Locoregional treatments for hepatocellular carcinoma: Current evidence and future directions
2019-09-05RiccardoInchingoloAlessandroPosaMartinMariappanStavrosSpiliopoulos
Riccardo Inchingolo, Alessandro Posa, Martin Mariappan, Stavros Spiliopoulos
Abstract Liver cancers are the second most frequent cause of global cancer-related mortality of which 90% are attributable to hepatocellular carcinoma (HCC).Despite the advent of screening programmes for patients with known risk factors,a substantial number of patients are ineligible for curative surgery at presentation with limited outcomes achievable with systemic chemotherapy/external radiotherapy. This has led to the advent of numerous minimally invasive options including but not limited to trans-arterial chemoembolization,radiofrequency/microwave ablation and more recently selective internal radiation therapy many of which are often the first-line treatment for select stages of HCC or serve as a conduit to liver transplant. The authors aim to provide a comprehensive overview of these various image guided minimally invasive therapies with a brief focus on the technical aspects accompanied by a critical analysis of the literature to assess the most up-to-date evidence from comparative systematic reviews and meta-analyses finishing with an assessment of novel combination regimens and future directions of travel.
Key words: Hepatocellular carcinoma; Cirrhosis; Liver; Interventional oncology; Transarterial chemo embolization; Selective internal radiation therapy; Ablation
INTRODUCTION
Hepatocellular carcinoma (HCC) represents 75%-90% of primary liver malignancies and is a major cause of worldwide mortality[1]. According to the National Cancer Institute, estimated HCC deaths for the year 2016 in the United States were 27170,while the incidence of the disease will continue to increase until 2030. Acknowledged risk factors are closely related with life-style choices and include chronic hepatitis B and C virus infection, fatty liver disease, cirrhosis, diabetes, obesity, and smoking[2-4].The prognosis of HCC remains poor, especially if diagnosed at an advanced stage,while mainstream curative options for very early and early stage HCC in good surgical candidates are liver transplantation and surgical resection. Nevertheless, at the time of diagnosis, a substantial number of patients are ineligible for surgical treatment, due to intermediate or advanced disease stage or severe comorbidities which increase the surgical risk[5]. Unfortunately, the prognosis of HCC following systemic pharmacotherapy, external radiotherapy or plain supportive treatments is also poor. As a result, various percutaneous, image-guided, locoregional therapies,have emerged in order to improve outcomes, initially among inoperable patients[6,7](Table 1). After decades of thorough investigation and clinical experience in the field of interventional oncology, numerous minimal invasive treatment options have been developed and include: (1) Curative modalities such as percutaneous radiofrequency ablation (RFA), microwave ablation (MWA), percutaneous ethanol injection (PEI),cryoablation (CA), irreversible electroporation (IRE); and (2) Palliative therapies such as bland trans-arterial embolization (TAE), conventional trans-arterial chemoembolization (TACE) or chemoembolization with drug-eluting beads (DEB-TACE)and more recently local endovascular radiotherapy via the trans-arterial delivery of beta-emitting microparticles (selective internal radiation therapy; SIRT). Moreover,the effectiveness of various combinations of locoregional treatments with or without systemic chemotherapy has been also investigated, aiming in down staging inoperable disease or increasing overall survival rates and improving quality of life[7-10]. This review analyses currently available locoregional treatment options for HCC and highlights their importance in the development of more efficient treatment algorithms.
TACE
Transcatheter arterial chemoembolization (TACE) represents the therapeutic goldstandard in patients unsuitable for surgery and for percutaneous ablation techniques,with multinodular HCC and preserved liver function, without vascular invasion or extra-hepatic spread (intermediate stage, BCLC-B)[11,12].
The TACE treatment is based on the occlusion of the arterial blood supply of the target neoplastic lesion by embolizing microparticles, combined with the injection of chemotherapeutic drugs in a super-selective manner, sparing the adjacent healthy liver[13-16].
There is great variety, in the literature, among therapeutic protocols, and middle-/long-term results are poor, mostly due to the tumour burden, incomplete embolization, and presence of undetectable satellite lesions[17,18].
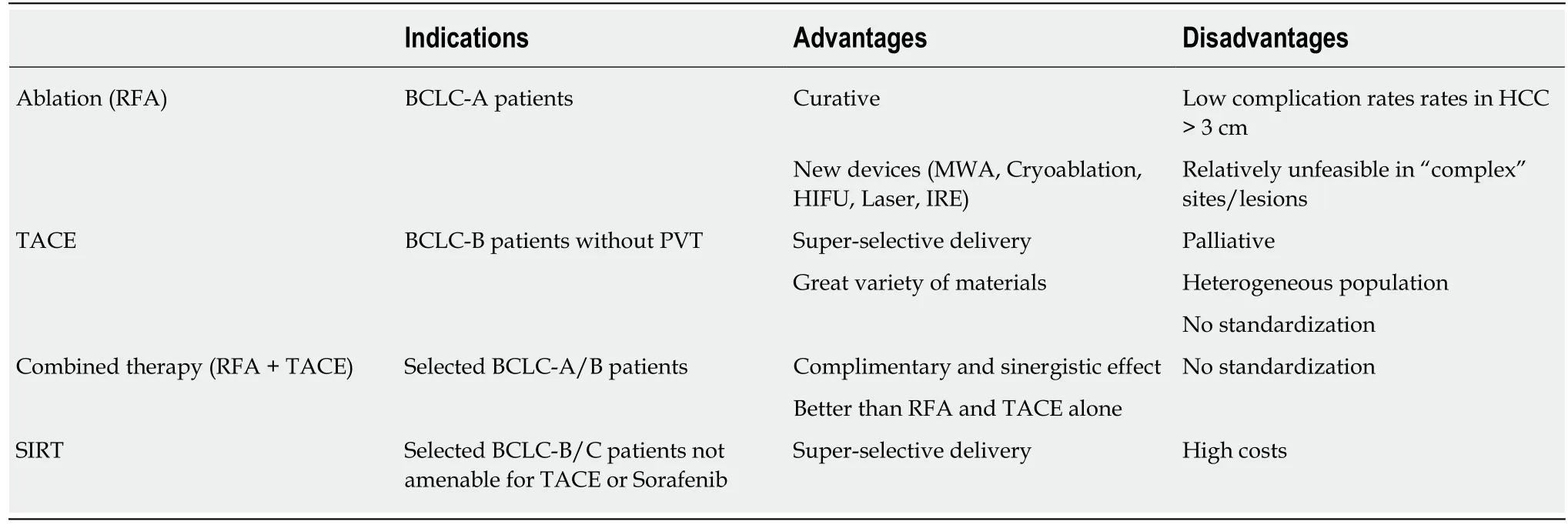
Table 1 Codified locoregional treatments: lndications, advantages and disadvantages
Nonetheless, BCLC stage B includes a heterogeneous population, with different tumour burden, as well as greatly different liver function (from Child-Pugh class A5 to B9), that cannot all be treated with the same weapons; there is, therefore, the need to perform individualized and personalized treatments. The ideal TACE target is represented by BCLC stage B asymptomatic patients with preserved liver function and without portal vein thrombosis, which are suitable for a more complete and effective treatment[19,20].
Less than 2% of patients obtain a complete response after the first TACE treatment,due to the presence of viable tissue and neo-angiogenesis which allows the continuous growth of the neoplasm; therefore, TACE should be performed more than once, at regular intervals[21], however, there is no consensus nor guidelines on the correct number on TACE treatments and on the time interval between TACE sessions,leaving the choice in the hands of the operators, with the expert's suggestion of “ondemand” treatments with 1- or 2-mo interval between sessions and of ceasing TACE after 2-3 unsuccessful sessions[22].
Meta-analysis and randomized controlled trials have demonstrated that treatment response is associated with a good 2-years patient's survival (about 60%), even though there is great heterogeneity among these trials in terms of patient's characteristics, treatment modalities and materials[23,24].
The principal contraindication to TACE treatment is the presence of a poor venous blood supply from the portal vein (mostly due to chemical or neoplastic thrombosis of the main portal vein or of its lobar and segmental branches, as well as porto-systemic anastomosis and hepatofugal portal flow), due to the increased risk of ischaemic necrosis of the liver and thus liver failure. In a similar manner, patients with advanced hepatic disease (Child-Pugh class B and C) should not be considered for TACE due to their increased risk of liver failure and death[25].
Adverse effects of selective transarterial administration of the chemotherapeutic drugs may be similar those seen with systemic administration: Nausea, vomiting,myelotoxicity, alopecia, and kidney failure.
Hepatic artery occlusion, causing acute ischaemia of the HCC lesion is associated,in more than 50% of the patients, with post-embolization syndrome, which usually lasts less than 48 hours and is characterized by fever, abdominal pain, and slowed peristalsis: Fever is determined by the tumoral necrosis with cytokines release. A small percentage of patients can present with infectious complications such as hepatic abscess or cholecystitis[20].
Various chemotherapic agents have been utilized for TACE, the most common choices being doxorubicin and cisplatin[20].
Liu et al[26]showed in a randomized trial that TACE with the use of more than one chemotherapic drug has a better efficacy on overall survival and overall response rates when compared to doxorubicin monotherapy.
One of the greatest matters of debate when dealing with TACE, is the choice between conventional TACE (c-TACE) and drug-eluting beads TACE (DEB-TACE). c-TACE is performed with the infusion of a suspension of iodized oil mixed with the chemotherapic drug; the iodized oil acts as a carrier for the drug, and undergoes selective uptake by the neoplastic cells, increasing HCC exposureto the drug,followed by administration of the embolic particles[25,27-29]. On the other hand, DEBTACE uses embolizing polyvinylchloride microspheres of different sizes, pre-loaded with the chemotherapic drug, which are injected in the tumour feeding artery releasing the drug in the neoplastic bloodstream, in a sustained, prolonged, and predictable manner; this determines a reduction of collateral effects due to the passage of the chemotherapeutic agent into the systemic circulation. Moreover, due to the predetermined microparticles' calibre, the arterial occlusion is a predictable and homogeneous process, increasing the anti-neoplastic activity and safety profile[30-35](Figure 1).
Burrel et al[34]observed that 1-, 3- and 5-years survival in a group of patients treated with DEB-TACE was 89.9%, 66.3% and 38.3% respectively, with a median survival time of 48.6 mo.
Various studies evaluated and comparedthe efficacy of c-TACE versus DEB-TACE,as the PRECISION-V trial, which showed higher - even though not significant -complete response, objective response and disease control rates in DEB-TACE, and a significant reduction in liver toxicity and doxorubicin-related side effects; other studies did not confirm that superiority. Therefore, the comparison between c-TACE and DEB-TACE is still a matter of debate[36-38].
A recent improvement to TACE was made with the introduction of embolizing microparticles containing iodine atoms in their structure, thus visible during fluoroscopy, granting a precise and controlled delivery of the chemotherapeutic drug during the treatment[39].
Furthermore, patients with intermediate-stage HCC (BCLC-B) and impaired liver function, or with portal vein thrombosis/invasion, can nowadays benefit froma particular form of chemoembolization, based on degradable starch microspheres(DSM-TACE), which carry the chemotherapic drug but are rapidly digested once delivered in the hepatic blood stream, reducing the ischaemic effect on the liver parenchyma[40,41]. This treatment has also been performed as a second-line treatment in BCLC-C stage patients ineligible forthe anti-angiogenic drug Sorafenib, with similar results in terms of progression-free survival (6.4 months) and overall survival (11.3 months), and of 1- and 16.5-months overall disease control of 80% and 52.5%respectively[42].
ABLATION
Ablative techniques using chemical or thermal energy have been developed and established in the loco-regional therapy of hepatocellular carcinomas over the last three decades[43,44]. The 2017 European association for the study of the liver (EASL)clinical practice guidelines on the management of HCCs recommend the use of ablative therapy in very early stage (single lesion < 2 cm) and early stage (single or 2-3 nodules < 3 cm) cancers amongst patients who are not candidates for surgical resection or transplant[44,45]. Prototypical amongst ablation therapies was PEI[46], used to cause coagulative necrosis of the lesion via cellular dehydration. By the early 90s however, the advent of RFA offered better survival and local disease control versus PEI[47,48]with the latter demonstrating local recurrence rates exceeding 43% in lesions >3 cm[49], but retaining a role in the management of tumours < 2 cm where thermal ablation is not feasible. RFA is now established as the first-line ablative therapy while concomitant advances have been made with MWA and CA. Newer technologies such as laser ablation (LA) and irreversible electroporation (IRE) remain under investigation for their efficacy[44-46].
In RFA, an alternating electric current between (460-500 kHz) is applied to the target lesion via a radio-frequency (RF) electrode placed under imaging/laparoscopic guidance and returning through grounding pads on the skin surface. An induced electromagnetic field causes oscillation of tissue ions and frictional heating leading to coagulative necrosis and cell death at temperatures of 60-100 °C[50,51]. Overall efficacy of RFA is limited by local tissue charring, which increases impedance, limiting the zone of ablation, and the well described ‘heat-sink' effect whereby, slow in vivo heat transfer from the electrode is counteracted by local high flow vascular perfusion[50].Strategies to mitigate these limitations include the use of internal electrode cooling and the use of bipolar mode with multiple electrodes to create overlapping ablation zones though these carry an increased risk of bleeding and adjacent organ damage[50,51]. Imaging guidance is generally achieved with the use of B-mode ultrasound (Figure 2), however one study by Kim et al[52]found up to a third of lesions were not visible on this modality alone with increased use of fusion imaging with volumetric CT/MRI data to circumvent this challenge[53]. An inadequate acoustic window may be improved by infusion of fluid into the pleural or peritoneal cavity with the added benefit of minimizing adjacent organ injury. Consideration must be given to the location of the tumour and RFA should be avoided in lesions with close proximity to other abdominal viscera or in a peripheral subcapsular location[54]. For patients with very early/early stage HCC (overall size < 3 cm) RFA is the principle loco-regional therapeutic option in contrast to transplantation or surgical resection(SR). Comparison of the outcomes between RFA and SR has yielded several inconclusive studies in the literature; however results from a 2014 Cochrane review by Weis et al and three other contemporaneous systematic reviews and meta-analyses[55-58]demonstrate similar overall survival at 1 and 3 years between RFA and SR groups for tumours < 2 cm in subgroup analysis. Conclusions about recurrence rates in this cohort are contradictory amongst the various studies with recurrence generally lower following resection which is associated with longer in hospital stay and more overall complications. Cucchetti et al[56]also demonstrate a favourable cost analysis of RFA over SR in this subgroup of patients. Amongst early stage tumours (2-3 cm, up to 3 nodules), Pompili et al[59]demonstrated no significant difference in survival (RFA 66.2% vs SR 74.4%, P = 0.353) or cumulative recurrence (RFA 57.1% vs SR 56.0%, P =0.765) at 4 years, though a trend was noted towards lower recurrence in the resection group. These findings were further confirmed with propensity score matching to minimise confounding factors for overall survival and recurrence (P = 0.450 and P =0.152 respectively) with similar results demonstrated in a more recent RCT of 218 patients by Ng et al[60]. RFA therefore remains the mainstay of ablative treatments very early and early stage HCC despite the dearth of large scale multi-centric RCTs in this field.
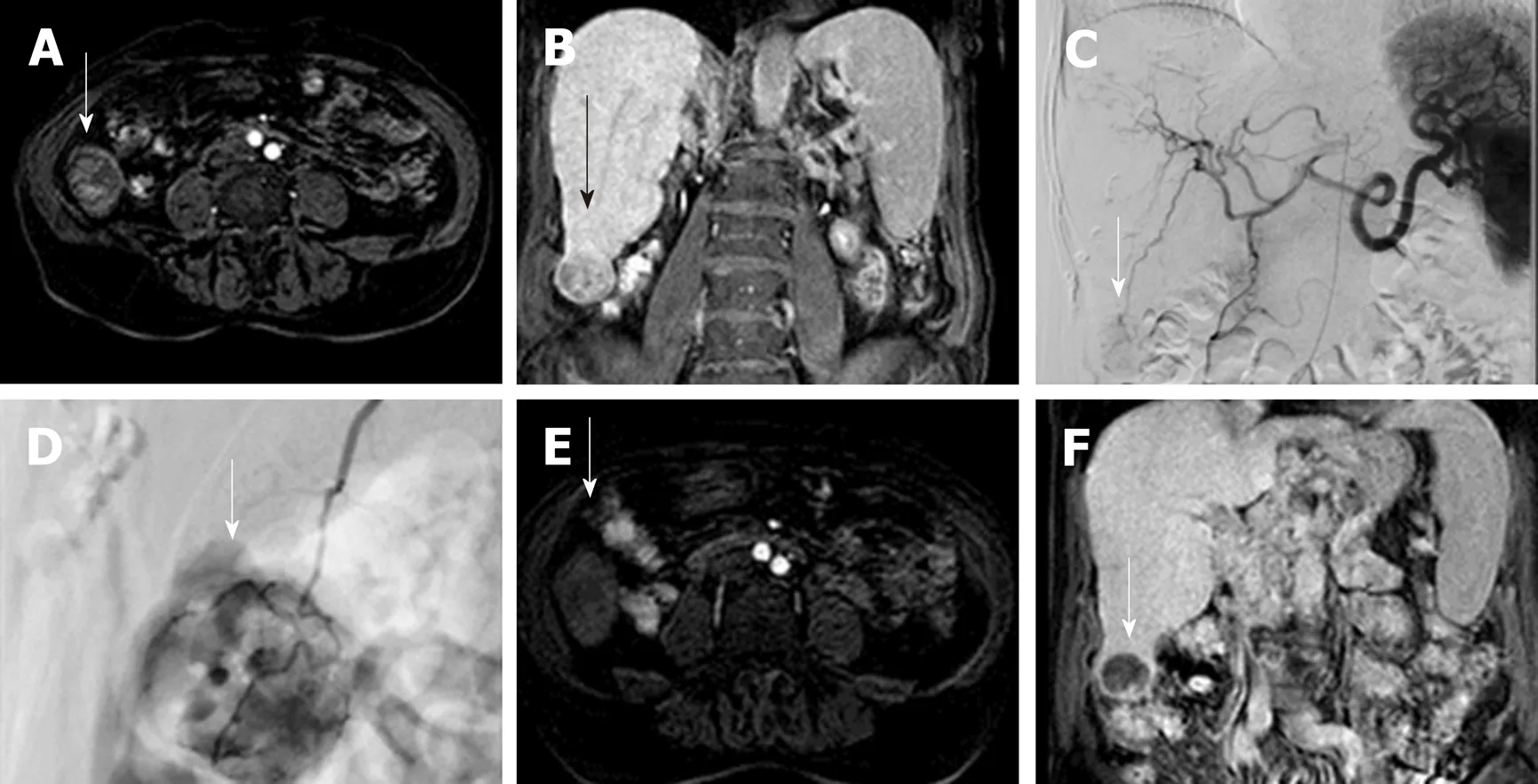
Figure 1 Drug Eluting Beads-Trans Arterial Chemo Embolization of 3 cm hepatocellular carcinoma. A: Gd-EOB- DTPA enhanced MR image of a 73 years old cirrhotic patient with a 3-cm exophytic liver nodule in segment VI(arrow), showing enhancement in the arterial phase; B: the nodule is hypointense in comparison to the surrounding liver parenchyma in coronal hepatobiliary phase, in keeping with HCC. C and D: Celiac axis DSA showing thehypervascular lesion (arrow); selective microcatheterization of the feeding vessel with infusion of doxorubicin-loaded drug-eluting beads (200 μm). E and F: 2-mo follow-up Gd-EOB- DTPA enhanced MR image demonstrating absence of arterial enhancement of the nodule and marked hypointensity in coronal hepatobiliary phase (arrow).
Microwave ablation was initially developed to work around the heat-sink and tissue impedance limitations of RFA within the liver[50]. While the underlying mechanism of cell death in MWA is similar to RFA, tissue temperature is raised by causing the continuous realignment of polar (principally water) molecules within an oscillating microwave field at frequencies of 915/2450 MHz[50,61]. Microwaves radiate equally through all biological tissue without impedance allowing a much larger volume of tissue to be heated with each application. The latest third generation systems incorporate antenna cooling and high-power generators in combination with different antenna designs, which contribute to variable size and shape of the ablation zones necessitating careful planning on the part of the operator. With these attributes,MWA shares a similar application profile as RFA but with advantages over the latter with regards to larger lesions or those closer to blood vessels and other visceral structures[62]. Given its relative novelty there is a lack of high powered studies comparing its efficacy to RFA and only 2 meta-analyses assessing outcomes of HCC treatment between the modalities[63,64]which largely included the same studies with similar cumulative numbers of approximately 400 patients in the MWA and RFA arms apiece. Chinnaratha et al[63]demonstrated no difference in local recurrence/progression between RFA and MWA with pooled OR (95%CI) 1.01 (0.67-1.50, P = 0.98) or overall survival at 3 years with pooled OR (95%CI) 0.76 (0.44-1.32, P= 0.33). Complete ablation was achieved in MWA at rates of between 91%-100%between studies. Of note, a subgroup analysis of the use of MWA in HCC beyond the Milan criteria (single tumor > 5 cm or > 3 nodules) in 450 patients demonstrated lower local tumor progression over RFA with pooled OR (95%CI) 1.88 (1.10-3.23, P = 0.02)supporting the use of MWA in larger lesions. Facciorusso et al excluded lower powered studies and congress abstracts finding no significant difference in local recurrence with OR (95%CI) 1.01 (0.53-1.87, P = 0.98), higher (though insignificant)overall survival at 3 years, OR (95%CI) 0.95 (0.58-1.57, P = 0.85) and a non-significant higher rate of complete ablation in MWA (P = 0.67). A systematic review by Lahat et al[65]analysing major complications (defined as symptoms persisting for > 1 wk,delaying discharge, causing significant morbidity/disability and death) following percutaneous ablation reported mortality of 0.15% and 0.23% for RFA and MWA respectively with major complications occurring in 4.1% and 4.6% of cases respectively the most common being haemorrhage but also including portal vein thrombosis, bile leak/duct injury, intestinal/diaphragmatic injury and liver abscess/dysfunction. Ding et al[66]found no statistically significant difference in types or number of complications between MWA and RFA in their large retrospective analysis of 879 patients (P > 0.05). Ultimately while MWA has shown promising results for local disease its proven benefits over RFA are limited and further study is required.
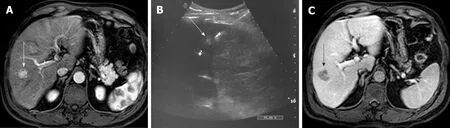
Figure 2 US-guided radiofrequency ablation Radio-Frequency Ablation of hepatocellular carcinoma. A: Gd-EOB- DTPA enhanced MR image of a 57 years old cirrhotic patient with a 1.5-cm liver nodule in segment VIII (arrow), showing enhancement in the arterial phase, in keeping with HCC. B: US image demonstrating the RF needle, with a 3 cm exposed tip, crossing the lesion (arrow). C: 1-mo follow-up portal-venous phase Gd-EOB- DTPA enhanced MR image demonstrating the oval shaped ablation zone (arrow).
In addition to RFA and MFA, several novel modalities of ablation are entering clinical practice including CA, LA, IRE and high-intensity focused ultrasound (HIFU).Cryoablation causes tumour necrosis by freezing at temperatures between -35 °C to-20 °C using the Joule-Thomson theory of expanding gases within a needle-like cryoprobe causing cooling at the probe tip[50]. Heat transfer to probe is by passive diffusion and so probe surface area limits cooling capacity. Procedures are therefore usually carried out with several probes with ablation times of up to 25-30 min with the advantage of precise intra-procedural monitoring of ice ball formation on image guidance (CT/MRI) and the ability to include larger ablative zones[67,68]. Cryotherapy is susceptible to the “cool-sink” effect whereby thermal energy exchange is disrupted near the cryoprobe owing to adjacent vascular structures[51]and the possibility of the serious and possibly life threating complication of Cryoshock whereby ablation of large tumours with large ablation volume eliciting an aggressive inflammatory response with pleural effusions, thrombocytopenia, haemorrhage, myoglobinemia and renal/respiratory failure[67]. A recent meta-analysis by Luo et al[69]including several cohort studies and one RCT demonstrated high rates of complete ablation(73.3%-100%) and no statistically significant difference in recurrence rates or overall survival between CA and RFA. In their RCT included within the meta-analysis, Wang et al[70], demonstrated improved 3 year survival (11% vs 7 %, P = 0.043), and for lesions> 3 cm, significantly lower local progression (7.7% vs 18.2%, P = 0.041) in patients treated with CA rather than RFA indicating some possible benefit with CA in larger lesions which needs to be weighed against the potentially serious associated complications. LA remains poorly studied in comparison to most other methods of ablations. Light is delivered via multiple flexible quartz fibers within water-cooled laser application sheaths and LA has been demonstrated to be safe and feasible[62]. Luo et al[69]found higher tumor recurrence, lower overall survival and complete ablation in LA vs RFA however none of these were statistically significant.
IRE is a recently developed non-thermal ablation technology that uses low-voltage,high-energy, electrical pulses to induce cell death by pore creation within the cell lipid membrane. The procedure is performed using multiple monopolar 19-gauge probes and, due to its non-thermal nature, represents a valid alternative to thermal for perivascular lesions and those in proximity to large bile ducts. However, despite the fact that initial results following IRE for HCC are optimistic for selected < 3 cm lesions,and the safety profile of the method has been established, data remain limited. As a result, application of liver IRE for the treatment of HCC requires further investigation and its clinical application for the moment remains limited[71].
The paucity of literature assessing HIFU limits conclusions that can be drawn about its effectiveness.
RFA remains the mainstay of ablative therapy at present with further high-quality randomised studies needed to evaluate the performance of the newer modalities in comparison.
SIRT
SIRT or also known as trans arterial radio-embolization (TARE) is a well-recognised loco-regional treatment modality used in patients with intermediate-stage or advanced stages of HCC (BLCL - B/C) in patients who are not eligible or cannot tolerate TACE/Sorafenib respectively[72,73]. Similar to TACE, delivery of treatment relies upon the hepatic arterial predominant blood supply of HCCs (80%) thereby reducing its effect on normal hepatic parenchyma which derives most (75%) of its supply from the portal vein[74]. In contradistinction to TACE which uses a combination of chemotherapy and ischaemia, SIRT has a minor effect from microembolisation and principally acts by irradiation from Yttrium-90(Y-90) bearing microspheres though other radioactive substances such as 131-iodine labelled lipiodol[44]may be considered.Emission of a beta particle (maximum energy 2.27 MeV; mean energy: 0.94 MeV) with decay of 90Y to 90Zr (Zirconium) is able to deliver targeted radiation to the lesion limiting radiation exposure to normal parenchyma while reducing the risk of radiation induced liver disease (up to 50% of patients with 40Gy delivered)[74]. High energy beta radiation triggers DNA double strand breaks resulting in tumour cell damage. Pre-procedure planning requires a separate angiographic procedure delivering 99mTc macroaggregated albumin (MAA) at the most ideal arterial position to target the tumour (Figure 3), followed by single photon emission computer tomography (SPECT) to detect extra-hepatic uptake and assess lung shunt fraction(proportion deposited in lung potentially causing radiation pneumonitis). Dosage of Y-90 to be delivered is calculated based on factors including type of microspheres being used, percentage involvement of tumour within the liver, lung shunt fraction and overall liver mass estimate from cross-sectional imaging with multiple formulas available but not completely evaluated at present[75]. Intra-arterial CT angiography(hybrid angiography-MDCT or cone-beam CT) allows volumetric assessment of tumour coverage by the chosen vessel and non-target vessels close to this should be selectively coil embolised to prevent extra-hepatic passage of Y-90. Careful administration of the Y-90 is carried out in conjunction with a physicist to reduce exposure to personnel.
Of both available prospective RCT results comparing SIRT and TACE amongst intermediate stage patients, PREMIERE (n = 43) and SIRTACE (n = 28), the former demonstrated significantly longer time to progression for SIRT (14.5 mo vs 6.4 mo, P =0.0019) and no significant difference in overall survival (23.8 mo vs 17.7 mo, P =0.9772) and the latter (pilot) study, by Kollig et al, demonstrated similar efficacy and health-related quality of life (HRQoL)[76,77]. All other studies being retrospective, a meta-analysis by Lobo et al[78]demonstrated comparable overall survival and complication rates with one study by Soydal et al[79]demonstrating a survival advantage for SIRT (39 mo vs 31 mo, P = 0.014). Kollig et al[77]also described a similar profile of safety between TACE and SIRT. Y-90 has also been demonstrated to have a role in early stage cancers as a bridge to liver transplantation and in order to downstage tumours from United network for organ sharing stage T3 to T2 (58% vs 31%, P = 0.023)[80,81].
Sorafenib remains the mainstay of treatment in advanced stage HCC (BCLC - C).Results from the SIRveNIB comparing SIRT with Sorafenib demonstrated no statistically significant differences in overall survival however progression free survival and time to progression in patients treated with SIRT vs Sorafenib with a similar trend demonstrated in the SARAH trial[82,83]. Complication rates of up to 4.9%and a mortality rate of 1.5% were reported in one multi-centre Australian study[84].The most common complications were post-embolisation syndrome (0-70%) and Radiation-induced liver disease (0-31%). Other complications include biliary system damage and pneumonitis. Given both the SIRveNIB and SARAH trials were not able to statistically demonstrate superiority and the studies were not aimed at simply demonstrating non-inferiority, the status quo of Sorafenib as first line treatment for advanced stage HCC remains and further study is suggested in specific patient cohorts where SIRT may prove useful such as those with tumoral portal vein thrombosis. Further large-scale trials are required prior to conclusive guidelines regarding the use of SIRT in advanced HCC.
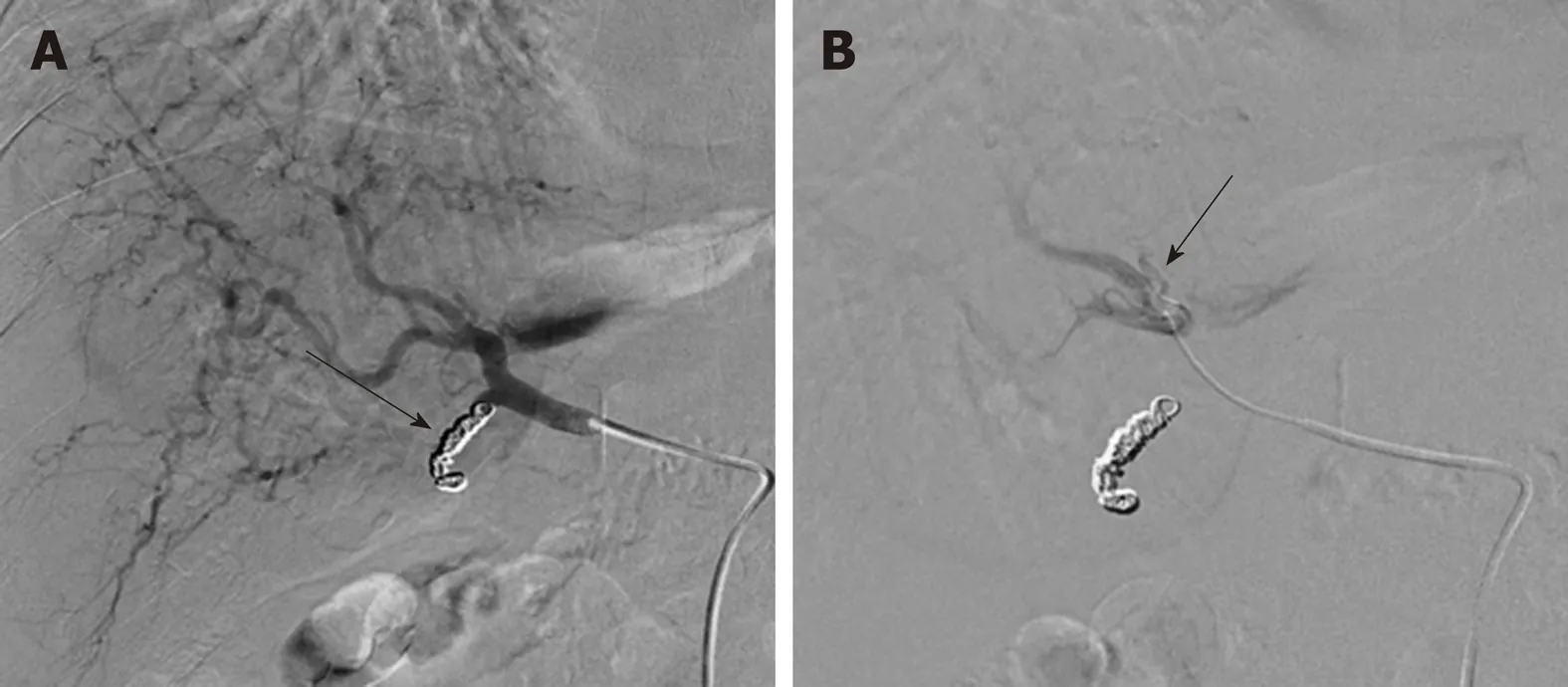
Figure 3 Tc-99m Macro-Aggregates of albumin mapping procedure prior to Y-90 radioembolization. A:Selective common hepatic artery DSA following coil embolization of the gastroduodenal artery (arrow); and B:subsequent infusion of MAA with documentation of the exact position of the tip of the microcatheter at the right hepatic artery (arrow).
COMBINED TREATMENTS
The combined treatment of HCC lesions implies the utilization of both RFA and TACE; this approach is used in early and intermediate stage (BCLC-A or -B) patients with large (> 3 cm) unresectable HCC nodules. Combined treatments were mostly investigated in retrospective studies, showing, however, better results when compared to RFA or TACE alone, both in terms of complete response and disease-free survival rates, as well as being less time and cost-consuming than performing the two treatments alone[85].
In fact, even if RFA provides excellent results in terms of local disease control and represents a curative treatment for HCC lesions up to 3 cm, it is ineffective in HCCs larger than 3 cm in size, with low rates of complete response, and high rates of local recurrence even after an initial complete response, as showed by Peng et al. in a prospective randomized trial, which compared combined treatment versus RFA alone in HCC up to 7 cm: The combined treatment group had significantly better overall survival and recurrence-free survival rates than the RFA-alone group, with a 1-, 3-and 4-year overall survival of 92.6%, 66.6% and 61.8% vs 85.3%, 59% and 45%respectively, and a recurrence-free survival of 79.4%, 60.6% and 54.8% versus 66.7%,44.2% and 38.9% respectively[86].
On the other hand, TACE is considered a palliative treatment, showing decreased effectiveness with increasing size of the target HCC lesion, with only a few treated HCCs obtaining a stable complete response. High rates of local recurrence, generally being due to an incomplete embolization of the target lesion or due to tumoral neoangiogenesis[33].
Many authors have demonstrated the efficacy of the combined treatment in achieving complete tumour necrosis and increasing patient's survival rates, especially in HCC lesions larger than 3 cm[85].
RFA and TACE can be combined in different but complimentary and synergistic ways; however, it is not clear which is the best combination and the optimal time interval between TACE and RFA to enhance the synergic effect and balance local therapeutic efficacy, with preservation of safety and liver function.
In particular, performing RFA first allows use of the sublethal heating area surrounding the central post-ablative necrosis, where the residual neoplastic cells have increased vascular permeability and blood flow. This in turn grants better delivery of the chemotherapeutic drug and improves efficacy due to a lower cellular resistance, as well as a better treatment of satellite nodules[87,88].
On the flipside, performing TACE first reduces the heat-sink effect secondary to the blood flow in the adjacent vessels, amplifying the RFA treatment, even though there could be a greater degradation of the chemotherapic drug when exposed to the high temperatures of the RFA. In addition, performing TACE first could lead to reduced ultrasound visibility of the target lesion, impairing the correct RFA electrode positioning; this issue has been partly overcome with the introduction of cone-beam CT (CBCT), which allows an accurate RFA electrode positioning using multiplanar and three-dimensional reconstructions[89,90].
Nonetheless, the individual steps of RFA and TACE, of combined treatment can be performed in a single session or with a wide time interval (ranging from 1 to 30 d)[86,91-93].
Various meta-analysis have shown that the combined treatment determines a significant increase of patient's 1-, 3-, and 5-years overall survival rates when compared to RFA alone (P = 0.0004, 0.0002 and 0.0001 respectively), in particular when dealing with HCC lesions larger than 3 cm, as shown by the meta-analysis of randomized controlled trials performed by Lu et al., whereas there was no difference in terms of overall survival rates in HCCs smaller than 3 cm[94]. Moreover, even if there are ambiguous results, when compared to surgical resection, combined treatment seems to grant the same overall survival, even though surgical resection has better disease-free survival rates, in particular in lesions larger than 3 cm[95,96].
In recent years, the introduction of a new ablative technique represented by MWA,which overcomes the RFA limitations and can produce greater necrosis volumes, has also expanded the possibilities combined treatments; MWA plus TACE has beendemonstrated to have good complete response rates in HCC lesions up to 5 cm,with a better efficacy when compared to RFA plus TACE, even if there are only a few studies comparing these two kinds of combined treatment. For example, Sheta et al.,showed 1-month recurrence rates of 0% and 5% for MWA+TACE and RFA+TACE respectively, with similar complication rates[97-103].
One other great advantage of the combined treatment is the possibility to overcome the classical contraindications of the ablative treatment; in particular, when dealing with complex lesions, such as nodules located in unfavourable positions and so with a greater risk of complications, as well as in “complex” patients (those with a high risk of bleeding due to their cirrhosis which leads to a low platelet count), performing TACE after the ablative treatment allows prompt treatment of eventual post-ablative bleedings[88,102,103]. Additionally, performing TACE after RFA allows, treatment of those not-so-rare hypovascular HCC lesions, thanks to the vasodilator and hyperaemic effects of thermal ablation.
When considering combined therapies, in HCC lesions larger than 3 cm, the recently-introduced treatment with RFA plus intravenous systemic lysothermosensitive liposomal doxorubicin (LTLD) is worth a mention: The liposomes contains doxorubicin, and the RFA-induced target heating, when performed for more than 45 min, determines a high target drug concentration, almost 25 times greater than that of free doxorubicin[104,105].
A final, special mention is deserved for the combination of TACE and systemic chemotherapy with Sorafenib: The TACE-induced ischaemia determines the production of neoplastic angiogenic growth-factors that can be promptly blocked by the anti-angiogenic action of Sorafenib, with a good tolerability for the patients[106].Most trials, however, failed to show a significant advantage of combined therapy versus TACE alone in terms of overall survival and time to progression[107,108]. On the other hand, the TACTICS trial showed a very favorable result of its primary end-point(progression-free survival rates) in the TACE+Sorafenib group versus TACE alone[109].The reason of this different trend in comparison to the other trials can be identified both in the different primary end-point (Time-To-Progression (TTP) for SPACE, PFS for TACTICS), and in the different definition of Time-To-UnTACEable-Progression(TTUP, the time until TACE is no more effective or feasible): Even if both the SPACE and TACTICS trial considered vascular invasion and extra-hepatic lesions as a sign of unTACEable progression, TACTICS trial did not considered the development of a new liver lesion as tumour progression, whereas the SPACE trial added Child-Pugh B, persistent ascites and low platelet count as other criteria, limiting the treatment possibilities and leading to a precocious stop in sorafenib administration; moreover,in TACTICS trial, TACE was administered “on-demand” at the growth of the viable lesions, whereas, in SPACE trial, TACE sessions were scheduled, leading to less treatments[106,109].
Alternatively to Sorafenib, Kudo et al. investigated the efficacy of combination therapy between doxorubicin-TACE and brivanib, an inhibitor of vascular-endothelial and fibroblast growth factor given as an adjuvant for TACE; the trial did not show improvements in terms of OS between TACE plus brivanib and TACE plus placebo[110].
FUTURE PERSPECTIVES
The optimal treatment algorithm for the management of HCC is still under meticulous investigation, as survival and recurrence rates are still far from satisfactory, and many unresolved issues remain to be determined. Surgery and liver transplantation have for years provided the best results. However, recent advances in minimal invasive locoregional treatments are continuously gaining ground not only in the management of non-operable HCC but also in the curative treatment of small T1 lesions. This is attributed to the fact that surgical options are often not indicated due to the advanced stage of underlying cirrhosis, or other severe comorbidities.Notably, in patients with HCC the prevalence of cirrhosis ranges between 85%-95%.However, despite the AASLD suggestion for surgical resection over RFA for T1 or T2 lesions in patients with Child- Pugh class A cirrhosis, the effectiveness of other ablative techniques, such as MWV or stereotactic body radiation as well as their combination with TACE and SIRT should also be evaluated compared to surgery[2].Recurrence of HCC, especially in cases in which the ablated treatment margin was deemed sufficient (1 cm), could be attributed to non-visible microscopic satellite nodules. Histopathology studies following curative hepatectomy for lesions measuring from 2.5 to 5 cm, have demonstrated the presence of non-detectable microsatellite disease (portal vein invasion or intrahepatic metastasis) in 46% of the patients with a mean distance from the primary tumor of 9 mm (range 8 to 30 mm).The authors believe that more potent ablative technologies achieving treatment margins beyond 1cm as well as the combination of ablative/embolization therapies,in lesions measuring over 2.5 cm, would improve overall survival and recurrence rates due to the possibility to expand the necrotic zone to also include non-identified satellite lesions. Interestingly, according to a recent network metanalysis of RCTs,comparing available percutaneous locoregional treatments combined with local ablative or adjuvant systemic treatments for non-operable HCC, TACE combined with external radiotherapy or local ablation significantly improve patient survival and tumor response compared with embolization therapies alone, indicating the utility of the synergic effect provided by different locoregional treatments. However, the quality of evidence remains low to moderate and future RCTs to provide further level A evidence are required. The combination of locoregional modalities in the same patient in order to improve both quality of life and overall survival should also be assessed. Future studies should focus on the effect of the sequential alternation of treatments such as ablation, TACE and SIRT, based on the existing staging of the disease and the realistic therapeutic target.
Another major issue remains the lack of high-quality evidence to verify a significant survival improvement of chemo-embolization over bland embolization, or the superiority of other TACE techniques versus conventional TACE for intermediate stage HCC. Therefore, carefully designed multicenter RCTs comparing various embolization options are still awaited.
The authors believe that a major breakthrough in the management of HCC treatment would be the genetic characterization of HCC in every-day clinical practice in order to enable a personalized treatment plan. Today, non-invasive, liquid biopsy by sequencing cell-free DNA in plasma is currently under investigation and aims in the identification of driver mutations and tumor heterogeneity as to enable targeted HCC therapy. Published studies demonstrated variability in the efficacy of targeted agents in different populations and high inter-patient heterogeneity attributed to genetic mutations[111]. Personalized medicine could contribute in patient selection and individualized decision-making could optimize the outcomes of locoregional treatments.
CONCLUSION
To summarize, future research direction should focus on the combination of loco regional therapies. High quality data from multi-center RCTs are required to investigate the possibility of improving the overall survival in unresectable HCC by applying the available ablative and embolization techniques. Crucial issues regarding the combination of minimal invasive therapies that remain to be determined by largescale trials, include the choice between bland TAE, particle-mediated TACE, or conventional TACE, the choice to embolize prior or after ablation, as well as the optimal timing of embolization (same session? after 2 wk, etc.). Moreover, the authors strongly believe that the investigation of aggressive sequential alternation of various locoregional therapies in the ambit of personalized patient selection will provide evidence that could modify the existing therapeutic protocols and improve both quality of life and survival outcomes. Finally, radioembolization is a very promising therapy and the initial failure to improve survival in patients with intermediate or advanced HCC should not discourage investigators. Well- designed trials with better patient selection would certainly define its role in HCC treatment.
杂志排行
World Journal of Gastroenterology的其它文章
- New Era: Endoscopic treatment options in obesity-a paradigm shift
- Chronic hepatitis delta: A state-of-the-art review and new therapies
- Eosinophilic esophagitis: Current concepts in diagnosis and treatment
- Review of current diagnostic methods and advances in Helicobacter pylori diagnostics in the era of next generation sequencing
- Exploring the hepatitis C virus genome using single molecule realtime sequencing
- Surgical management of Zollinger-Ellison syndrome: Classical considerations and current controversies
