Optimizing pH-sensitive and time-dependent polymer formula of colonic pH-responsive pellets to achieve precise drug release
2019-09-12LijunSongLipingLingXioyingShiHonglngChenShuminZhoWenfengChenRuoxiZhouWenchngZho
Lijun Song , Liping Ling Xioying Shi Honglng Chen Shumin Zho Wenfeng Chen Ruoxi Zhou Wenchng Zho
, *
a School of Pharmacy, Guangdong Medical University, No.1, Xincheng Road, Dongguan 523808, China b Guangdong Key Laboratory for Research and Development of Natural Drugs, Guangdong Medical University,Zhanjiang 524023, China
Keywords:ES100-ERS NCO ERS-ES100 TCO Total alkaloids of sophora alopecuroides Colon targeted delivery pellets Drug release
ABSTRACT Time-sensitive and pH-dependent polymers are generally employed to prepare colon-site delivery system, and their coating thickness and order are very important in controlling the drug release. The traditional colon-site delivery systems consist of time-dependent polymers as inner layer and pH-sensitive polymers as outer layer. However, they suffer from low drug-loading rate and immature drug release. In this study, total alkaloids of sophora alopecuroides(TASA)-loaded pellets were prepared by extrusion-spheronization method and coated with Eudragit RS30D and Eudragit S100. Pellets using Eudragit RS30D as inner layer and Eudragit S100 as outer layer were named as ERS-ES100 TCO, while pellets with Eudragit S100 as inner layer and Eudragit RS30D as outer layer were ES100-ERS NCO. Both types of formulations with varying coating ratios and orders of Eudragit S100 and Eudragit RS30D were designed and prepared. The following in vitro drug release and SEM studies indicated that ERS-ES100 TCO(F2) with 12.8% Eudragit RS30D as inner layer and 21% Eudragit S100 as outer layer released up to 42% drug in 5 h. Interestingly, ES100-ERS NCO (F4) coated with 12.8% Eudragit S100 and 14.8% Eudragit RS30D showed optimal drug release in colon.In conclusion, ES100-ERS NCO colonic delivery system achieved reduced coating thickness and improved colonic targeting compared with traditional delivery system (ERS-ES100 TCO).In addition, the similarity factors ( f 2 ) value of sophoridine and matrine for investigated formulation were within 50-100 and > 80, demonstrating that sophoridine and matrine in all formulations achieved a synchronous release.
1. Introduction
Oral drug is the most convenient formulation for patients [1].Colon-targeted oral pellets offer various advantages in treating colonic disease, such as ulcerative colitis, amoebiasis, irritable bowel syndrome and colorectal cancer [2-5]. Moreover,colonic drug delivery systems can reduce the undue side effects and increase the local drug concentrations. As the consequences the therapeutic effect and patient appliance will be improved [4 ,6]. There are various approaches to achieve drug colon specific delivery, including pH-based, time-dependent and bacterial degradable systems.
Time-dependent pellets for colon targeting are made of insoluble polymers (hydroxypropyl cellulose or hydroxypropyl methyl cellulose) to overcome the great variation of gastric empty time. According to the various pH values of the gastrointestinal tract in different physiological conditions (stomach pH 2-3, small intestine pH 6.5-7.0, colon pH 7.0-8.0 [7,8],pH-sensitive systems are rationally designed and prepared.However, it is still a challenge to avoid the premature release of drug for single pH-sensitive systems. Therefore, both timedependent polymer (Eudragit RS30D) and pH-sensitive polymer (Eudragit S100) were utilized to prepare the novel colon drug delivery systems in our research.
Total alkaloids of sophora alopecuroides(TASA) is a mixture of extract from the roots or seeds of sophora alopecuroide,which has been used in the treatment of the acute enteritis for over 100 years in China. It contains aloperine, sophoridine, oxymatine, oxysophocarpine, matrine, sophocarpine,lehmannine, cytisine, and other alkaloids( Fig. 1 ) [9-11] . TASA possesses a variety of pharmacological features, such as antiinflammatory, anti-tumor, anti-cachectic and immunity regulation properties [12-17]. Recently, Zhao and coworkers reported that TASA showed therapeutic effect on colitis through regulating cytokine balance [17-19]. Based on the above considerations, the aim of this study is to directly deliver TASA to colon. Herein, we reported that TASA, as a model drug, was encapsulated with Eudragit RS30D and Eudragit S100 in different coating order and thickness. Swelling and dissolving experiments of free films of Eudragit S100 or Eudragit S100 coated with Eudragit RS30D were carried out. The results indicated that pellets of ES100-ERS NCO system led to reduced coating thickness and improved colonic drug release than that of ERSES100 TCO system.
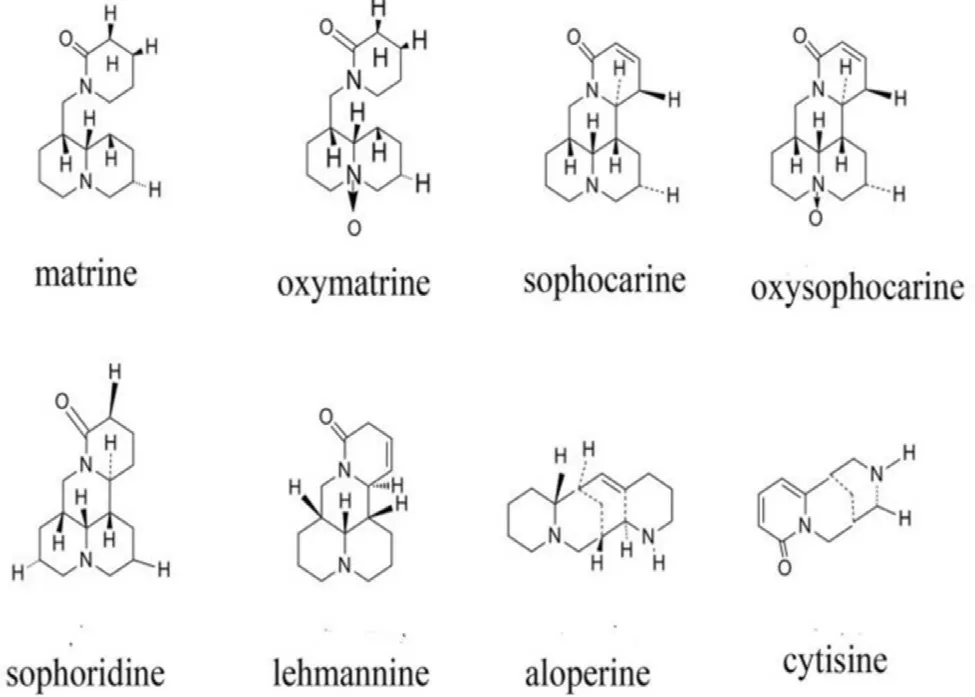
Fig. 1 -Structures of alkaloids of sophora alopeucuroides .
2. Materials and methods
2.1. Materials
TASA with a purity of 95% was purchased from Bauhinia pharmaceutical Co., Ltd. (Ningxia, China), bletilla striata polysaccharide(BSP) with a purity of 80.12% were from SenFu Biological Technology Co., Ltd.(Shanxi, China), microcrystalline cellulose (MCC) PH 101, talc powder (Tal) and triethyl citrate (TEC)were from XIYA Reagent. Reference compounds of sophoridine, oxymatine and cytisine with the purity ≥98% were supplied by Nology Co., Ltd.(Chengdu, China), reference compounds of sophocarpine and matrine with the purity ≥98%by DuShun Biological Chemical Co., Ltd.(Ningxia, China). Eudragit RS30D and Eudragit S100 were from Evonik industry Co.,Ltd.
2.2. Swelling and dissolving experiments of polymer films
Eudragit S100 and Eudragit RS30D polymer suspensions were prepared according to Table 1 . Eudragit S100 suspension was transferred into a teflon plate and stored at 40 °C for 24 h to cure a complete film. Then, the whole film was cut into three pieces of 1 cm2films (film A-C) and weighted. Film B and C were coated with different thickness of Eudragit RS30D and stored at 40 °C for 24 h. The three films were weighted and immersed into 100 ml medium at 37 °C. Then the samples were withdrawn from the medium and weighted after wiping the surface water by a filter paper at specific intervals. After 52 h, the swelling and dissolving curve, and linear fit were carried out separately. The constant (a) of linear denotes the swelling ability and dissolving ability of films per unit time.The medium used in this experiment were simulated gastric fluid (SGF) (2 h), simulated intestinal fluid (SIF) (3 h) and simulated colonic fluid (SCF) (47 h). All experiments were carried out in triplicate.
2.3. Preparation of TASA pellet core
TASA pellet cores were prepared via the extrusionspheronization method using Mini-250 (Xinyite, China).TASA, BSP and MCC were put together to amount of 480 g(7:1:12, w/w) to prepare wet mass. TASA and BSP were dissolved with 50% ethanol using ultrasound and kneaded withMCC. All the wet mass were loaded into the extruder (an axial type, single screw extruder equiped with a 1.0 mm scree for both the orifice diameter and screen thickness),and the extrusion speed was set up at 30 rpm. The resulting extrudates were spheronized for 6 min at a speed of 1500 rpm using a spheronizer with a cross-hatched geometry friction plate. The pellets were oven-dried for 24 h at 60 °C.
2.4. Determination of sophoridine and matrine released from pellets
A HPLC method has been established to determine the content of sophoridine and matrine released from pellets in different medium. The Agilent 1200 series HPLC system was equipped with a quaternary pump, a degasser, an auto-sampler, a column heater and a tunable wavelength UV detector. The separation was performed at 30 °C using a kromasil 100-5C column (4.6 mm ×250 mm, 5 μm). The mobile phase was acetonitrile (A) and 0.05 mol/l KH2PO 4 (with 2 ml/l triethylamine)as solution (B) in gradient elution at a flow rate of 1.0 ml/min.The gradient is as follows: 0 -25 min -60 min -65 min: 5%(A) -5% (A) -12% (A) -5% (A). The detection wavelength is 205 nm. Good linearity was observed in the range of 0.0033-0.052 mg/ml and 0.026-0.42mg/ml for sophoridine with a high correlation coefficient (A1 = 36362X-45.436, r2= 0.9991;A2 = 37852X-96.443, r2= 1.0000) (if the peak area of sophoridine in the drug release experiment below 887 mAu equal to 0.026 mg/ml of sophoridine, Equation A1 was chosen to determine the release of sophoridine, otherwise, equation A2 is selected). Moreover, good linearity was observed in the range of 0.0031-1.6 mg/ml for matrine with a high correlation coefficient (A = 42957C + 191.92, r2= 0.9995). The RSD values of precision and repetition for sophoridine were 0.236% and 2.92%,while for matrine were 0.157% and 3.56%. The stability data of sophoridine and matrine within 48 h were 0.735% and 1.33%,respectively.
2.5. The coating of pellets
Pellet cores (400 g) were transferred into a mini-250 multifunctional pelletizing coater (equipped with a bottom spray,Xinyite, China) and coated by polymer suspensions ( Table 1 )until getting the desired coating thickness. During coating, the aqueous dispersions were continuously stirred in order to prevent the sedimentation of the insoluble particles. The operating conditions were given in Table 2 . The polymer dispersions used in coating process contain 30.32% Eudragit RS 30D (w/w)or 9.32% Eudragit S100 (w/w) (related to the content of insoluble polymer).
2.6. In vitro drug release studies
Drug release from coated TASA pellets were determined using the basket method on Chinese Pharmacopoeia (2015 ED)at a speed of 50 rpm. Pellets (5 g) corresponding to 1.75 g TASA were placed in 900 ml 0.1 N HCl (pH 1.2) for 2 h, then phosphate buffer solution (pH 6.8) for 3 h. After that, the pellets were immersed into phosphate sodium buffer (pH 7.4) for the predetermined time at a temperature of 37 ±0.5 °C. The samples
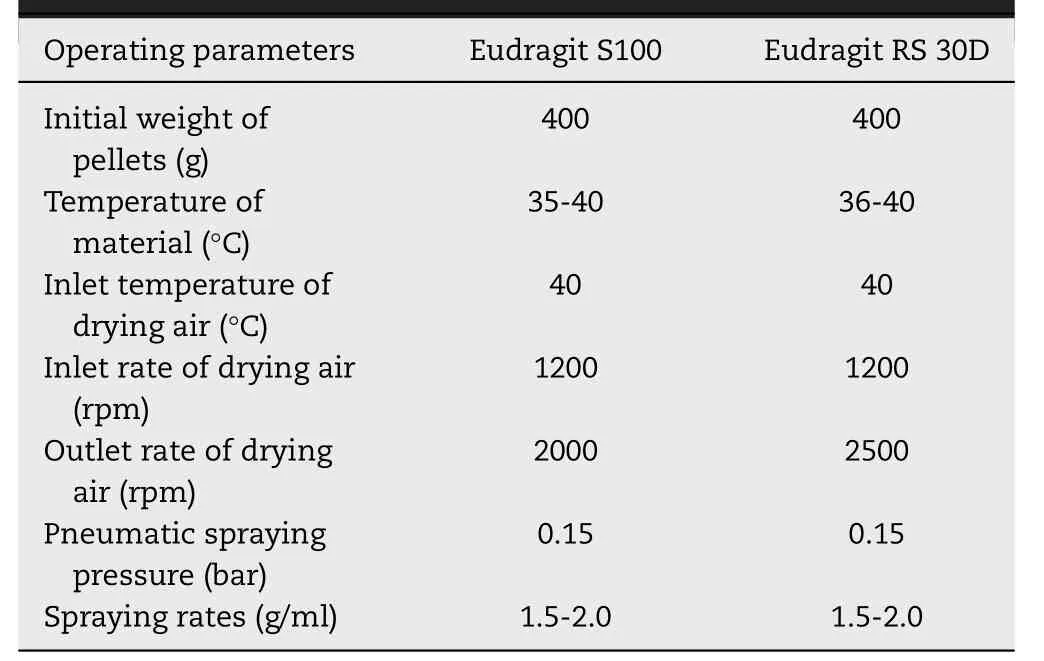
Table 2 -Operating conditions prevailing during the coating processes of the pellets.
(5 ml) were withdrawn at 2, 4, 5, 6, 8, 10, 12, 16, 20 and 24 h respectively, and were filtered with 0.22 μm membrane filter for HPLC measurements. Meanwhile fresh medium (5 ml) should be supplied. All experiments were performed in triplicate and the results were expressed as an arithmetic mean.
2.7. Kinetics of drug release
In order to investigate the most suitable drug release model,the drug release data was fitted into different models using linear regression analysis, the following models were used:
Zero order [20]
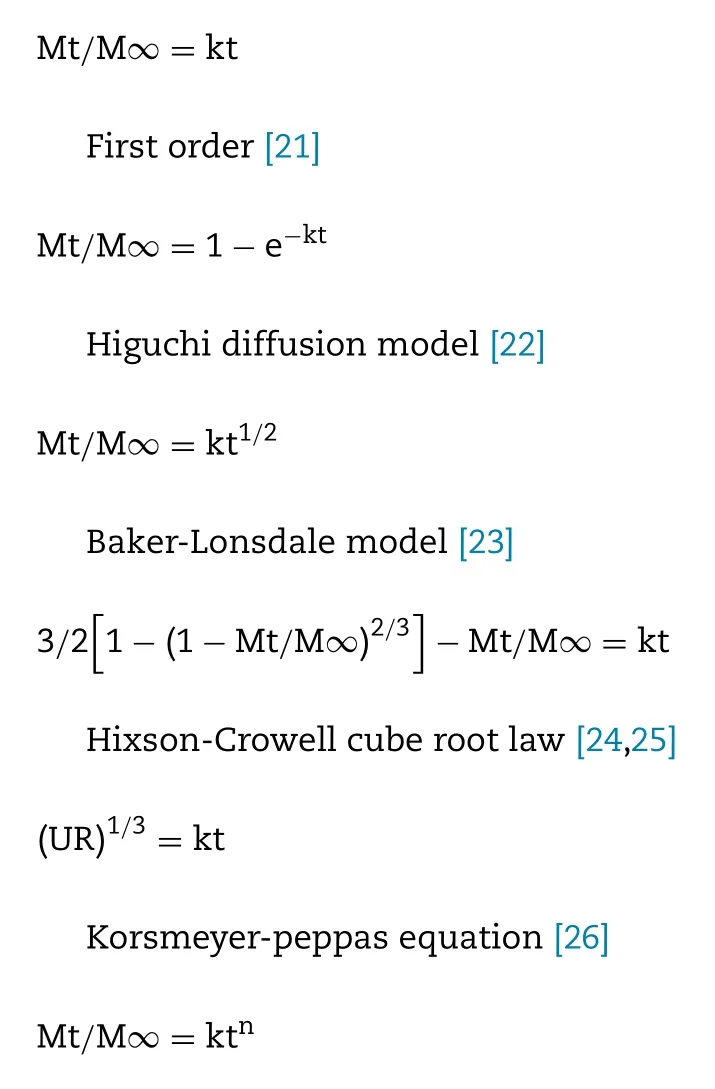
Where Mt/M ∞ denotes the fraction of drug released at time t , k is the rate constant corresponding to each model and n represents the diffusional exponent of the release mechanism.
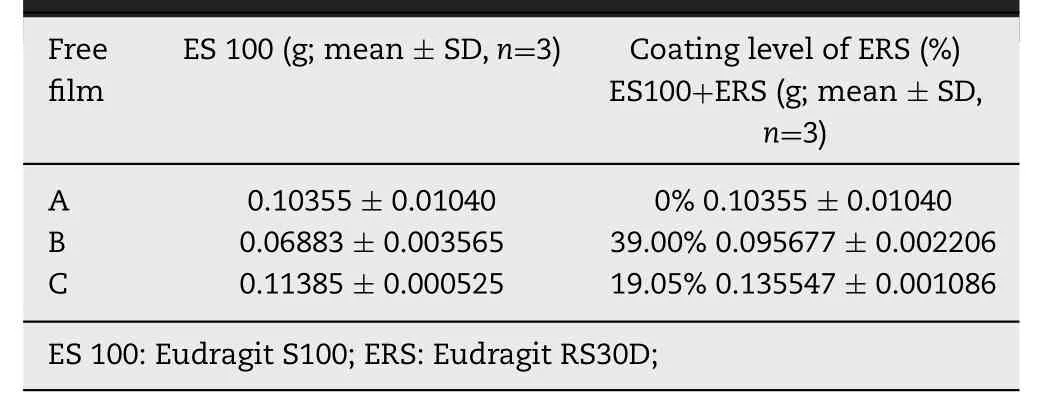
Table 3 -Composition of free films in swelling and dissolve experiments.
2.8. Scanning electron microscopy ( ×100 magnifications)
Scanning electron microscopy (SEM) was used to observe the changes of pellet surface morphology between the coated and uncoated pellets in different medium (before and after in vitro dissolution testing). SEM images were recorded using a scanning electron microscopy (SU8020, Japan). Sputter coating with gold for all SEM samples was performed before measurements.
2.9. The release behavior of sophoridine and matrine in formulations
As mentioned above, sophoridine and matrine are both compositions of TASA. In order to investigate whether they reach synchronous release in pellets, difference factor ( f 1 ) and similarity factor (f 2 ) [27] were applicated in this research. Similarity factor has been exploited by the center for drug evaluation and research (FDA) and human medicines evaluation unit of the European agency for the evaluation of medicinal products(EMEA), to be a criterion for the assessment of the similarity between two in vitro dissolution profiles [28].
3. Results and disscussion
3.1. Determination of swelling and dissolving for polymer films
The purpose of the experiments was to evaluate the preventing effect of Eudragit RS30D and Eudragit S100 polymer in the swelling or dissolving progress. The swelling data and dissolving data of polymer films (A-C) were analyzed by swelling and dissolving curve and linear fitting. The composition of three polymer films was shown in Table 3 . The swelling and dissolving curves and linear fittings were shown in Figs. 2 and 3 , respectively. As shown in Fig. 2 , film A reached the max swelling within 7 h, and dissolved completely within 18 h. Whereas,the max-swelling times of film B and C were 20 h and 22 h,respectively. They did not dissolve completely even after 52 h.
The results indicated that the max-swelling time and complete-dissolving time of films are related to the preventing effect provided by eudragit RS30D polymer. As shown in Fig. 3 , the swelling constant (a s ) values of A, B and C are 0.01894, 0.00258 and 0.00485, respectively, which is: A (a s ) >C (a s ) > B (a s ). The dissolving constant values of A, B and C are 0.02044, 0.00269 and 0.00497, superlatively , which is consistent with a s : A (a d ) > C (a d ) > B (a d ).
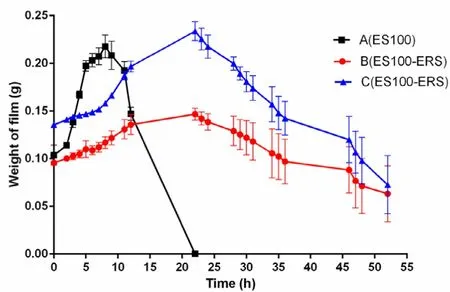
Fig. 2 -Swelling and dissolving curves of A, B and C(mean±SD, n = 3).
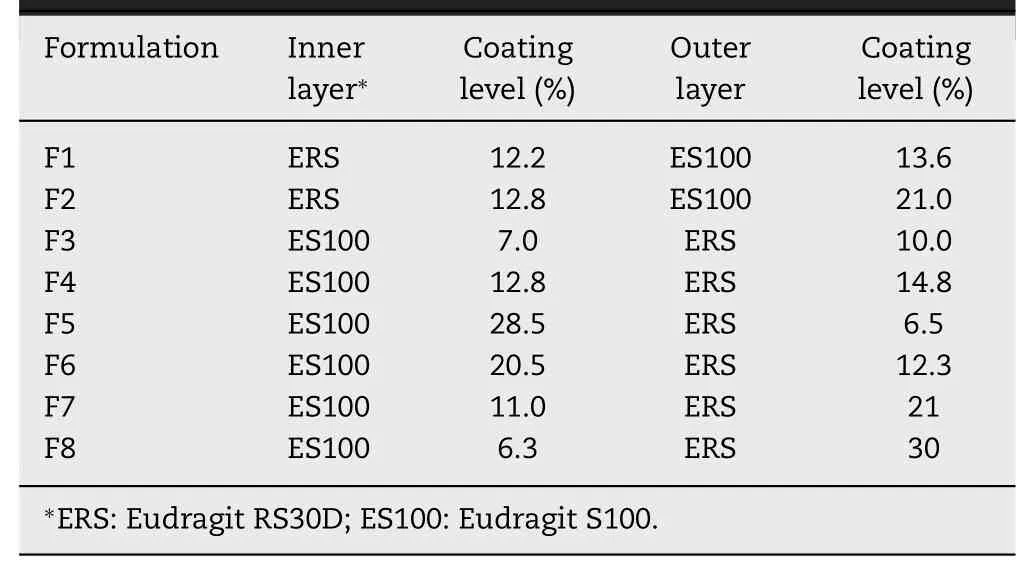
Table 4 -Coating level of experimental formulations.
3.2. Determination of sophoridine and matrine in pellets formulations
The hybrid reference substance and TASA sample were analyzed using a HPLC method described above. As shown in Fig. 4 , the chromatographic peak of sophoridine (peak 3) and matrine (peak 6) were both higher than other chromatographic peaks in TASA sample. In consideration of minimum drug release upper gastrointestinal simulated gastric fluid,which should reach the detection limit and the quantitation limit, sophoridine and matrine as main components in TASA were selected to investigate drug release. In this research, different kinds of mobile phases including H2O, 0.01% phosphate solution (pH was adjusted between 5 and 6 by triethylamine),methanol and 0.05 mol/l KH 2 PO 4 (2 ml/l triethylamine) were investigated. The image showed that 0.05 mol/l KH 2 PO 4 (2 ml/l triethylamine) would not interfere the detection of samples and reference in the condition of HPLC ( Fig. 4 A).
3.3. In vitro drug release studies
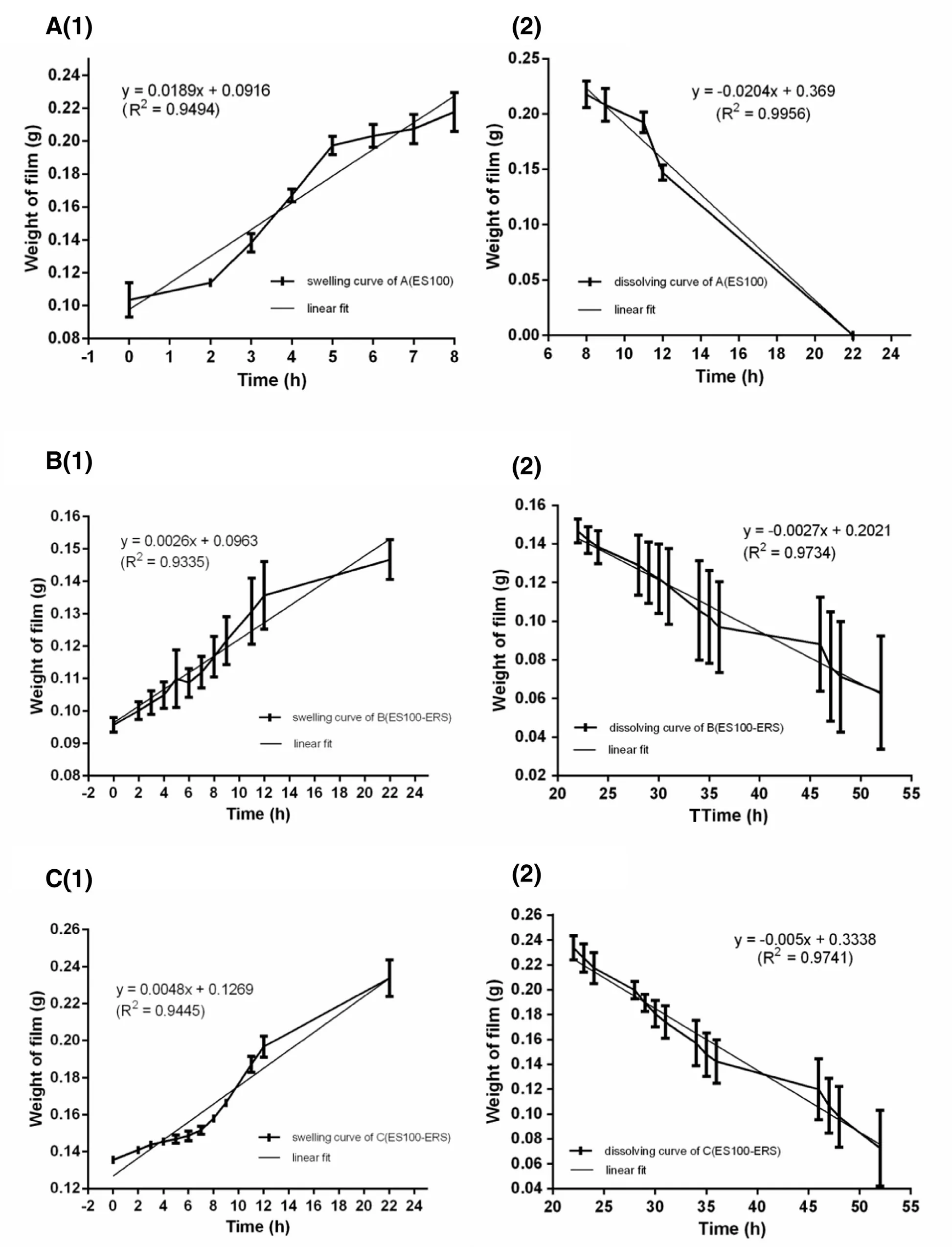
Fig. 3 -Linear fit for swelling curve or dissolving curves of A, B and C (mean ±SD, n = 3).
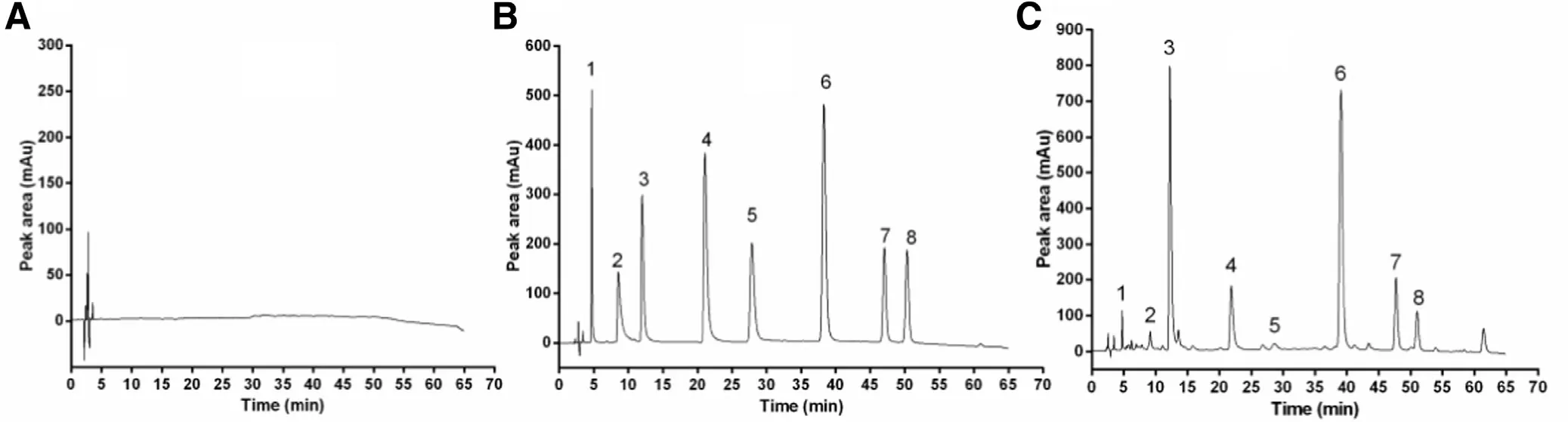
Fig. 4 -HPLC of (A) baseline of KH 2 PO 4 (2ml/l trimethylamine), (B) hybrid reference substance and (C) TASA sample.Chromatographic peak attribution: 1-cytisine; 2-aloperine; 3-sophoridine; 4-oxymatine; 5-oxysophocarpine; 6-matrine;7-sophocarine; 8-lehmannine.
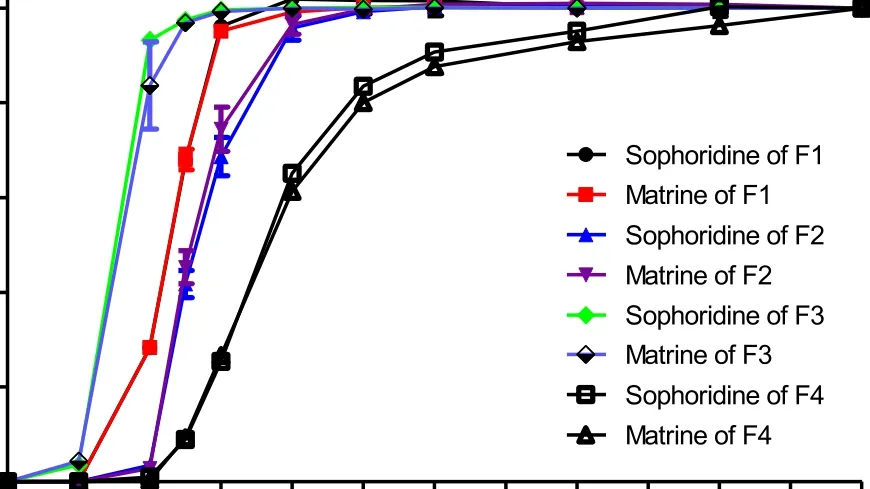
Fig. 5 -Drug release of (A) F1 to F4 and (B) F5 to F8 (mean ±SD, n = 3).
A total of 8 formulations were designed in this experiment and the details of coating thickness were shown in Table 4 . Pellets release profiles of different formulations were shown in Fig. 5 . For ERS-ES100 TCO (F1, F2), constant drug release was observed from 4 h to 12 h. Although the coating thickness of ES100 was up to 21%, the drug released up to 42% within 5 h(F2). In contrast, a slow drug release in ES100-ERS NCO was observed. There was no drug release within 2 h and no more than 9% within 5 h. Surprisingly, 90% of sophoridine and matrine was released within 12 h (after releasing in SCF for 7 h), and completely released within 24 h (F4). Although the total coating thickness of ERS-ES100 TCO (F2) and ES100-ERS NCO (F6)were both 33%, there was a significantly difference between their drug release profiles within 5 h. For F6, a negligible drug release was observed within 4 h and 19% drug release was observed within 5 h, whereas F2 represented 42% drug release within 5 h.
When comparing drug release profiles of F2, F4 and F6, it indicates that ES100-ERS NCO showed reduced coating thickness compared with ERS-ES100 TCO when realized colonic drug release. Then, formulations of F5 to F8 (ES100-ERS NCO)were designed to investigate the effect of the thickness of eudragit S100 and eudragit RS30D in drug release. As shown in Fig. 5 B, the total coating thicknesses of F5-F8 were controlled from 32% to 36%. The drug release rates of F5-F8 were about 35%, 19%, 18% and 8.5% within 5 h. When decreasing the coating thickness of eudragit S100 (inner layer) and increasing the coating thickness of eudragit RS30D (outer layer), drug releasebecame slower within 5 h. For all the formulations (F5-F8),there were negligible drug release within 4 h and complete released within 12 h.

Table 5 -Dissolution profiles between formulations F5 to F8.
Taking the release of sophoridine in F5 as a reference to compare the similarity of drug release behavior in F6, F7, F8,and the difference factor ( f 1 ) and similarity factor(f2) were employed. Generally, to confirm the similarity of the drug dissolution profiles, f 1 values should be lower than 15 (0-15) and f 2 values should be higher than 50 (50-100) [28]. The results( Table 5 ) showed that F5, F6 and F7 presented similar drug release profiles, while F8 appeared a different drug release profile compared with F5.
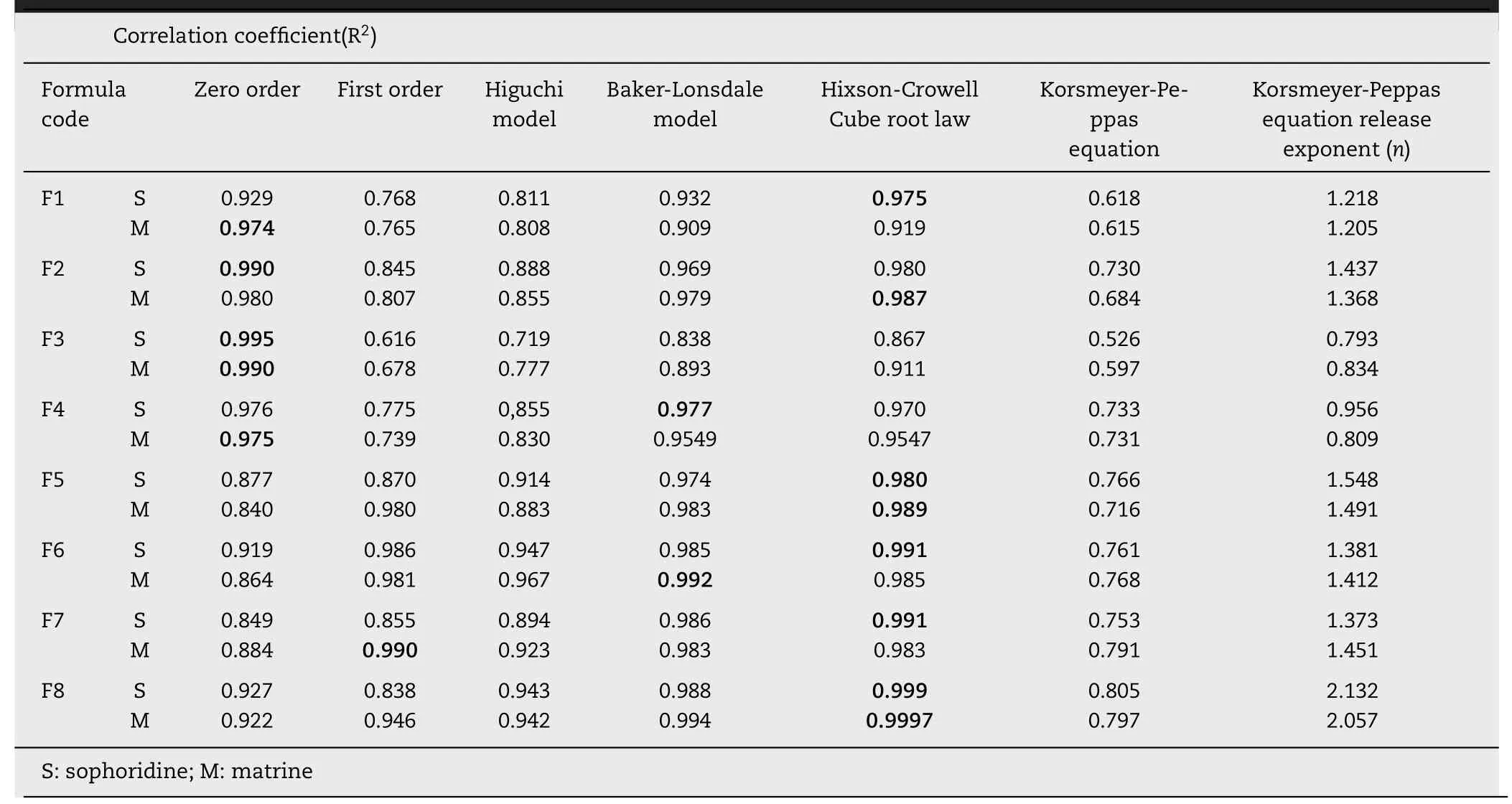
Table 6 -Kinetic assessment of drug release data from different formulation according to various kinetic models.
3.4. Kinetic of drug release
To explore the mechanism of TASA releasing from coated pellets, the dissolution data were fitted into zero order, first order,Higuchi model, Baker-Lonsdale model, Hixson-Crowell cube root law and Korameyer-Peppas equation ( Table 6 ).
For the release of matrine, a comparative evaluation of the correlation coefficients showed that zero order was the most appropriate to describe the kinetics of matrine releasing from F1, F3 and F4, which indicated a constant matrine release from coated pellets. The Hixson-Crowell cube root law provided the best fit to F2, F5, F8, and Baker-Lonsdale model was suitable for F6, first order was fit for F7.
For the release of sophoridine, the correlation coefficients confirmed that zero order was most suitable for F2 and F3,while Hixson-Crowell cube root law was suitable for F1, F5, F6,F7, F8, and F4 exhibited Baker-Lonsdale model. The Hixson-Crowell model is used to assume the release of drugs that controlled by the dissolution rate of the drug particles. It is used to describe the release profiles taking place in the diminishing surface of the drug particles during the dissolution [29].Baker-Lonsdale model is used to describe the drug controlled release from a spherical matrix. When the drug-loading rate is higher than 20%, the drug release profiles of most TASA coated pellets are limited by the diffusion that occurs through the interconnection channel of the polymeric matrix [30] .
3.5. Scanning electron microscopy ( ×100 magnifications)
The photomicrographs of uncoated pellets ( Fig. 7 A), coated pellets ( Fig. 7 B) and coated pellets after immersing in different mediums ( Fig. 7 C -G) were shown in Fig. 7 . Compared the surfaces of uncoated pellets with coated ones, it indicated that rough and rugged surface of uncoated pellets caused in spheronizing progress ( Fig. 7 A). However, the texture disappeared on the surfaces of coated pellets ( Fig. 7 B) and polymeric films could be observed clearly.
In drug release studies, ES100-ERS NCO samples and ERSES100 TCO samples were withdrawn from different medium in predetermined time to compare the differences of their surfaces ( Fig. 7 C and D). After immersing in SGF (pH 1.2) for 2 h and SIF (pH 6.8) for 3 h, ES100-ERS NCO and ERS-ES100 TCO maintained integral surface. The reason was that eudragit ES100 did not dissolve at acid environment and maintained its barrier function. After immersing in pH 7.4 medium(SCF) for 19 h, the surface of these two pellets both became unsmooth ( Fig. 7 E and F). There were some rough cakes appeared on the surface of ERS-ES100 TCO, which might because that the skeleton support effect of eudragit ES100 film disappeared as eudragit ES100 dissolved in the pH 7.4 medium(SCF).However, there were no cakes on ES100-ERS NCO pellets although they shrank to some extent, which benefited from the protection effect of eudragit RS30D.
3.6. The release behavior of sophoridine and matrine
Some methods have been adopted to compare the drug release profiles, such as statistical methods, model-independent methods and model-dependent methods. The f 1 and f 2 are the pair-wise procedures of model-independent method. Generally, in order to confirm the similarity of the dissolution profiles, f 1 values should be lower than 15 (0 -15) and f 2 values should be higher than 50 (50 -100) [28] . Results of the f 1 and f 2 of the investigated formulations were showed in

Table 7 -Results of f 1 and f 2 in investigated formulations.
Table 7 . As the results confirmed, sophoridine and matrine achieved synchronous release in all investigated formulations.
3.7. Discussions
In the determination of swelling and dissolving for free films,a s and a d represented the ability of swelling and dissolving per unit time, respectively. The increment of the values will lead to improved swelling ability or dissolving ability. The results showed that film A (Eudragit S100) presented a stronger swelling and dissolving ability than film B and film C (Eudragit S100 and Eudragit RS30D). The explanation is that the film A contacted with medium directly without the protection of Eudragit RS30D. In addition, a s and a d also related to the coating thickness of Eudragit RS30D. When the coating thickness increased, the values of a s and adboth decreased. The coating thickness of Eudragit RS30D in film B and film C were 39.00% and 19.05%, respectively. The a s and a d value of film C both were nearly twice as the value of film B. The reason might be that Eudragit RS30D film is a penetration film. As the coating thickness of Eudragit RS30D increased, the path of medium penetrated outward to Eudragit S100 film became longer, which would decrease the swelling rate and dissolving rate per unit time. In a word, the increment of the coating thickness of Eudragit RS30D led to the decrement of the swelling or dissolving ability. According to the SEM results of ERS-ES 100 TCO and ES100-ERS NCO, mechanisms of drug release from ERS-ES 100 TCO and ES100-ERS NCO could be speculated. Their drug release mechanisms showed an inconsistency in medium pH 6.8 and pH 7.4.
The drug release mechanism of ERS-ES100 TCO was interpreted in Fig. 6 A. As a kind of methacrylic acid copolymer, Eudragit S100 would not dissolve in pH 1.2 (SGF) and could maintain its barrier function to provide gastric resistance. When test performing in pH 6.8 medium (SIF), the outer layer film(Eudragit S100 film) began to swell in this environment, despite that the pH value was close to neutrality. As the test went on, the film fell off. For ERS-ES100 TCO, the swelling of Eudragit S100 film would led more medium to infiltrate into inner layer (Eeudragit RS30D film) and pellet core, which would cause more drug release in pH 6.8 (SIF). The outer layer (Eudragit S100) would dissolve and shed completely when pellets in pH 7.4 (SCF). The path for medium penetrated into the inner layer (Eudragit RS30D film) or pellet core would become shorter, which resulted in drug dissolving and releasing rapidly.
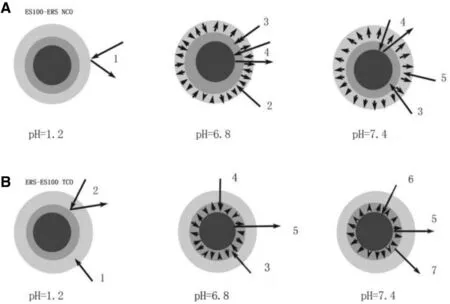
Fig. 6 -Mechanism of drug release from ES100-ERS coated pellets: 1 swelling of Eudragit RS30D; 2 gastric resistance; 3 swelling of Eudragit S100; 4 penetration of water into the swelling of Eudragit S100; 5 release of drug; 6 dissolution of Eudragit S100; 7 penetration of water into the pellet core.
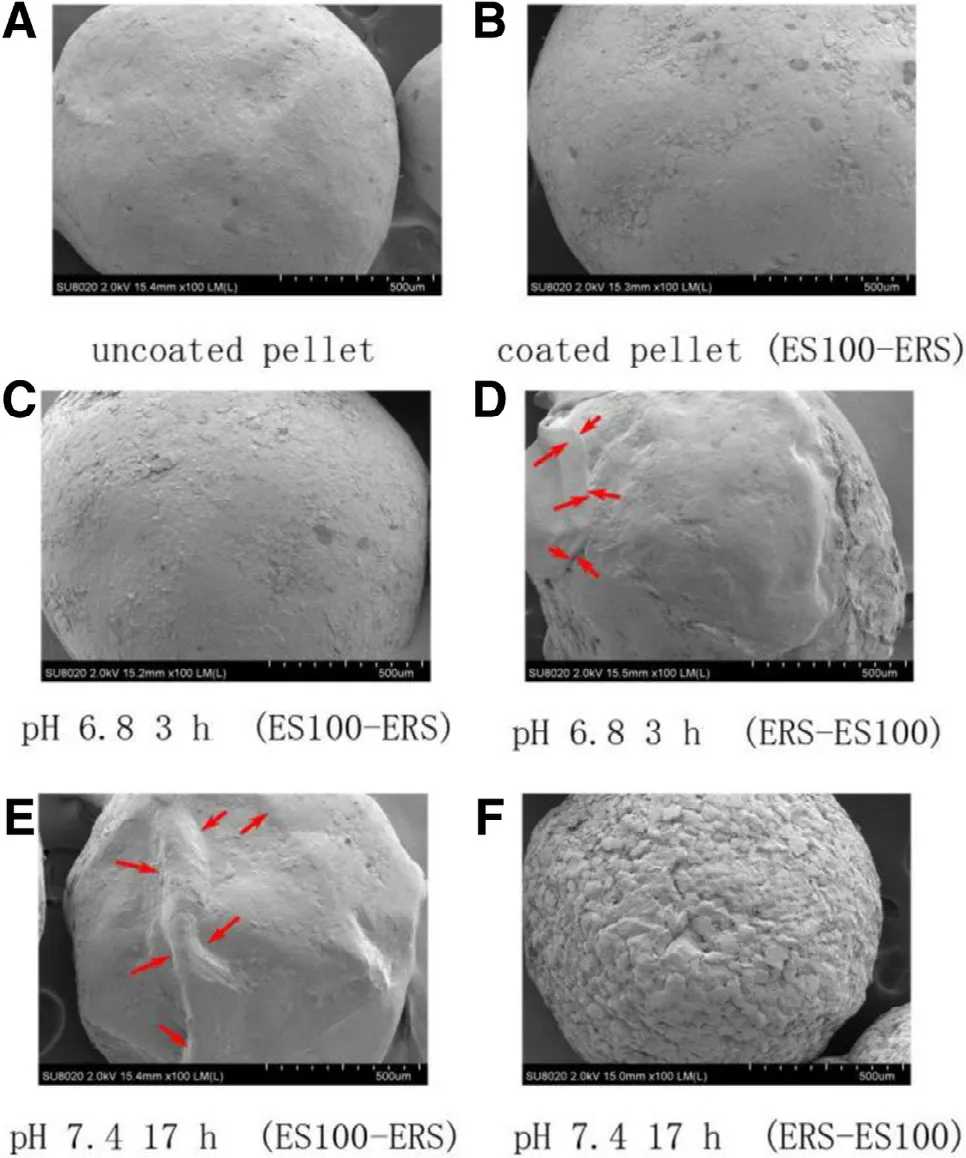
Fig. 7 -Scanning electron microscope photographs ( ×100 magnifications) of uncoated pellets and coated pellets after immersed in different pH medium. (A) uncoated pellet; (B)coated pellet; (C) Formula F4 after immersed in pH 1.2 2 h;(D) Formula F4 after immersed in pH 6.8 3 h.; (E) Formula F2 after immersed in pH 6.8 3 h; (F) Formula F4 after immersed in pH 7.4 17 h; (G) Formula F2 after immersed in pH 7.4 17 h.
For ES100-ERS NCO( Fig. 6 B), the medium (pH 1.2, SGF) could penetrate through the outer layer (Eudragit RS30D film). However, it could not penetrate into inner layer (Eudragit S100 films) as the pH value did not reach to the dissolving point of Eudragit S100. Therefore, the inner layer (Eudragit S100 films)could maintain its barrier function and provide gastric resistance. When pellets transformed into pH 6.8 medium (SIF),the medium would penetrat through the outer layer (Eudragit RS30D films) and lead to the swelling of the inner layer (Eudragit S100 films). Finally, the medium reached to pellets core which would lead to the drug dissolving and release. Release mechanism of ES100-ERS NCO in pH 7.4 (SCF) was the same as pellets in pH 6.8 (SIF).
The different release mechanisms of these two coating systems could be explained by the swelling and dissolving experiments of polymer films. For ES100-ERS NCO, the rate of swelling and dissolving of Eudragit S100 was controlled by Eudragit RS30D film. The increment of the thickness of Eudragit RS30D film leads to a decrease of the swelling and dissolving rate of Eudragit S100 film and less drug release. For ERS-ES100 TCO, the swelling and dissolving of Eudragit S100 film were only influenced by pH value of the experimental medium,which would lead to a faster drug release from pellet core than ES100-ERS NCO.
To avoid drug release in the upper digestive tract for colonspecific drug release system, drug coated with pH-sensitive polymers as outer layer maybe interest the colon specific applications. However, a high coating thickness of pH-sensitive layer is always necessary to prevent drug release in upper gastric tract. Several published literatures have responded to this problem. Xu reported that coating thickness of the outer layer(Eudragit S100 film) reached up to 28% to achieve colon targeting release [31]. Cui investigated a dosage of pH-time based and enzyme-degradable pellets for colonic delivery. For the pH sensitive layer of Eudragit L30D-55, the 30% increase of coating weight was necessary [32].
Take F2 as an example, for ERS-ES100 TCO system, 42%drug released within 5 h even the coating thickness reached 21%. In contrast, 12.8% coating thickness was necessary (F4)when using the Eudragit S100 as inner layer,. Therefore, the total coating thickness would be decreased, which was essentially helpful for the economic production and improved patient appliance.
4. Conclusions
In conclusion, we prepared ES100-ERS NCO with reduced coated thickness by changing the order of polymeric coatings, and achieved optimized drug release in colonic site. Additionally, ES100-ERS NCO maintained the complete shapes,and their drug release properties were in accord with osmotic pump release mechanism. We concluded that the penetration of outer time-dependent layer (Eudragit ERS layer) might control the swelling and dissolving of inner pH-sensitive layer(Eudragit ES100 layer) in the ES100-ERS NCO system. Moreover, the outer layer (Eudragit ERS layer) decreased the rate of swelling and dissolving in a coating thickness dependent manner. In the future, the in vivo drug release profile should been carried out for further research.
Conflicts of interests
The authors declare that there is no conflicts of interest.
Acknowledgments
The experiment was supposed by major science and technology projects of Guangdong province, China(2013A022100039),science innovation projects of higher school(2012KJCX0060),the technology bureau of Zhanjiang, Guangdong, China(2011C3108015), Guangdong province sail plan project of high level talents in 2014, the National Natural Science Foundation of China ( 81473401 ) and Guangdong provincial innovation and entrepreneurship training program for college students in 2016 no. 196.
杂志排行
Asian Journal of Pharmacentical Sciences的其它文章
- The appliances and prospects of aurum nanomaterials in biodiagnostics, imaging, drug delivery and combination therapy
- Current developments in drug delivery with thermosensitive liposomes
- The application of nitric oxide delivery in nanoparticle-based tumor targeting drug delivery and treatment
- Delivery of docetaxel using pH-sensitive liposomes based on D - α-tocopheryl poly(2-ethyl-2-oxazoline)succinate: Comparison with PEGylated liposomes
- Amino functionalized chiral mesoporous silica nanoparticles for improved loading and release of poorly water-soluble drug
- A hybrid genipin-crosslinked dual-sensitive hydrogel/nanostructured lipid carrier ocular drug delivery platform
