Colorectal cancer surveillance in inflammatory bowel disease:Practice guidelines and recent developments
2019-08-26WilliamClarkeJosephFeuerstein
William T Clarke, Joseph D Feuerstein
Abstract Patients with long-standing inflammatory bowel disease (IBD) involving at least 1/3 of the colon are at increased risk for colorectal cancer (CRC). Advancements in CRC screening and surveillance and improved treatment of IBD has reduced CRC incidence in patients with ulcerative colitis and Crohn’s colitis. Most cases of CRC are thought to arise from dysplasia, and recent evidence suggests that the majority of dysplastic lesions in patients with IBD are visible, in part thanks to advancements in high definition colonoscopy and chromoendoscopy. Recent practice guidelines have supported the use of chromoendoscopy with targeted biopsies of visible lesions rather than traditional random biopsies. Endoscopists are encouraged to endoscopically resect visible dysplasia and only recommend surgery when a complete resection is not possible. New technologies such as virtual chromoendoscopy are emerging as potential tools in CRC screening.Patients with IBD at increased risk for developing CRC should undergo surveillance colonoscopy using new approaches and techniques.
Key words: Inflammatory bowel disease; Colorectal cancer screening; Ulcerative colitis;Crohn’s disease; Colonoscopy; Chromoendoscopy
INTRODUCTION
Patients with long-standing inflammatory bowel disease (IBD) involving at least 1/3 of the colon are at increased risk for developing colorectal cancer (CRC). Traditional CRC screening and surveillance for these patients at increased risk with ulcerative colitis (UC) and Crohn’s colitis included random four quadrant biopsies every 10 cm.The 2015 SCENIC guidelines from the American Gastroenterological Association(AGA) and American Society for Gastrointestinal Endoscopy provided updated recommendations on how to screen for CRC[1]. This review will focus on updates for CRC screening in patients with IBD since the publication of the 2015 SCENIC guidelines, with an emphasis on high-definition (HD) scopes, dye and virtual chromoendoscopy (CE), and random versus targeted biopsies.
BACKGROUND AND EPIDEMIOLOGY
CRC in IBD patients is thought to be preceded by unequivocal neoplastic epithelial changes known as dysplasia. Early detection of dysplasia is a primary goal of endoscopic surveillance. Riddell and colleagues described a classification system of no dysplasia, indefinite for dysplasia, low-grade dysplasia (LGD), and high-grade dysplasia (HGD) that is still used today[2,3]. When the pathologist cannot distinguish between dysplastic and non-dysplastic atypia or inflammatory-associated changes,the sample is considered indefinite for dysplasia. LGD and HGD are differentiated based on the distribution of nuclei within the mucosa[4]. There is high inter-observer variability in grading dysplasia among even experienced gastrointestinal pathologists,so guidelines recommend all cases of suspected dysplasia be reviewed by a second gastrointestinal pathologist[5,6]. All dysplasia should be defined as invisible if obtained by random biopsies or visible if identified and removed or sampled by targeted biopsies[7]. Furthermore, visible lesions should be classified by the endoscopist as polypoid or non-polypoid, as per the Paris classification[1,8].
The incidence rate of CRC in IBD is approximately 18% after 30 years of colitis[9-11].However, recent population-based studies show a decreasing risk of CRC in IBD with improved medical therapy and CRC surveillance[12-15]. The risk of CRC begins approximately 7 years after diagnosis and increases linearly thereafter. Factors increasing the risk of CRC include diagnosis at a young age, longer duration of disease, and severity of intestinal inflammation[16-18]. Colonic strictures, pseudopolyps and a fore-shortened colon are all likely markers of prior inflammation and are associated with an increased risk of CRC[2,16,19-21]. Family history increases the risk of CRC in IBD patients approximately 2-3 fold[22,23]while primary sclerosing cholangitis(PSC) increases the risk of CRC and dysplasia with an odds ratio of 3.24 when compared to patients with IBD without PSC[24].
ENDOSCOPIC SURVEILLANCE
Multiple case-control studies and population-based cohort studies have shown that endoscopic surveillance improves CRC-related survival in IBD patients at increased risk for colon cancer[25-28]. Endoscopic surveillance is widely recommended by international gastrointestinal societies for the early detection and resection of dysplasia or CRC[1,2,29-32]. Societal recommendations differ in details including when to perform initial and subsequent surveillance colonoscopies, the optimal methods of detecting dysplasia, and the management of dysplastic lesions (Table 1). There is consensus that patients with PSC should undergo annual surveillance. Otherwise,societies recommend surveillance intervals ranging from every 1-5 years based on a number of risk factors including personal history of dysplasia, active inflammation,family history, and anatomic abnormalities such as inflammatory pseudopolyps,foreshortened colon and strictures.
Most guidelines recommend an initial screening colonoscopy with staging biopsies for all IBD patients 8 years after onset of symptoms to evaluate the disease extent and determine the need for ongoing surveillance[2,29,32]. All societies recommend ongoing surveillance colonoscopies for patients with UC and Crohn’s involving one-third of the colon or more than one segment. Dysplasia in IBD was previously thought to be flat and difficult to detect, so the historic recommended screening modality was white light endoscopy (WLE) with random four quadrant biopsies every 10 cm.
HD colonoscopy produces images with more pixels than standard definition (SD)colonoscopy, resulting in greater image detail. HD also allows for faster image refresh rates than SD, improving the display of moving objects[33]. HD colonoscopy has been shown to result in higher adenoma detection than SD colonoscopy in patients undergoing screening colonoscopy[34].
CE applies a blue contrast dye of indigo carmine or methylene blue to the colon epithelium, enhancing areas of mucosal irregularity and delineating borders of suspected lesions. Early studies including a 2013 meta-analysis by Soetikno et al[35]found CE with targeted biopsies of abnormal appearing mucosa detected dysplasia 8.9 times more often than WLE alone. Other studies showed most dysplastic lesions are visible and targeted biopsies are superior to random biopsies[35-37].
The 2015 SCENIC international consensus statement provided updated recommendations on how to screen for CRC in IBD with a focus on the use of HD colonoscopy and CE[1]. Since the publication of these guidelines in 2015, many further studies have been published to further investigate the ideal colonoscopy surveillance method for patients with IBD.
Chromoendoscopy
SCENIC recommends CE over WLE when using SD colonoscopy but only suggests the use of CE over WLE when using HD colonoscopy[1]. However, new evidence is conflicting as to the benefit of CE over WLE. Mooiweer et al[38]from the Netherlands published a retrospective study in 2015 of more than 2200 colonoscopies over nearly 14 years and found no benefit in dysplasia detection from the use of CE. A 2017 metaanalysis by Iannone and colleagues showed that CE is superior to WLE only when compared to SD WLE; when compared to HD WLE, there was no benefit to CE, and CE was associated with longer procedure times[39].
In support of CE is a prospective cohort study from Spain published in 2018.Carballal et al[40]evaluated each colonic segment first with WLE and then with CE; the authors reported that 57.4% of dysplastic lesions were identified only with CE. Wan and colleagues published a 2019 meta-analysis including eleven studies that found CE was superior to WLE in detecting nonpolypoid dysplastic lesions and that the incremental yield of CE for detection of dysplasia was 9%[41]. While statistically significant in both groups, the advantage to CE was greater in SD than in HD colonoscopy (relative risk 2.04 vs 1.60). A more recent study from Sekra et al[42]evaluating 110 consecutive patients in a New Zealand tertiary care center found higher rates of dysplasia detection (risk difference 11.8%, P = 0.008) and dysplasia detection rates per patient (risk difference 20.6 lesions per 100 patients, P = 0.003)when using CE.
Similarly, a meta-analysis by Feuerstein et al. showed that CE was more effective in finding dysplasia per patient undergoing colonoscopy compared to SD but not when compared to HD colonoscopy. The study further showed that when evaluating studies with randomized control design methodology there was no difference between CE and HD. However, when CE was compared to non-randomized control design methodology CE was significantly more effective than SD and HD colonoscopy. However, this finding was likely more related to study design bias[43].
Virtual chromoendoscopy
New technology in the field of virtual CE (VCE) is being actively investigated as an alternative to traditional dye-based CE. The SCENIC guidelines did not recommend the use of VCE with narrow band imaging (NBI) in place of WLE or CE[1], but new studies have shown promising results.
In 2017, Bisschops et al[44]published data showing no difference between NBI and CE with methylene blue in a multicenter prospective randomized clinical trial (RCT)of 131 patients with UC. In another RCT, Iacucci et al[45]studied 270 patients and found the Pentax (Tokyo, Japan) branded VCE called HD iSCAN was non-inferior to traditional CE and HD WLE for detection of neoplastic lesions. Based partly on this data, the 2019 American College of Gastroenterology Clinical Guidelines for UC recommend CE or NBI when using HD colonoscopes[46].
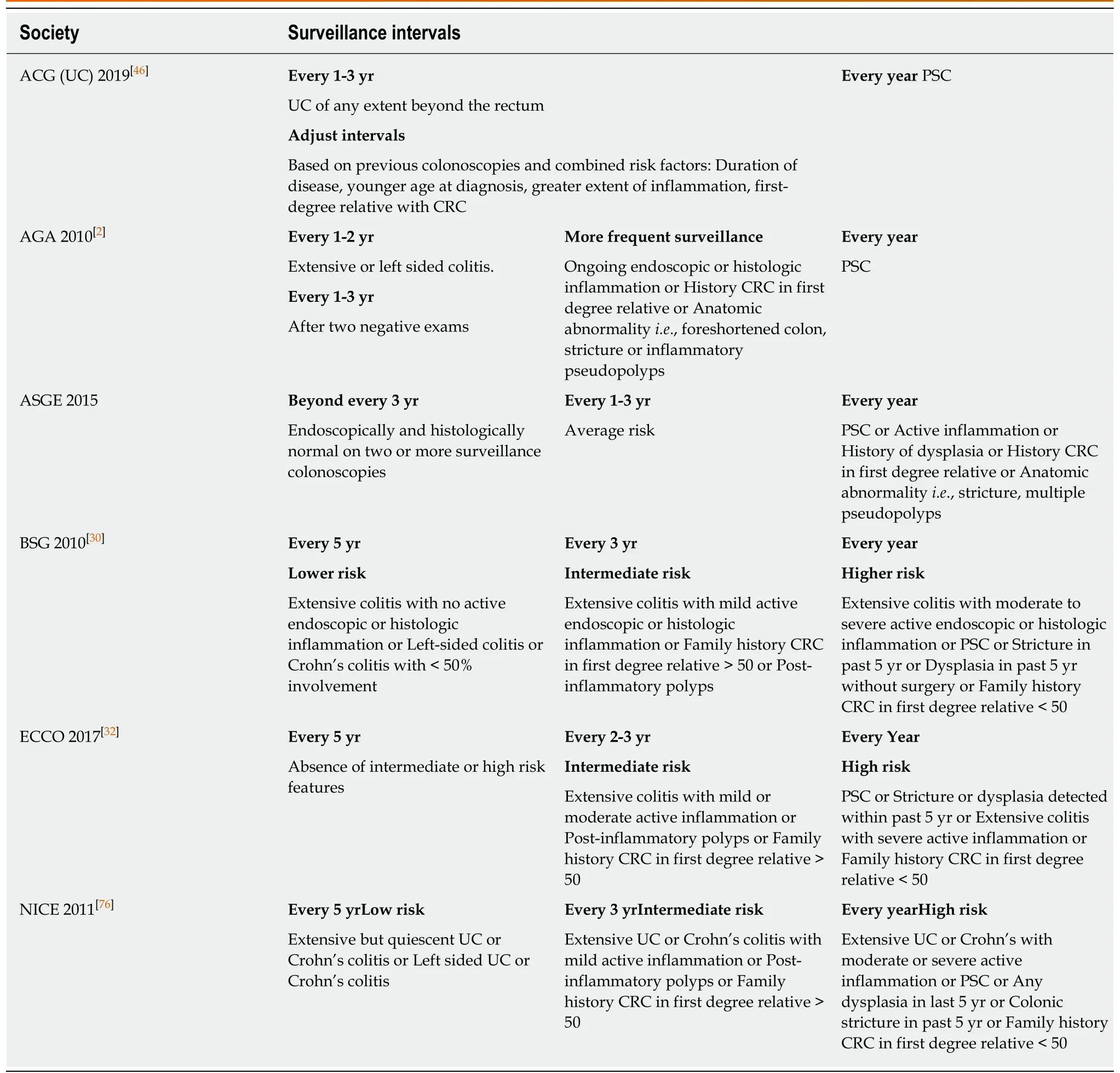
Table 1 Societal recommendations for colorectal cancer surveillance
In non-IBD patients, NBI was shown to increase adenoma detection rate over WLE in the general population[47], and iSCAN was shown to increase polyp detection in patients with Lynch syndrome, another group at high risk for CRC[48]. While the SCENIC guidelines suggest that NBI is not beneficial in the evaluation of CRC screening and surveillance in IBD, multiple studies have shown a potential benefit of this technique. A meta-analysis of these studies show no difference in dysplasia per patient when comparing NBI and dye based CE. Based on this data there may be a role for NBI when evaluating potentially suspicious dysplastic lesions.
VCE has potential uses beyond dysplasia detection. To assess the accuracy and interobserver agreement of pit pattern recognition, endoscopists were given pictures of lesions using CE with methylene blue or NBI. There was superior interobserver agreement differentiating between neoplastic and non-neoplastic lesions using NBI in comparison to CE[49]. Another study by Iacucci et al[50]demonstrated iSCAN assessment of mucosal inflammation correlated strongly with histology.
New technologies
There is ongoing research into other new technologies to improve dysplasia detection.Panoramic views during colonoscopy were obtained by adding two side-viewing cameras to the traditional forward-viewing camera. This “full-spectrum endoscopy”significantly improved dysplasia detection when compared to traditional forward view colonoscopy[51]. Another new technology called autofluorescence was shown to be inferior to CE in a 2018 randomized controlled trial of 210 patients in Europe[52].
Random biopsies
The benefit of random biopsies in surveillance colonoscopy is another area of controversy and ongoing research. The yield of neoplasia detection with random biopsies has been shown to be very low[36,53]. Watanabe and colleagues performed a multi-center RCT of 246 patients comparing dysplasia detection in UC patients by random versus targeted biopsies. The authors found non-inferiority between the random and targeted biopsy groups although patients undergoing random biopsies had longer procedures and more biopsy samples[53].
In a prospective, randomized, multicenter study with tandem colonoscopies,Leifeld et al[54]found no difference in dysplasia detection between WLE with 40 random biopsies and NBI with 10 random biopsies; colonoscopies performed with NBI resulted in far fewer biopsy specimens (11.9 vs 38.6, P < 0.001) and a shorter withdrawal time (23 vs 13 min, P < 0.001). Results from a study by Gasia et al[55]published in 2016 suggest random biopsies are still beneficial when using SD WLE but targeted biopsies are preferred over random biopsies in HD WLE, CE and VCE.Random biopsies in addition to CE are not currently recommended by the 2010 AGA or 2017 European Crohn’s and Colitis Organization guidelines[2,32].
However, there are still circumstances in which random biopsies are beneficial.Random biopsies for histologic staging can still guide IBD treatment[29,56]. In special circumstances such as a personal history of dysplasia, concomitant PSC or a foreshortened colon, random biopsies are still recommended regardless of the screening method[57].
DYSPLASIA
Endoscopically visible dysplasia
Visible dysplastic lesions in parts of the colon uninvolved by colitis should be managed with standard polypectomy techniques, and surveillance should continue based on the patient’s underlying IBD risk without any need for increased surveillance or surgical resection[2,29]. For polypoid and non-polypoid visible lesions with clear margins, endoscopic resection is recommended only if complete resection is possible[58]. Features of underlying malignancy include ulcerated lesions, inability to lift with submucosal injection, and surrounding neoplastic changes and are associated with failed resection[35]. In cases where the lesions are not endoscopically resectable,total proctocolectomy should be recommended[2,7].
Referral to providers experienced in the removal of colorectal lesions in IBD patients should be considered as advanced techniques such as endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD) may be necessary[59].However, only small studies have demonstrated success with these techniques[60-63].Importantly, the long-term efficacy of these techniques in preventing surgery or malignancy is still unclear. In all cases, whenever a larger polyp is removed, a tattoo should be placed to aid in locating the lesion and future surveillance. Guidelines also recommend obtaining additional biopsies of the flat mucosa surrounding the polypectomy site to evaluate for adjacent dysplasia[2,7,56]. However, studies from The Netherlands in 2017 and England in 2018 report that these additional biopsies are rarely beneficial[64,65].
Patients with dysplastic polypoid lesions that have been completely resected should undergo close endoscopic surveillance, although the ideal timing of subsequent procedures is unclear[66,67]. In cases of EMR and ESD, the Global Interventional IBD Group recommends a follow-up surveillance colonoscopy with CE and biopsies at the resection site three months after resection[58].
Endoscopically invisible dysplasia
Endoscopically invisible dysplasia is associated with a high rate of synchronous CRC,up to 22% with invisible LGD and 45%-67% with invisible HGD[10,68-71]. However, in many cases of invisible dysplasia in older studies would possibly be visible today with HD WLE and CE.
Any endoscopically invisible dysplasia discovered at the time of random biopsies should be confirmed with a pathologist experienced in IBD given the significant interobserver variability in the diagnosis of IBD associated dysplasia[5,72]. Guidelines from 2015 also recommend patients with invisible dysplasia be referred to an experienced provider for a repeat HD colonoscopy with CE and repeat random biopsies[1,29]. If visible lesions are present repeat colonoscopy, resection and further surveillance can be considered. If LGD or no dysplasia is present, discussions about the risks and benefits of continued vigilant surveillance versus proctocolectomy should be initiated.Studies in this group are limited, but Navaneethan and colleagues reported in 2013 on 102 patients with LGD and found that with a median follow-up of 36 months, only 5 patients (4.9%) progressed from LGD to either HGD or CRC[73]. In cases of endoscopically invisible HGD or multifocal LGD, total proctocolectomy should be recommended[2,7].
POUCH SURVEILLANCE
For IBD patients who have undergone colectomy with ileal pouch anal anastomosis(IPAA), development of dysplasia in the anorectal or ileal pouch mucosa is rare. A 2014 study of 1200 patients with IBD and IPAA in the Netherlands over 20 years found only 1.8% developed pouch neoplasia and 1.3% developed adenocarcinoma[74].Risk factors for dysplasia following IPAA include a history of dysplasia or CRC,history of PSC, refractory pouchitis, and severely inflamed atrophic pouch mucosa[74,75]. Patients with these risk factors should be considered for annual surveillance including biopsies in the pouch and within the anal transition zone[29].Many suggest surveillance every 3 years for patients with IPAA but without risk factors, but the optimal interval is unknown.
CONCLUSIONS AND FUTURE RESEARCH
Patients with UC and Crohn’s colitis involving more than one-third of the colon are at increased risk for CRC and should undergo regular surveillance colonoscopies as early identification of dysplasia is critical to prevent CRC. Advances in technology have allowed for better identification of dysplasia, and recent data suggest that the majority of dysplastic lesions are visible. With the use of HD endoscopy, there will be continued debate over the role of CE with targeted biopsies versus HD WLE with random biopsies. With improved identification of dysplasia, there is an increasing effort to remove any endoscopically resectable visible dysplasia and only recommend surgical resection when endoscopic resection is not possible. Continued research is needed into the outcomes of endoscopically resected dysplasia, new technologies such as VCE and whether traditional surveillance intervals are still appropriate.
杂志排行
World Journal of Gastroenterology的其它文章
- Exhaled breath analysis in hepatology: State-of-the-art and perspectives
- Miniature gastrointestinal endoscopy: Now and the future
- Issues and controversies in esophageal inlet patch
- Role of hepatocyte nuclear factor 4-alpha in gastrointestinal and liver diseases
- G protein-coupled estrogen receptor in colon function, immune regulation and carcinogenesis
- Helicobacter pylori and cytokine gene variants as predictors of premalignant gastric lesions
