a 30X30 μm2area with a step size of 3 μm;(b~e)the intensity of Raman vibration at 610,1 310,1 362,and 1 647 cm-1of R6G solution in Raman scattering spectra showed in(a),respectively
To detect the uniformity of the prepared SERS substrates,SERS mapping-scan spectra on 120 spots were collected (Fig.5).The Raman mapping was scanned over a 30 μmX30 μm area with a step size of 3 μm.It was demonstrated that the Raman signals obtained from the 120 spots show similar intensity.The relativestandard deviation(RSD)values for the vibration at 610,1 310,1 362 and 1 647 cm-1are 12.1%,12.1%,12.0%,and 11.9%,respectively,which shows that the obtained Ag nanoparticlesarray substrates have reasonable uniformity.The relatively lowerRSD values are related to the high uniformity of the SERS pattern with microscale pattern. For SERS investigation,the usual laser spot size is about~1 μm for 532 nm laser;at this size scale,the obtained pattern is uniform,thus showing high SERS signal uniformity with low RSD values (lower than those of Ag nanoparticles loaded on pristine ITO,51%~75%).
It was generally accepted that SERS is predominantly originated from two mechanisms,electromagnetic and chemicaleffects.The electromagnetic enhancement is related to surface plasmon resonance occurring in the nanogapsbetween nanoparticles(known as"hot spots"),which induce stronger electromagnetic field and thusstrongerSERS signals.Compared with flat substrate[26],honeycomb-patterned film with larger surface area can immobilize more Ag nanoparticles and create more "hot spots",thus inducing higher enhancement.On the other side,it is also believed that chemical enhancement also provides some contribution to the SERS performance of our prepared Ag nanoparticles array on honeycombpatterned film substrates.Usually,the peaks at 1 362 and 1 647 cm-1due to the aromatic C-C stretching vibrations show the relatively highest intensity in Raman spectrum of R6G[27].However,in our case,the peak at 610 cm-1resulting from C-C-C ring in-plane bending vibration is stronger than the above two,which maybecaused bytheuniquemolecular orientation of analytes on the Ag nanoparticles array due to the weak interaction between the analyte molecular and the polymer[28]. This suggests the possible contribution of chemical enhancement to the SERS.
Compared to other SERS substrates prepared with various chemical routes,our prepared honeycomb SERS substrate has several advantages.Firstly,the preparation route is quite simple without complex instrument or surface modification.It is easily scaled up.Secondly,the honeycomb pattern with larger surface area can form amounts of "hot-spots",thus showing high EF value.Especially,the 4-vinylpyridine part in the polymer can not only adsorb more Ag+ions on the surface but also will possibly enrich the analyte molecular,both ofwhich are favorable ofthe enhanced SERS performances.
3 Conclusions
In summary, this study demonstrates the fabrication of Ag nanoparticle array SERS substrate with the assistance of PS-b-P4VP film obtained by static breath figure method.The prepared SERS substrate shows EF value as high as 1.31X109and limit of detection low to 10-10mol.L-1for R6G.In addition,the smaller relative standard deviation values of~12% indicate the favorable uniformity.It is believed that the route to patterned metal film would also extend to other metal composition for various potential applications.
