Delivery of docetaxel using pH-sensitive liposomes based on D - α-tocopheryl poly(2-ethyl-2-oxazoline)succinate: Comparison with PEGylated liposomes
2019-09-12ShuHnRuiyngSunHongSuJingLvHunXuDiZhngYunshnFub
ShuHn,RuiyngSun,HongSu,JingLv,HunXu,*,DiZhng,YunshnFub,**
a Department of Pharmacy, School of Chemistry and Chemical Engineering, Liaoning Normal University, Dalian 116029, China b Department of Anatomy, College of Basic Medical Science, Dalian Medical University, Dalian 116044, China
Keywords:d- α-tocopheryl poly(2-ethyl-2-oxazoline) succinate Liposomes pH-sensitive PEGylation
ABSTRACT This study aimed to investigate the ability of the novel materials D - α-tocopheryl poly(2-ethyl-2-oxazoline) succinate (TPOS) to construct pH-sensitive liposomes. TPOS was initially synthesized and characterized by TLC, FTIR, and 1 H-NMR. The buffering capacity of polyethylene glycol- distearoyl phosphatidylethanolamine (PEG-DSPE) and TPOS was determined by acid-base titration, and TPOS displayed a slower downtrend and gentler slope of titration curve than PEG-DSPE within pH 7.4-5.0. Studies on the in vitro drug release demonstrated that TPOS modified docetaxel (DOC) liposomes (TPOS-DOC-L) had a slower drugrelease rate at pH 7.4 similar to PEGylated-DOC liposomes (PEG-DOC-L), whereas the release rate reached approximately 86.92% ±1.69% at pH 6.4. In vitro cellular uptake assays by microplate reader, and flow cytometry revealed that TPOS modified coumarin 6 liposomes(TPOS-C6-L) had stronger cellular uptake at pH 6.4 than that at pH 7.4 ( P < 0.01). Conversely,for PEGylated C6 liposomes (PEG-C6-L) and conventional C6 liposomes (C6-L), very similar cellular uptakes were exhibited at different pH values. Confocal laser scanning microscopy images showed that PEG-C6-L and C6-L were mainly located in lysosomes. By contrast,TPOS-C6-L showed broader cytoplasmic release and distribution at 4 h. MTT assay showed that the cytotoxicity of TPOS-DOC-L was similar to that of PEG-DOC-L and conventional DOC liposomes (DOC-L) at the same DOC concentration and at pH 7.4, but was much lower than those at pH 6.4 after 48 h of incubation. The apoptosis of PEG-DOC-L and DOC-L had no remarkable improvement with decreased pH from 7.4 to 6.4. Meanwhile, TPOS-DOC-L
1. Introduction
The chemotherapy is an important approach for clinical cancer treatment by inhibiting the growth of primary tumor and suppressing the proliferation of metastatic tumor cells [1,2] . However, most chemotherapy drugs (e.g. paclitaxel, doxorubicin) pose some problems such as low bioavailability, weak selectivity, poor solubility and cytotoxic side effects [3,4] . In recent years, liposomes were widely used to deliver chemotherapy or gene drugs. Polyethylene glycol(PEG) was usually coated on the surface of liposomes to improve pharmacokinetic and pharmacodynamic properties of liposomes [5,6] .
However, with further research, PEGylated liposomes produced a series of negative effects. When liposomes were modified by amphiphilic PEG-lipids, the surface aqueous layer and conformation cloud of PEG moiety inhibited the uptake of the nanocarrier by the target cells. Therefore, the ability of cellular uptake was weak [7,8] . Furthermore, for the gene or proteinloaded pH-sensitive liposomes, the shielding effects of PEG moiety resulted in poor endosomal escape via membrane fusion, followed by degradation of drugs in lysosomes [9,10] .These serious issues regarding the use of PEG in nano-drug delivery were referred to as the “PEG dilemma”[11-15] . “PEG dilemma”presented a barrier in the research of PEGylated liposome and their use in clinics.
Poly(2-ethyl-2-oxazoline) (PEtOz), a polymer of 2-ethyl-2-oxazoline monomers that is safe, non-toxic, amphipathic, and biocompatible, has been approved by the United States Food and Drug Administration. The extracellular pH values of most solid tumors range from 5.7 to 7.8, whereas the pH of normal tissue is at pH 7.4 [16,17] . PEtOz would change from hydrophilic to hydrophobic under low pH conditions. Changes in the polymer chain conformation resulted in the destabilization of modified liposomes at low pH due to the liposomes fusion and ultimately led to the release of the encapsulated drugs. Therefore, PEtOz is suitable in fabricating pH-sensitive drug delivery. The authors have applied PEtOz,such as PEtOz-cholesterol methyl carbonate (PEtOz-CHMC)[18,19] and PEtOz-cholesterol hemisuccinate (PEtOz-CHEMS)to construct pH-sensitive DDS [20] . Compared with conventional and PEGylated liposomes, PEtOzylation could effectively promote the endosomal escape of liposomes. In addition, in vivo experiments in rats further demonstrated that PEtOz not only has equivalent acid sensitivity but also stability level to PEG [18,21,22] .
In this study, a novel amphiphilic pH-sensitive copolymer named D - α-tocopheryl poly(2-ethyl-2-oxazoline) succinate(TPOS) was synthesized through esterification between D -α-tocopheryl succinate and poly(2-ethyl-2-oxazoline) [molecular weight (MW) = 2 kDa]. Liposomes modified with TPOS(TPOS-L) possessed both stable and pH-sensitive properties( Fig. 1 ). TPOS could endue liposomes with a strongly hydrophilic shell and conformation cloud, which increased the stability of TPOS-L significantly. TPOS-L could response to the slightly acidic circumstance and increase the uptake of TPOS-L by tumor cells. In this study, the liposomes were prepared for anticancer drug delivery with docetaxel (DOC) as a model anticancer drug and coumarin 6 (C6) as a fluorescence probe. The physicochemical properties of liposomes including in vitro release, intracellular uptake, and endosomal escape were evaluated.
2. Materials and methods
2.1. Materials
Poly(2-ethyl-2-oxazoline) (MW = 2 kDa) was prepared by our own laboratory. D - α-tocopheryl succinate and C6 were purchased from Aladdin Industrial Corporation (Shanghai,China). 4-(Dimethylamino) pyridine (DMAP) was provided by Shanghai Medpep Co. Ltd. (Shanghai, China). Soybean phosphatidylcholine (S100) and egg yolk lecithin (EPC) were purchased from LIPOID (Ludwigshafen, Rhineland-Palatinate,Germany). PEG-DSPE was purchased from Shanghai Advanced Vehicle Technology Co. Ltd. (MW of PEG is 2 kDa; Shanghai,China). DOC was provided by Beijing Huafeng United Technology Co. Ltd. (Beijing, China). Cholesterol, Sephadex G-50 and dicyclohexylcarbodiimide (DCC) were obtained from Biosharp(Japan). Dialysis bag (MWCO 8-14 kDa) was obtained from Spectrum (USA). Triton X-100 were obtained from Sinopharm Chemical Reagent Co., Ltd, (Shanghai, China).
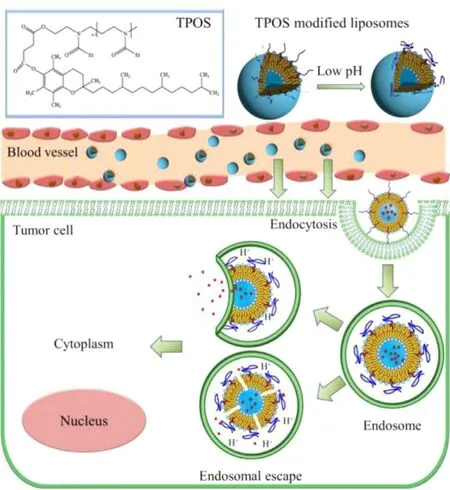
Fig. 1 -Schematic representation of TPOSylated liposomes.
The human red blood cells (RBCs) were provide by medical office of Liaoning Normal University.
For cell culture, human cervical carcinoma cell line HeLa was provided by the cell bank of the Chinese Academy of Sciences (Shanghai, China). The cell culture reagents(DMEM),namely, 3-(4,5-dimethylthiazol-2-yl) -2,5-diphenyl tetrazolium bromide (MTT) and the albumin from Bovine serum albumin (BSA) were purchased from Sigma-Aldrich (St. Louis,MO, USA). 4′,6-Diamidino-2-phenylindole (DAPI), immunol staining fix solution, and anti-fade mounting medium were obtained from Beyotime Biotechnology Company (Nantong, China). Dimethylsulfoxide (DMSO), acetone, and other reagents (AR grade) were purchased from Tianjin Kemiou Chemical Reagent Co., Ltd. (Tianjin, China).
Human cervical carcinoma cell line (HeLa) was cultured using DMEM supplemented with 10% FBS and 1% penicillinstreptomycin solution in a cell culture dish at 37 °C and 5% carbon dioxide in the incubator. The morphology and cell growth conditions were observed daily and the culture medium was replaced accordingly.
2.2. Synthesis of TPOS
PEtOz2k was synthesized in our laboratary [20] . The synthesis of TPOS is shown in Fig. 2A . In a nitrogen atmosphere, 1.2 mmol D - α-tocopheryl succinate dichloromethane was added to PEtOz2k (1.0 mmol), then DMAP (0.4 mmol) and triethylamine (0.4 mmol) were added. After reacted for 24 h at 24 °C, the mixture was dealt with distilled water, HCl (1 M),and saturated sodium chloride solution, respectively. The organic layer was dried in vacuum with anhydrous magnesium sulfate. The crude product was purified by silica gel column and dichloromethane: methanol (5:1, v:v) was used as eluent.The chemical structures of TPOS was confirmed by IR and1H NMR. IR ν/cm -1 ( Fig. 2 B): 1732 (ester carbonyl, C = O), 1628 (tertiary amine), 1580 (carbonyl, C = O), and 2920 (saturated hydrocarbons, -CH 2 -); 1 H NMR(CDCl 3 , 500 MHz) δ( Fig. 2 C): 0.87(s,3H, H-4), 1.13(d, J = 5.70 Hz, 3H, H-1), 1.26(d, J = 3.95 Hz, 3H, H-2), 1.55(d, J = 6.80 Hz, 3H, H-3), 2.41(s, 3H, H-5), 2.59(s, 3H, H-9),3.73(q, J = 7.05 Hz, 2H, H-7,8), 5.30(s, 1H, H-6).
2.3. Preparation of DOC or C6 loaded liposomes
Conventional DOC/C6 loaded liposomes (DOC-L, C6-L), PEGDSPE coated DOC/C6 loaded liposomes (PEG-DOC-L, PEG-C6-L), and TPOS-coated DOC/C6 loaded liposomes (TPOS-DOC-L,TPOS-C6-L) were prepared by the lipid film hydration method as previously described [20] . DOC/C6, S100, cholesterol, and PEG-DSPE/TPOS (added only in the preparation of coated liposomes, molar ratio of S100: cholesterol = 3:1, PEG-DSPE/TPOS with 5 mol%, C6 with 0.68 mol% and DOC with 3.68 mol% of the total lipids) were dissolved in 5 ml chloroform. After the chloroform was removed at 37 °C, a thin lipid film was formed and then further dried in vacuum overnight to remove any traces of the remaining solvent. The lipid film was hydrated in 0.01 M PBS at 37 °C. The suspensions were filtered through 0.45 and 0.22 μm polycarbonate membranes after sonication with an ultrasonic cell disruptor (JY92-2D probe sonicator, Ningbo,China).
2.4. Characterization of liposomes
2.4.1. Particle size, polydispersity and zeta potential analysis of liposomes
The size, polydispersity, and zeta potential of liposomes were measured by using a Zetasizer (Nano ZS, Malvern Instruments, Malvern, UK) via the photon correlation spectroscopy(PCS). All samples were conducted in triplicate.
2.4.2. Encapsulation efficiency (EE) of liposomes
EE of DOC loaded liposomes was measured by highperformance liquid chromatography (HPLC, Agilent LC1100).A reverse-phase HPLC column (Agilent Eclipse XDB-C18,4.6 mm ×200 mm, 5 μm) was used. The column effluent was identified with a UV detector at 230 nm. The EE of C6 loaded liposomes was measured by using a model F-7000 fluorescent spectrophotometer (Hitachi, Japan) at an excitation wavelength of 455 nm and an emission wavelength of 506 nm. The EE of DOC and C6 loaded liposomes were calculated through the following equations:

where w and w 1 are the amounts of the drug in the liposomes after and before passing, respectively, over the sephadex G-50 column. All experiments were performed in triplicate, and the results are expressed as mean ±SD.
2.4.3. Morphology of liposomes
Surface morphologies of liposomes (DOC-L, C6-L, PEG-DOC-L,PEG-C6-L, TPOS-DOC-L, TPOS-C6-L) were imaged by a Su8010 scanning electron microscope (Hitachi, Japan). After sonication, sample suspensions were diluted and dried on a copper tape for 5 min. Before imaging, samples were coated with a platinum layer for 45 s using JFC-1300 automatic fine platinum coater (JEOL, Tokyo, Japan) for 40 s at 20 mA. The morphologies of liposomes were observed using transmission electron microscopy (TEM, JEM-2000EX, JEOL, Japan). The samples were negatively stained with 4% phosphotungstic acid and dried on carbon-coated grids for observation.
2.4.4. Differential scanning calorimetry (DSC) measurement DSC analysis was performed to investigate the physical status of DOC inside the TPOS-DOC-L by using a DSC STA449F3 thermogravimetric analyzer (Germany). The freeze-dried liposomes were purged with dry nitrogen. A temperature range of 30 to 250 °C was scanned at a heating rate of 10 °C/min.
2.5. Buffering capacity of polymer
The buffering capacity of PEG-DSPE and TPOS was determined by acid-base titration [23] . Briefly, PEG-DSPE or TPOS was dissolved in 0.01 M NaCl (4 mg/ml) and the solution was adjusted to pH 12 with 1 M NaOH. The 0.01 M HCl was added into the solution sequentially to obtain the titration profile. Solution pH was measured with a pH meter after each addition of acid.The slope of the plot of pH versus the amount of HCl indicates the buffering capacity.
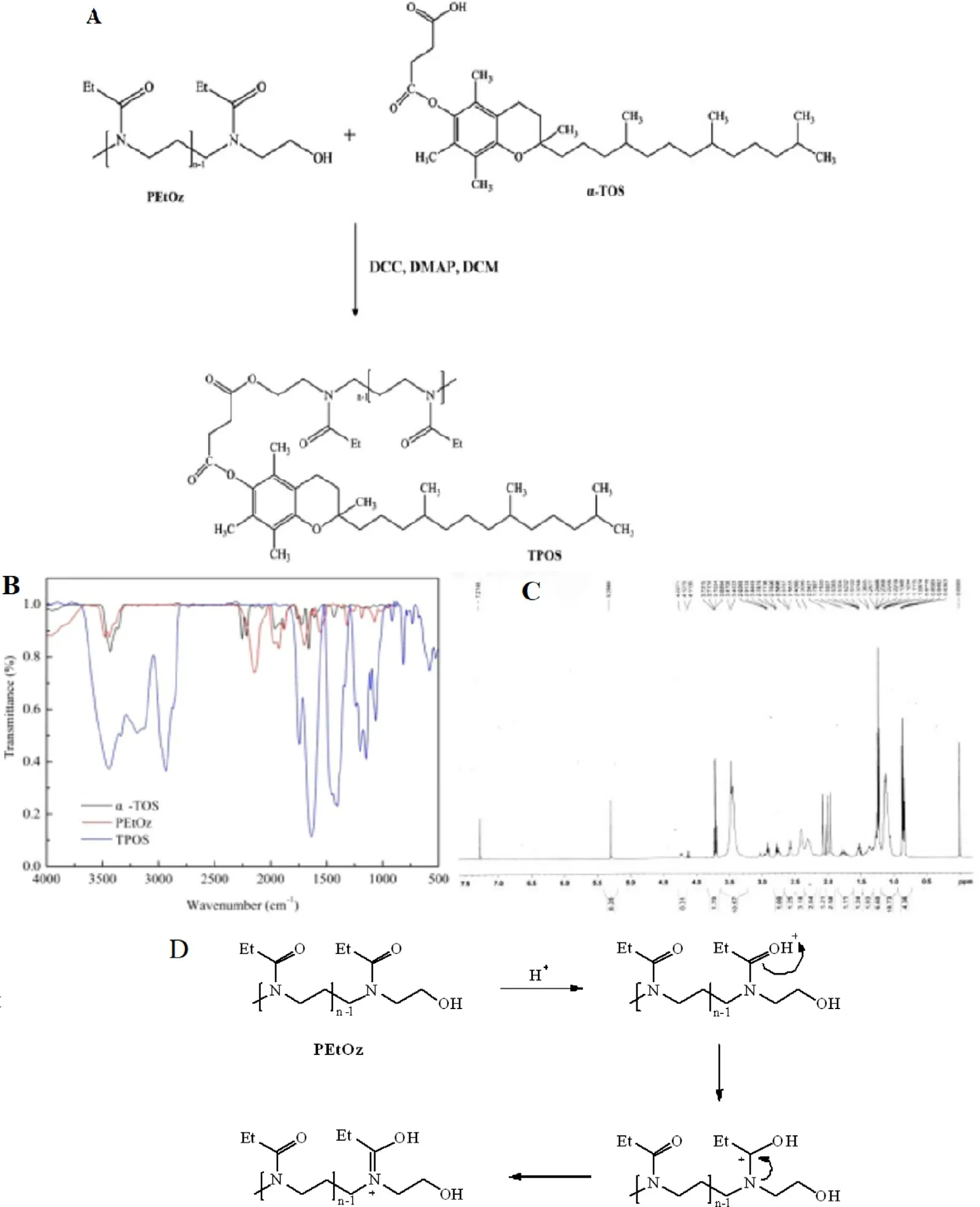
Fig. 2 -(A) The schematic of the synthesis of TPOS, (B) FTIR spectrum of α-TOS, PEtOz and TPOS and (C) 1H NMR spectrum of TPOS, (D) pH-sensitive mechanism of PEtOz.
2.6. In vitro drug release
For investigating the in vitro drug release profile of DOC-loaded liposomes, drug release study of liposomes was conducted[23] . A total of 3.0 ml DOC-loaded liposomes were sealed in a dialysis bag (MWCO 8-14 kDa) and incubated in 70 ml pH 7.4 and 6.4 PBS containing 0.1% (w/v) Tween 80 at 37 °C. A total of 250 μl of release medium were completely removed,and the same volume of fresh PBS was supplemented at predetermined time intervals. The DOC samples content in the samples was determined by HPLC as described in the liposomes EE determination. The drug release profiles were calculated.
2.7. In vitro hemolysis of TPOS or PEG-DSPE micelles
Hemolysis studies were performed to evaluate the safety of the polymer for application in vivo [24,25] . All experiments were conducted in accordance with the guidelines of the local Animal Welfare Committee. The red blood cells (RBCs) were harvested by centrifugation at 1000 ×g for 10 min after the collection of the rat's blood in heparin sodium containing tubes.The erythrocytes were washed three times with PBS buffer.The RBCs were resuspended in the buffer (pH = 7.4). Different concentrations of PEG-DSPE and TPOS were incubated in 10% erythrocyte suspension at 37 °C for 1 h, then the samples were centrifuged at 1000 ×g for 5 min. The absorbance of the supernatants was measured at 570 nm by UV-Vis spectrophotometry.
The observed hemolysis of RBCs in the physiological saline and 10% Triton X-100 were used as a negative and positive control, respectively. The hemolysis rate (HR) was calculated according to the following equation:
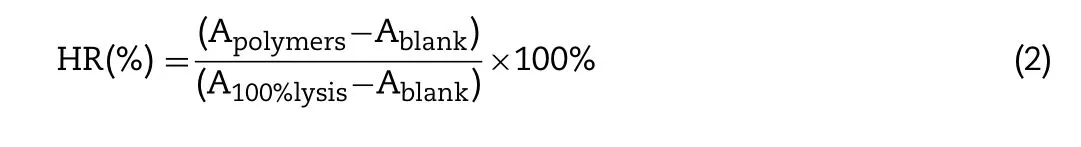
where A polymers is the absorbance value of the hemoglobin released from the RBCs treated with the polymer solution; A blank is the absorbance value of the hemoglobin released from the RBCs treated with physiological saline; and A 100%lysis is the absorbance value of the hemoglobin released from RBCs treated with 10% Triton X-100.
2.8. In vitro cytotoxicity of liposomes
in vitro antiproliferative activities of DOC-L, PEG-DOC-L, and TPOS-DOC-L against HeLa cells at pH 7.4 and 6.4 were estimated by MTT assay. A total of 100 μl of HeLa cells at 3 ×104/well was seeded in 96-well plates and incubated for 48 h to allow the cells to adhere to DMEM culture medium(containing 10% FBS). The spent medium was discarded, and the cells were incubated with DOC-L, PEG-DOC-L, and TPOSDOC-L at 0.025, 0.05, 0.25, 0.5, 2.5, 5 and 25 μg/ml equivalent drug concentrations for 48 h at pH 6.4 and 7.4, respectively. Cell layers were washed with cold PBS and further incubated with 10 μl of MTT solution (5 mg/ml in PBS) for 4 h at 37 °C. After the MTT medium was removed, 150 μl of DMSO was added to each well of transformed MTT crystals, and the absorbance of the transformed MTT solution in the wells was measured at 490 nm by using a microplate reader (Molecular Devices, USA).The cell survival rate was calculated by using the following formula.
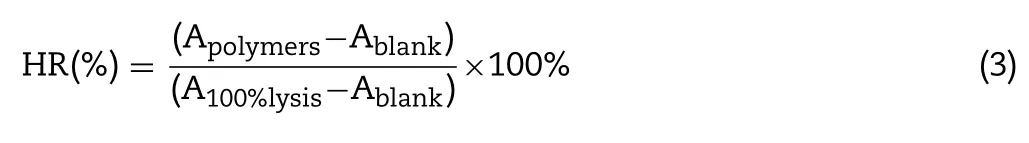
where OD liposomes is the absorbance intensity of the cells treated with different DOC-liposomes and OD control is the absorbance intensity of the cells incubated with the culture medium.
2.9. Qualitative analysis of cellular uptake by fluorescence microplate reader
For qualitative cellular uptake analysis, HeLa cells at 1 ×105/well were seeded in six-well plates (with cell climbing pieces) and incubated for 24 h in an incubator (Thermo Scientific, USA) at 37 °C under an atmosphere of 5% CO2and 90%relative humidity. After the cells reached 80% confluence, the medium was changed to the suspension of C6-L, PEG-C6-L,and TPOS-C6-L at C6 concentration of 100 ng/ml, and then incubated for 1, 4, and 6 h at pH 6.4 or 7.4, respectively. The spent medium was then discarded and the cells were washed three times with PBS. Finally, the cells were immersed in 50 μl of 0.5% Triton X-100 in 0.2 N NaOH solutions for cell lysis. After 15 min incubation under gentle shaking, the fluorescence intensities were measured with a microplate reader (Genios,Tecan, Switzerland) at an excitation wavelength of 455 nm and an emission wavelength of 506 nm. Cellular uptake efficiency was expressed as the percentage of cells-associated fluorescence after washing versus the fluorescence present in the feed suspension.
2.10. Quantitative analysis of cellular uptake by flow cytometry
The flow cytometry analysis was used to estimate the cellular uptake of the C6-L, PEG-C6-L, and TPOS-C6-L. Typically,HeLa cells at 1 ×105/well were seeded in six-well plates, After 70% -80% confluence was reached, the medium was changed to the suspension of C6-L, PEG-C6-L, and TPOS-C6-L at C6 concentration of 100 ng/ml, then incubated for 4 h at pH 6.4 or 7.4 at 37 °C, respectively. After incubation, the cells were washed thrice with cold PBS. Then, the cells were collected by adding 1 ml trypsin solution to each well, and the cell suspension was centrifuged at 1000 ×g for 5 min. Another 500 μl of PBS buffer was added to restructure the cells and was measured by a flow cytometry analysis (Beckman Coulter, USA). The events collected were ten thousand.
2.11. Intracellular delivery
The intracellular delivery of the liposomes in HeLa cells, such as endosomal escape, was evaluated by confocal laser scanning microscopy (CLSM) (ECLIPSE-Ti, Nikon, Japan). The cells(1 ×105/well) were seeded in six-well plates (Costar, USA)and were cultured at 37 °C for 24 h, followed by cell incubation with different C6-loaded liposomes at C6 concentration of 100 ng/ml for 4 h. The spent culture medium was discarded, and the cells were washed thrice with cold PBS.The nuclei were stained by incubating with DAPI for another 10 min prior to microscopic observation. The cells were fixed with 98% acetone after washing twice with cold PBS for 1 h. The cell monolayer was observed by CLSM with imaging software.
2.12. Apoptosis assay
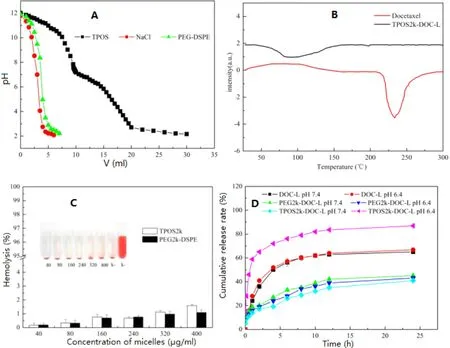
Fig. 3 -(A) Acid titration profiles obtained by titrating aqueous solutions of PEG-DSPE and TPOS (4 mg/ml) in 0.01 M NaCl solution(pH 12, adjusted with NaOH) with 0.01 M HCl. (B) DSC assay of DOC and DOC-loaded liposomes. (C) Hemolysis of the different TPOS or PEG-DSPE micelles at various concentrations. Data are represented as the mean ±SD ( n = 3). (D) in vitro DOC release profile from the DOC-L, PEG-DOC-L and TPOS-DOC-L. pH 7.4 or 6.4 PBS (0.1 M) with 0.1% (w/v) Tween 80 was employed as the release medium. Results are presented as mean ±SD (n = 3).
HeLa cells at 1 ×105/well were seeded in 24-well plates in 500 μl of culture medium and allowed to attach for 24 h.Then, the culture medium in wells was replaced with serumfree medium adjusted to pH 7.4 or pH 6.4 containing different DOC-loaded liposomes (DOC-L, PEG-DOC-L and TPOSDOC-L) with the DOC concentrations of 1 μg/mL; PBS solution was used for the negative control group. The incubation of the cells was at a humidified atmosphere containing 5% CO 2 at 37 °C for 12 h. Then the cells were washed with PBS solution twice and stained with Hoechst 33258 for 30 min under the protection from light. After washing with PBS twice, the apoptosis assay was performed by fluorescence microscope.
2.13. Statistical analysis
The data are expressed as the mean ±SD. Statistical significance was determined by using one-way analysis of variance.Statistical significance for all tests was set at*P < 0.05 and**P < 0.01.
3. Results and discussion
3.1. Buffering capacity of PEG-DSPE and TPOS
Nanoparticles (liposomes or macromolecule polymers) entered the tumor cells mainly through energy-dependent endocytosis, followed by transporting into the endosomes/lysosomes [26-28] , and these nanoparticles were degraded easily by the acid and hydrolase from endosomes/lysosomes. Estimation of the buffering capacity of vectors was essential to determine whether liposomes could induce endosomal escape and release the drugs into the cytoplasm. As shown in Fig. 3 A, TPOS displayed a slower downtrend and gentler slope in the titration curve than PEG-DSPE over the pH range 7.4-5.0, indicating that TPOS exhibited a good buffering capacity. pH was consistent with the pH from the physiological condition of lysosomes, whichmeant that TPOS would be pH-sensitive in the acidic pH environment of cancer cells.
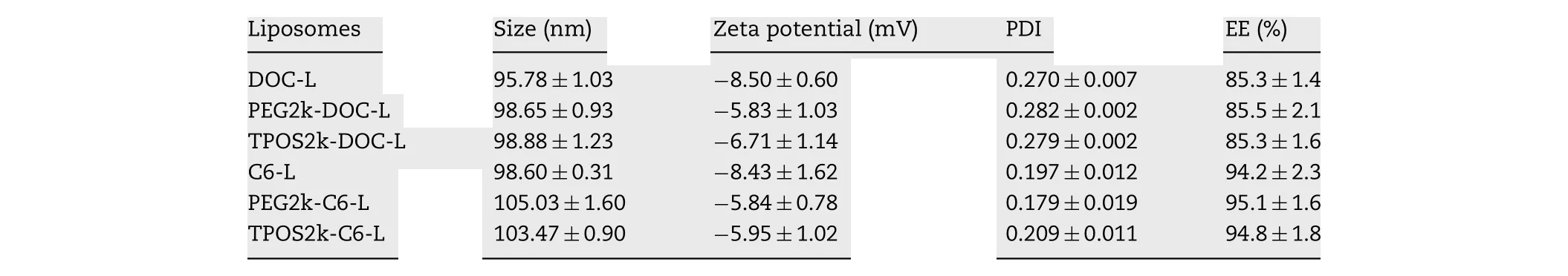
Table 1. -Particle size, polydispersity, zeta potential and encapsulation efficiency of the liposomes.
To obtain a sustained and controlled drug delivery, a liposomal formulation with a newly synthesized TPOS polymer was developed in this study. In comparison with PEG-L,the characteristic of TPOS-L in vitro was evaluated. Nanoparticles (liposomes or macromolecule polymers) entered the tumor cells mainly through the energy-dependent endocytosis, followed by transporting into the endosomes/lysosomes[24,25] , and these nanoparticles were degraded easily by the acid and hydrolase from endosomes/lysosomes. The estimation of the buffering capacity of TPOS was essential to determine whether TPOS-L could induce the endosomal escape and release drugs into the cytoplasm. In this study, TPOS exhibited a satisfactory buffering capacity over the pH range 7.4-5.0.pH was consistent with the pH from the physiological condition of endosomes and lysosomes [17,29] , which meant that TPOS would be pH-sensitive in the acidic pH environment of the cancer cells. The pH sensitivity of TPOS was attributed to PEtOz. As shown in Fig. 2 D, the carbonyl group hada nonshared electron pair. When pH decreased, the carbonyl group binds to the hydrogen proton and undergoes protonation, and the charge transfer changes “N”to “N+”, which induces the molecular chain possesses positive charges. The transfer of charge density and the greater steric hindrance around the carbonyl group make the polymer molecules change from hydrophilicity to hydrophobicity in an acidic environment. As a result, the hydrophobic polymer would be embedded in the lipid bilayer and would cause the damage of the liposomes,followed by the release of the drugs into the cytosol. In our earlier study, we found that the liposomes that were modified by PEtOz with different lipids also showed pH sensitivity[18-20] .
3.2. Particle size, zeta potential and EE analysis
The particle size and surface charge affect the phagocytic uptake of liposomes and determine the behavior of liposomes in vitro and in vivo [17] . Research showed that particles with a diameter of less than 200 nm could be accumulated in the tumor tissue passively through the EPR effect for the special structure of tumor vessels [30,31] . The mean particle size, polydispersity, and zeta potential of the liposomes are listed in Table 1 . Several types of liposomes have similar average diameters at approximately 95-103 nm, which was favorable for tumor accumulation via the EPR effect [32] . The liposome diameters increased slightly when the liposomes were modified by PEG-DSPE or TPOS. The zeta potential showed that all the liposomes are negatively charged. The absolute value of the zeta potential of PEG-DSPE and TPOS-coated liposome decreased compared with non-coated liposomes. The EEs of DOC-L and C6-L were 85.3% ±1.4% and 94.2% ±2.3%, respectively,and the addition of TPOS or PEG-DSPE had no influence on EE.
3.3. Morphology of liposomes
The morphology of liposomes was examined by Su8010 scanning electron microscope (Hitachi, Japan). The images showed that all the liposomes are generally spherical in shape with a mean diameter of approximately 100 nm ( Fig. 4 ).
3.4. DSC analysis
DSC analysis was conducted to investigate the physical state of DOC in the TPOS-coated liposomes, which could influence both the in vitro and in vivo drug release profile of the liposomes. As shown in Fig. 3 B, the melting endothermic peak of free crystalline DOC appeared at approximately 230 °C. However, thermograms of TPOS-coated DOC liposomes have no such melting peak. Therefore, DOC in liposomes possibly exists as an amorphous or disordered crystalline phase, or in the solid solution state.
3.5. In vitro hemolysis of TPOS or PEG-DSPE micelles
As shown in Fig. 3 C, the hemolysis rate of the two micelles increased slightly with an increase in the concentration, but all of them were less than 5%. In this study, hemolysis of synthetic TPOS was evaluated using the human red blood cells(RBC), which was more convincing than the animal RBC, and the human RBC could simulate the in vivo environment more accurately. From the results of in vitro hemolysis test, TPOS and PEG-DSPE weresimilar and considering an increase in their amounts, the hemolysis rate could be controlled within 5%.The TPOS is similar to PEG-DSPE, which is safe to meet the requirements of intravenous injection.
3.6. In vitro drug release
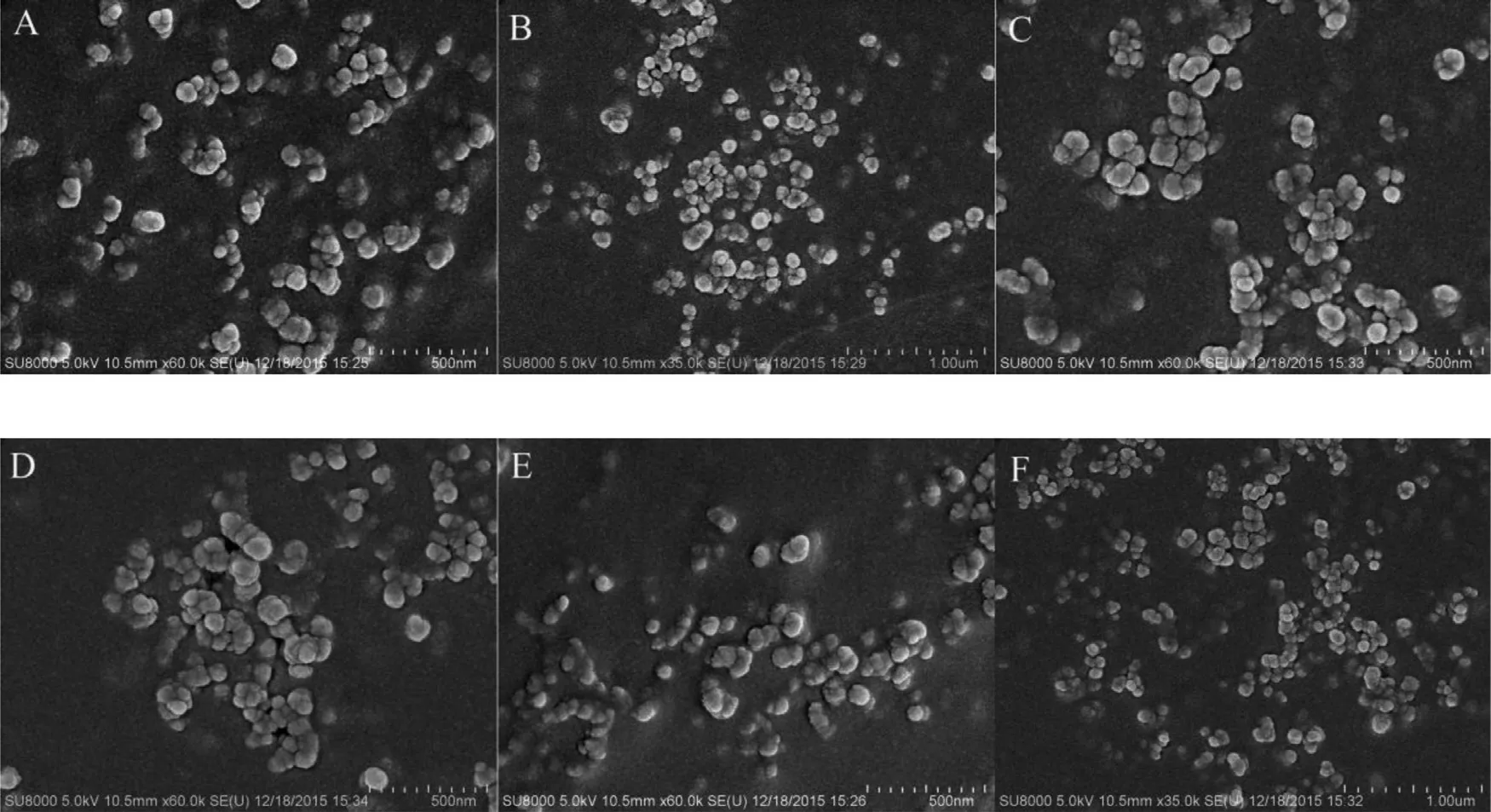
Fig. 4 -SEM observation of liposomes. (A) C6-L; (B) PEG-C6-L; (C) TPOS-C6-L; (D) DOC-L; (E) PEG-DOC-L; (F) TPOS-DOC-L.
Fig. 3 D shows the accumulated percentage release of DOC from the DOC-L, PEG-DOC-L, and TPOS-DOC-L in PBS (pH 7.4 or 6.4) containing 0.1% (w/v) Tween 80. The result showed that no significant initial burst release occurs at pH 7.4 for all liposomes, suggesting that these liposomes were stable at the physiological condition. The change of pH had no pronounced effect on the release profile of DOC-L and PEG-DOC-L. Notably, TPOS-DOC-L showed a slower drugs release kinetics at pH 7.4 (40.91% ±1.12% within 24 h incubation) similar to PEGDOC-L, whereas the release amount reached approximately 86.92% ±1.69% at pH 6.4. The release condition could be attributed to the structural conversion of PEtOz under acidic conditions, resulting in the disruption of the liposomes and encapsulated drug release.
Scientific rigorous in vitro experiments could help the researchers in evaluating the in vivo properties of the preparation. in vitro release test is a kind of drug release experiment in which the preparation is carried out under the environment of simulated temperature, medium condition, and movement state. In this manuscript, the pH sensitivity of the TPOS-modified liposomes was determined by an in vitro release test in different pH. The results showed that the release of TPOS-DOC-L increased significantly with decreased pH, and DOC-L and PEG-DOC-L pH sensitivity was not observed under acidic conditions. Thus, TPOS could provide the liposome a satisfactory pH-sensitivity.
The environment in vivo is complex and varied, the conventional liposomes are easy to be absorbed and degraded in the process of in vivo circulation, which leads to decreased drug efficacy and also the side effects on the normal tissues. To improve the stability of the conventional liposomes, the researchers modified the surface of the liposome with the amphiphilic polymer, such as PEG-DSPE [30,31] . To induce the protective effects of PEG chain, the coating on the surface of the liposomes should achieve a minimum thickness. The coating thickness is usually related to the MW, chain density, and conformation of PEG [6] . Most studies have shown that the MW of PEG chains on the surface of the liposomes is more than 2000 Da to avoid the endocytosis of mononuclear phagocyte system (MPS). Researchers also have found that the minimum effective hydrodynamic layer's thickness is approximately 5% of the nanoparticle diameter or more than twice the hydrodynamic radius of the polymer in the dilute solution[33,34] . The liposome stability, the modification of the chain length and the length of the polymer chain on the surface of the liposome need further study. In the present study, the MW of PEG-DSPE and TPOS were both 2 kDa, and both of them were 5% for liposome.
3.7. Qualitative analysis of cellular uptake by fluorescence microplate reader
The intracellular uptake of liposomes at 1, 4, and 6 h were examined by fluorescence microplate reader. As shown in Fig. 5 A, the cell uptake of C6-L, PEG-C6-L, and TPOS-C6-L did not vary between 4 and 6 h, indicating that the uptake of the three liposomes reached saturation after 4 h, and prolonging the incubation time which had no significant effect on cell uptake. Under the similar time and pH conditions, the order of cell uptake of different liposomes was as follows: TPOS-C6-L > C6-L > PEG-C6-L. Compared with C6-L and PEG-C6-L, the uptake of TPOS-C6 at pH 6.4 is significantly higher than that of TPOS-C6-L at pH 7.4, which further demonstrates that TPOSC6-L has pH-sensitivity.
3.8. Quantitative analysis of cellular uptake by flow cytometry
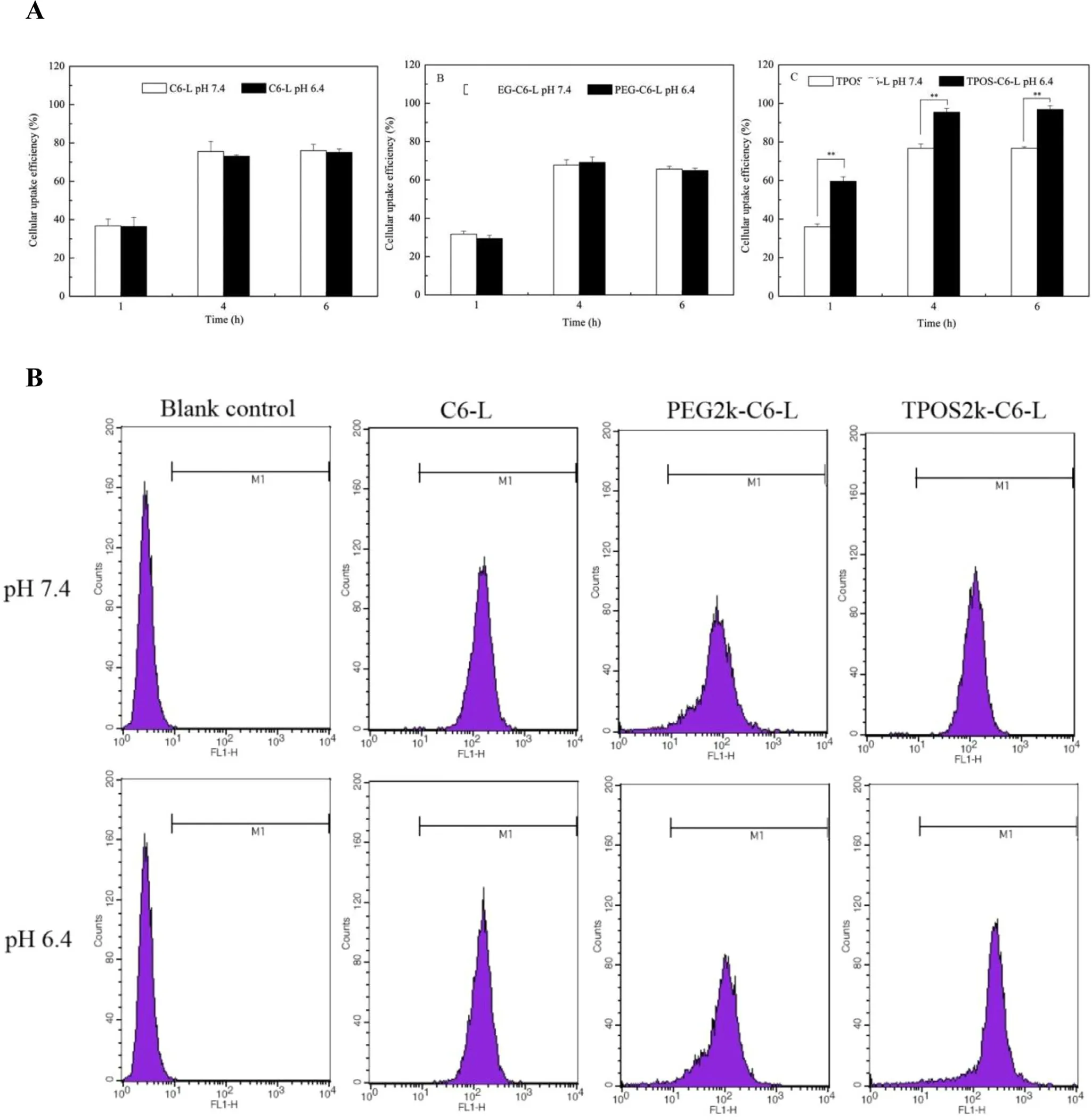
Fig. 5 -(A) Cellular uptake efficiency of C6-L, PEG-C6-L and TPOS-C6-L at different time points. Results are presented as mean ±SD, n = 3 ( *P < 0.05, **P < 0.01). (B) Flow cytometry measurement of C6 internalized into Hela cells after incubated with different C6-loaded liposomes for 4 h at pH 6.4 or 7.4.
In the attempt to demonstrate quantificationally that TOPS-L could facilitate the tumor cellular uptake, HeLa cells were incubated with different C6-loaded liposomes at pH 7.4 and 6.4.As shown in Fig. 5 B, the cellular uptake of TPOS-C6-L at pH 6.4 was higher than that at pH 7.4 suggesting that the uptake of TPOS-C6-L is pH-dependent. In contrast, the cellular uptake of PEG-C6-L and C6-L showed no noticeable change at pH 7.4 and 6.4. Remarkably, compared with TPOS-C6-L,PEG-C6-L exhibited a weaker cellular uptake as a result of the reduced affinity to the cells by the PEG shell, regardless of pH.
3.9. Endosomal escape of the liposomes
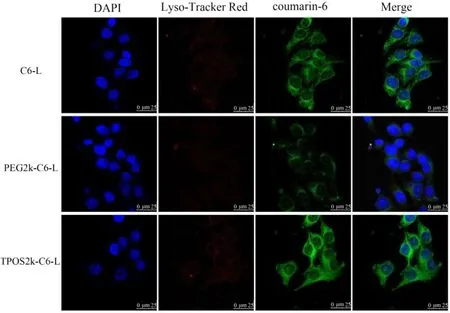
Fig. 6 -Confocal laser scanning microscopy (CLSM) of Hela cells after 4 h incubation with the C6-L, PEG-C6-L, TPOS-C6-L.The cell nuclear were stained by DAPI. The late endosomes and lysosomes were stained by LysoTracker Red. Blue fluorescent: cell nuclear, red fluorescent: endosomes/lysosomes, green fluorescent: coumarin-6. Scale bar represents 25 μm.(For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
The intracellular trafficking and “endosomal escape”of the different liposomes were evaluated by CLSM in HeLa cells after 4 h of incubation. In the CLSM images, lysosomes were selectively stained by LysoTracker Red as a red fluorescence,whereas the liposomes encapsulating C6 were observed as a green fluorescence. Co-localization of the liposomes with the endosomes was viewed as a yellow fluorescence. The nucleus was stained as a blue fluorescence. As shown in Fig. 6 , PEG-C6-L and C6-L had relatively high co-localization spots of the green fluorescence and red lysosomes at 4 h,indicating that the PEG-C6-L and C6-L were mainly located in the lysosomes, and thus, these liposomes had a difficulty in entering the endosomes. By contrast, TPOS-C6-L showed a broader cytoplasmic release and distribution at 4 h, judging from the nearly total separation between the green and red fluorescence. Green fluorescence intensities of TPOS-C6-L were stronger than that of PEG-C6-L and C6-L at the same period, in accordance with the results of the flow cytometry and fluorescence microscopy.
Cellular uptake was investigated either qualitatively or quantitatively in vitro . C6 has the advantages of a high laser conversion rate, stable performance, and high fluorescence intensity [35-37] . In addition, C6 is a lipophilic drug, which can be prepared by thin-film dispersion method to obtain liposomes with a high EE. Therefore, C6 can be used as a model drug to characterize the intracellular behavior of the liposomes. In this study, C6 was selected as a fluorescent probe to investigate the molecular biology of liposomes. The results indicated that the cellular uptake of TPOS-C6-L was increased with decreased pH compared with C6-L and PEG-C6-L ( Fig. 5 ). The intracellular distribution of liposomes was further observed by CLSM. Contrary to PEG-C6-L, the green fluorescent of C6-L and TPOS-C6-L was localized in the cytoplasm ( Fig. 6 ). These results proved that the modifications of TPOS introduced into the liposomes play a role in the improvement of the cellular uptake capabilities outside the tumor cells. When liposomes were internalized into the cells, the TPOS promoted the liposome destabilization, leading to the endosomal escape and cytoplasmic release for the efficient intracellular trafficking in mildly acidic endosome microenvironment. Here,the cellular uptake or endosomal escape of PEG-C6-L was the weakest.
3.10. In vitro cytotoxicity of DOC-loaded liposomes
The MTT assay was employed in HeLa cells to evaluate the cytotoxicity of the TPOS-DOC-L, PEG-DOC-L, and DOC-L under the similar conditions of pH 7.4 or pH 6.4. As shown in Fig. 7 , the cell viability of TPOS-DOC-L, PEG-DOC-L, and DOC-L reduced with an increase in the DOC concentration at different pH values. The cytotoxicity of the TPOS-DOC-L was comparable to that of PEG-DOC-L and DOC-L at the same DOC concentration at pH 7.4, and much lower than those at pH 6.4 after 24 h of incubation. IC 50 value of TPOS-DOC-L at pH 7.4 was 0.876 μg/ml, and nearly 0.051 μg/ml at pH 6.4. However,IC50value of the other two liposomes had no obviously change at different pH (IC 50 values of PEG-DOC-L at 7.4 and 6.4 were 2.249 and 1.694 μg/m, and IC 50 values of DOC-L at 7.4 and 6.4 were 1.488 and 1.200 μg/ml, respectively). These results indicate that more DOC was delivered by the TPOS-DOC-L,and the internalized DOC was effective in killing the tumor cells.
3.11. Cell apoptosis
Cell apoptosis is a determining factor for the drugs to work. Here, cell apoptosis triggered by different DOC-loaded liposomes was identified qualitatively by observing the mor-phology of the stained nuclei using fluorescence microscope.Fig. 8 shows the fluorescence images obtained for apoptotic cells at pH 7.4 and 6.4. In apoptosis, the condensation and the fragmentation of chromatin occur. Then, the nuclei lose their round or oval shape and become crescent shape followed by fragmented-apoptotic bodies. The apoptosis of PEG-DOC-L and DOC-L had no remarkable improvement from pH 7.4 to pH 6.4. In contrast, the TPOS-DOC-L significantly induced the apoptosis of HeLa cells with the decrease in pH. TPOS-DOC-L has enhanced the apoptosis more significantly than PEGDOC-L and DOC-L at pH 6.4 after 12 h of treatment defined by the occurrence of crescent nuclei and apoptotic bodies, which is consistent with the MTT analysis. Thus, the TPOS-DOC-L could achieve much better in vitro therapeutic effects than the PEG-DOC-L and DOC-L at the microenvironment of tumor cells.
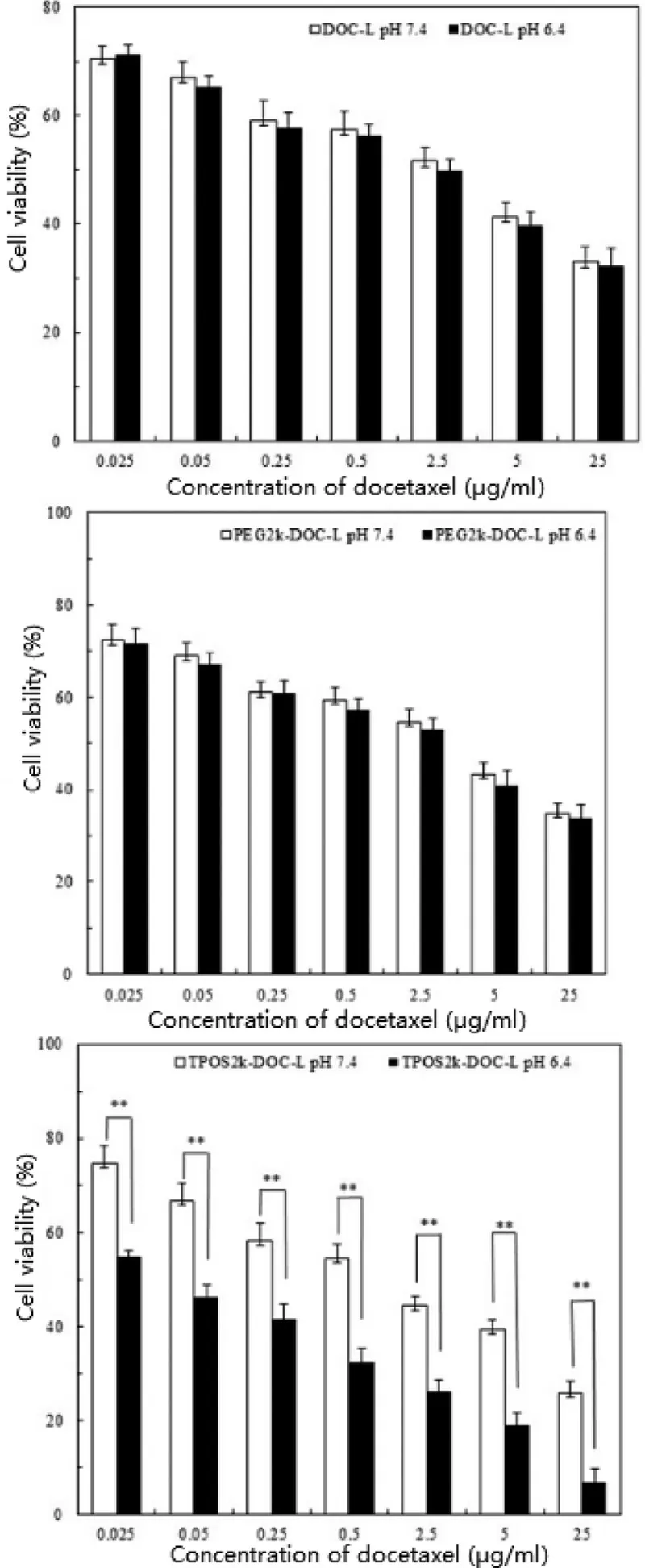
Fig. 7 -Viability of Hela cells was determined by MTT assay when cells were incubated with liposomes encapsulated different concentration of DOC at various pH for 48 h.Results are presented as mean ±SD, n = 3 ( **P < 0.01).
D- α-tocopheryl hemisuccinate ( α-TOS) is a semisynthetic prototype of α-tocopherol representing the most developed VE analog and is extensively studied as the model agent used in various biological and physicochemical experiments[38] . Recently, it has also been reported that α-TOS has anticancer properties against a wide variety of human cancer cells [39] . Furthermore, researchers found that the antiproliferative activity of α-TOS was less potent in normal cell types [40,41] .These results suggested that α-TOS could be clinically useful as an anticancer agent. However, its therapeutic efficacy was limited by the low solubility in aqueous solvents. Amphiphilic TPGS was synthesized by conjugation PEG with α-TOS to improve the bioavailability of α-TOS. Mi et al.[42] found that the empty TPGS2k carrier material could also show cytotoxicity to MCF7 breast cancer cells compared with drugs loaded with TPGS2k micelles. Youk et al. [43] proposed that the apoptotic activity and the antitumor efficacy of TPGS were more potent than that of TOS. They assumed that apoptotic activity of TOS could be altered positively or negatively depending on the type of modification created in its succinyl moieties. Hydrophilic modifications, such as PEG conjugation,could exert positive effects, whereas hydrophobic modifications could negatively affect the apoptotic activity of TOS.Similar to PEG, PEtOz is a safe, non-toxic, amphipathic, and biocompatible polymer. Thus, we further estimated the viability of the empty TPOS-L ( i.e. with no docetaxel) of the same content as those in the four designed drug concentrations at cell viability assay. As expected, the empty TPOS-L showed certain cytotoxicity compared with docetaxel-loaded TPOS-L after 24 h of incubation (data not shown). On this basis, we intuitively evaluated the effects of an empty TPOS-L ( i.e. with no docetaxel) or TPOS-L ( i.e. with docetaxel) on the induction of the apoptosis of HeLa cells by fluorescence microscopy. Surprisingly, empty TPGS2k-L could also facilitate the cell shrinkage and chromatin condensation of HeLa cells and induce the cell apoptosis at the same conditions. These results provided a new knowledge for the application of nanocarriers that the use of TPOS as a delivery system for anticancer drugs could improve the therapeutic efficacy of cancer treatments. Liposomes modified by TPOS could play a synergistic effect with an antitumor drug such as docetaxel. The ability of α-TOS and TPGS in inducing the cell apoptosis is associated with its increased ability to induce ROS generation and apoptosis [43] .Such process could be suppressed by the normal cells because tumor cells are generally more resistant to oxidative stress[44] . The inhibition mechanism of the empty TPOS-L to tumor cells needs further research.
4. Conclusion
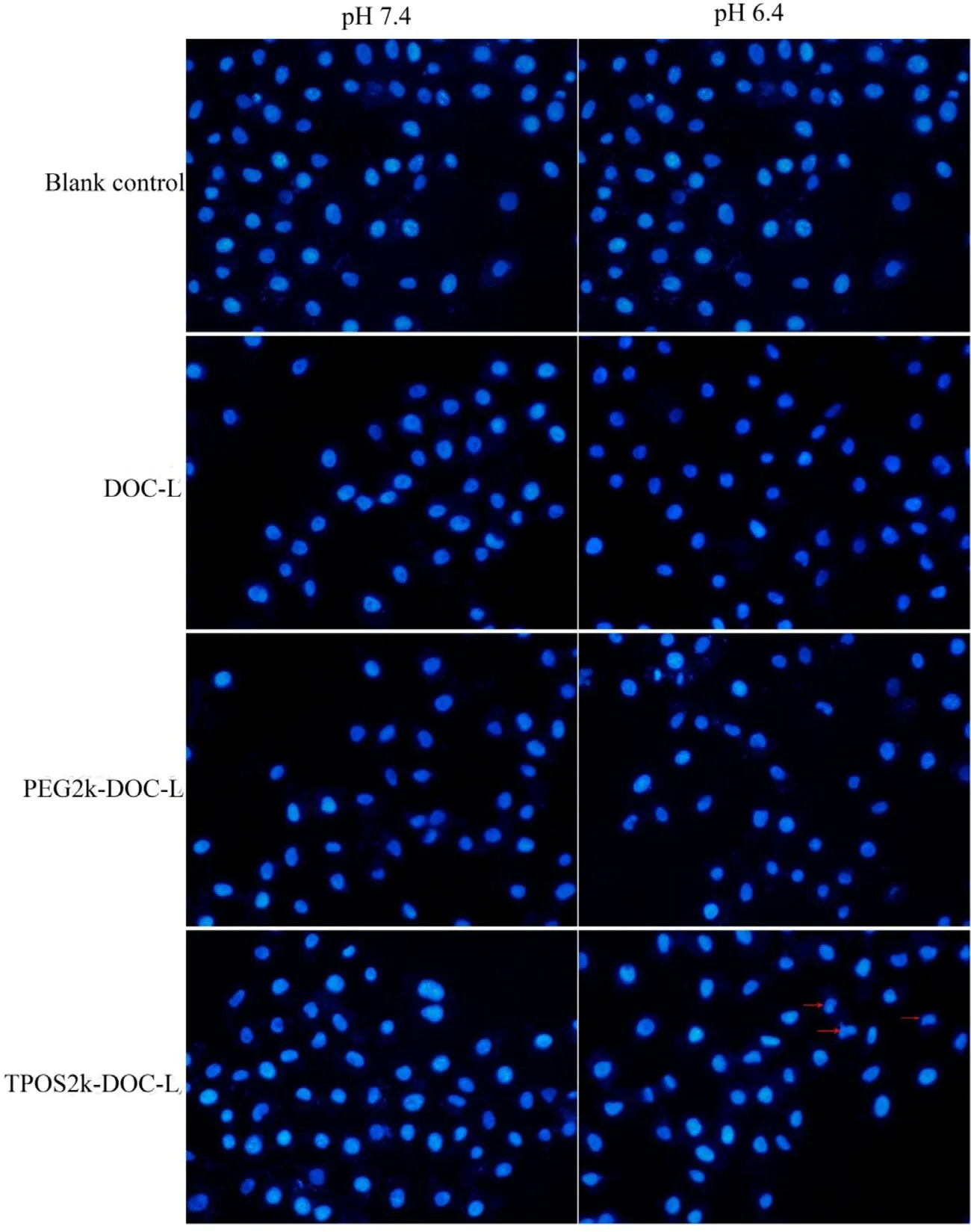
Fig. 8 -Apoptosis assay of Hela cells after 12 h incubation with the DOC-L, PEG-DOC-L, TPOS-DOC-L at pH 7.4 or 5.4 by fluorescent microscope. Red arrows indicate nuclei shrinking and noticeable nuclear condensation. The nuclei were stained by Hoechst 33258. Cells treated with PBS were used as blank.
In summary, PEtOz was conjugated with α-TOS by esterification reaction to obtain TPOS and was applied for the modification of liposomes. PEtOz is a kind of water-soluble polymer with acid sensitivity and stability. Similar to most of the polymer materials such as PEG and PEtOz with certain MW could form a three-dimensional barrier in the shell of the preparation (hydrophilic protective film and steric hindrance). In this study, the authors investigated whether TPOS could overcome the “PEG dilemma”that were reported in recent years.The experimental results showed that TPOS had satisfactory biocompatibility and stability and pH sensitivity. The conventional and PEGylated liposomes were used as controls. TPOS could significantly improve the stability of liposomes and promote the endosomal escape of liposomes in an acidic environment and the rapid release of drugs to the cytoplasm. In general, the amphiphilic TPOS is a promising polymeric material in developing a drug delivery vehicle.
The molecular weight of polymer TPOS was determined to be 2000 and the amount of modification to liposome was 5%,and the effect of the polymer on liposomes was evaluated.There is no definite conclusion about the favorable molecular weight and amount of TPOS chain modification on DOCliposome surface according to the literature. A series of study on TPOS will be carried out in the future.
Conflicts of interest
The authors declare that there is no conflicts of interest.
Acknowledgement
This work was supported by National Natural Science Foundation of China ( 81102394 ) and Natural Science Foundation of Liaoning Province ( 20170540575 ).
杂志排行
Asian Journal of Pharmacentical Sciences的其它文章
- The appliances and prospects of aurum nanomaterials in biodiagnostics, imaging, drug delivery and combination therapy
- Current developments in drug delivery with thermosensitive liposomes
- The application of nitric oxide delivery in nanoparticle-based tumor targeting drug delivery and treatment
- Amino functionalized chiral mesoporous silica nanoparticles for improved loading and release of poorly water-soluble drug
- Optimizing pH-sensitive and time-dependent polymer formula of colonic pH-responsive pellets to achieve precise drug release
- A hybrid genipin-crosslinked dual-sensitive hydrogel/nanostructured lipid carrier ocular drug delivery platform
