Miniature gastrointestinal endoscopy: Now and the future
2019-08-26JohnMcGoranMarkMcAlindonPrasadIyerEricSeibelRehanHaidryLaurenceLovatSarmedSami
John J McGoran, Mark E McAlindon, Prasad G Iyer, Eric J Seibel, Rehan Haidry, Laurence B Lovat,Sarmed S Sami
Abstract Since its original application, gastrointestinal (GI) endoscopy has undergone many innovative transformations aimed at expanding the scope, safety, accuracy,acceptability and cost-effectiveness of this area of clinical practice. One method of achieving this has been to reduce the caliber of endoscopic devices. We propose the collective term “Miniature GI Endoscopy”. In this Opinion Review, the innovations in this field are explored and discussed. The progress and clinical use of the three main areas of miniature GI endoscopy (ultrathin endoscopy, wireless endoscopy and scanning fiber endoscopy) are described. The opportunities presented by these technologies are set out in a clinical context, as are their current limitations. Many of the positive aspects of miniature endoscopy are clear, in that smaller devices provide access to potentially all of the alimentary canal, while conferring high patient acceptability. This must be balanced with the costs of new technologies and recognition of device specific challenges.Perspectives on future application are also considered and the efforts being made to bring new innovations to a clinical platform are outlined. Current devices demonstrate that miniature GI endoscopy has a valuable place in investigation of symptoms, therapeutic intervention and screening. Newer technologies give promise that the potential for enhancing the investigation and management of GI complaints is significant.
Key words: Ultrathin endoscopy; Capsule endoscopy; Single fiber endoscopy
INTRODUCTION
Gastrointestinal (GI) endoscopy has for many decades been an essential component in the practice of diagnosing and managing digestive diseases. Current endoscopic practice began with Schindler’s development of the flexible gastroscope in 1932[1].Since that innovative step, approaches have been taken to expand the abilities of endoscopy, improve its safety and present exciting challenges to what further can be done. One aspect of this is the development of miniature endoscopes which have aimed to address various clinical problems. The progress of miniature endoscopic devices is largely dependent on that of optical technology and its resultant incorporation of that into clinical application.
The use of an endoscopic device with a smaller caliber has many advantages in clinical practice. Tolerance and safety of invasive GI procedures can be improved,potentially leading to greater uptake and enhanced trust in a care provider. This feature of miniature endoscopy, combined with the potential for more portable devices, could have benefits for wider access to population screening for various GI diseases. Devices that demand less sedation and carry fewer complications add to arguments for the cost-effectiveness of miniature endoscopes. These features as well as the authors’ vision for future applications are outlined in this Opinion Review.
The authors have both academic and clinical expertise in the development and use of miniature endoscopes to enhance patient care. They are aware of the present challenges to clinical practice, including rapid access to screening and diagnostics,improving early cancer detection rates and developing less invasive therapeutic interventions. Miniature endoscopy may have a place in addressing all of these challenges.
The 3 main areas explored in the review are: ultrathin endoscopy; wireless capsule endoscopy; and scanning fiber endoscopy.
ULTRATHIN ENDOSCOPY
Ultrathin endoscopes have many uses in gastroenterology and they are lauded as safe,cost-effective and easy-to-use tools which carry benefits that standard endoscopes do not[2]. The first recorded use of unsedated ultrathin endoscopy (UTE) was in 1994 when twenty healthy volunteers underwent esophagogastroduodenoscopy (EGD)using an Olympus GIF-N30 device[3]. Since then, its use has expanded into common practice in most endoscopy departments. In addition to diagnostic procedures, UTE has been used to varying degrees of success in therapeutic scenarios, such as selfexpanding metal stent insertion, long intestinal tube placement for small bowel obstruction and some endoscopic retrograde cholangiopancreatography (ERCP)cases[4-6].
The conventional design of ultrathin endoscopes is similar to that of standard endoscopes. However, shaft diameters tend to be around 6 mm or less, allowing insertion through the nasal cavity to perform transnasal endoscopy (TNE)[2]. Portable and disposable models of ultrathin endoscopes have the potential to change the approach taken to clinical practice. The newest devices have disposable sheaths which eliminate the need for instrument decontamination and allow multiple examinations to take place using the same device in quick succession. The light source, processor and screen are integrated into a portable digital processing unit[7](Figure 1). The employment of a portable system can obviate transfer to a hospital unit, which in itself can cause inconvenience and distress to patients and carry significant cost to time and resources. The endoscopic test can instead take place in a setting that is more acceptable to such individuals[8]. One therapeutic procedure for which this may be pertinent is percutaneous endoscopic gastrostomy (PEG) insertion. Ultrathin transnasal endoscopes can be used to insert feeding tubes using the introducer method, which inserts the tube directly into the gastric lumen and eliminates the need for passage through the mouth[9]. This technique, combined with use of a portable endoscope serves to reduce the risk of cardiorespiratory side effects in selected at-risk cases[10].
Using tolerability assessment scores, unsedated transnasal endoscopy (TNE) is reported by patients as comparable to sedated conventional EGD (C-EGD)[11]. The tolerability, safety and effectiveness of UTE lends itself well to use in endoscopic screening for esophageal disorders such as Barrett’s esophagus (BE) and esophageal varices[11-13]. BE can be reliably diagnosed using UTE and the yield for intestinal metaplasia using smaller biopsy forceps is comparable to those used in C-EGD[14]. The productivity of a screening program is enhanced by portable, disposable models,opening up the possibility that screening using UTE can be a cost-effective measure.In a United States based Barrett’s screening study of 209 patients, unsedated TNE was significantly lower in cost compared to sedated C-EGD, with mobile endoscopy costs proving less costly than TNE delivered in a hospital setting[15]. This applied to both direct and indirect medical costs. Options for the setting of this test could also expand,with office-based esophagoscopy becoming a potential reality[13].
UTE does carry drawbacks and limitations. Low-caliber endoscopes carry less capacity for constituent components (such as access channels for biopsy and therapeutic interventions); relatively lower image resolution and angle of view compared to C-EGD. There is some evidence that biopsies taken through an ultrathin channel carry comparable diagnostic yield for dysplasia to standard biopsies but larger studies are necessary[16]. The diagnostic accuracy of UTE for early superficial gastric cancers also continues to be prone to scrutiny in countries with high incidence such as Japan, albeit UTE being used for gastric cancer screening in this region[17].
CAPSULE ENDOSCOPY
The use of wireless capsule endoscopy (WCE) was first described in 2000 and has enjoyed widespread use since Food and Drug Administration approval in 2001[18]. The common application of WCE for identifying small bowel bleeding follows evidence that it is a superior diagnostic test to push enteroscopy and barium contrast studies[19].It is a reliable test for Crohn’s disease, with a diagnostic yield as high as 71% and a high safety profile provided the risk of capsule retention is lowered by sufficient imaging or patency studies[20,21]. Improvements in diagnostic yield continue to be developed by widening field of view and increasing the number of recorded images,including the development of adaptive frame rates[22,23]. Various software tools have been developed to reduce reading time while maintaining the diagnostic yield. Their properties include omission of almost identical images, provisional selection of the most standout images and multiple-view modes[24]. The limitations of even very experienced and skilled readers in identifying pathologies are acknowledged. In response to this, the place of artificial intelligence in WCE is now recognized as a very real prospect. Applicable technology remains in the embryonic stages but over time,this, as well as patient and physician acceptance, are seen as barriers that can be overcome[25].
Beyond diagnosing small bowel pathology, colon capsule endoscopes (CCE) have been produced in response to concerns over the resource intensiveness driven by increased demand for colonoscopies, the chance of failure of cecal intubation and suboptimal patient uptake due to the poor tolerance of more conventional endoscopy[26]. The second generation CCE-2 has two optical cameras at each end giving a 172° view and adaptive frame rate up to 35 frames per second. This provides bidirectional views in real time. Its dimensions are 31.5 mm by 11.6 mm and its recording capacity is ten hours. The software contains a polyp size estimation function and a flexible spectral imaging color enhancement for enhanced visualization[27]. Provided bowel preparation is excellent, detection of polyps > 6 mm and > 10 mm for the CCE-2 carries sensitivity and specificity rates of over 85%,supporting claims that this may be applicable in a screening setting[28]. In controlled settings, CCE have also been comparable to colonoscopy in assessing the colonic mucosa of those with inflammatory bowel disease[29,30].

Figure 1 The “EG Scan II” system. A: The portable case with four main parts; B: The image processor (top left),disposable probe (top right), air tube (bottom right) and hand-held controller (bottom left); C: The system connected and ready for use; D: Close view of the capsule probe tip. Reproduced with permission from Sami SS et al. Copyright John Wiley and Sons.
Esophageal diseases such as BE and esophagitis may be detected using a capsule device. A blinded study comparing EGD as gold standard with the PillCam ESO 2 device (Given Imaging Ltd., Yoqneam, Israel) yielded promising detection rates for BE and esophagitis with a sensitivity of 100% and a specificity of 74%, and a sensitivity of 80% and a specificity of 87%, respectively[31]. In an attempt to overcome the impairment to diagnostic sensitivity exacerbated by rapid esophageal transit,tethered wireless capsule endoscopes have been developed. For the detection of Barrett’s esophagus, early results have been mixed and further large scale studies in relevant populations are advocated[32]. WCE has also been trialed in the emergency setting of acute upper GI bleeding. A prospective study found it to be a feasible and safe way of detecting and stratifying such cases[33]. It may also have a place in future practice for screening and surveillance of esophageal varices. The current literature,with a pooled sensitivity of 72%, does not support its use over EGD[34].
Active locomotion in capsules using mechanical actuation, in a crawling, inchworm or swimming motion has been proposed as a way of controlling transit through the GI tract and resisting peristalsis in cases where prolonged inspection of an abnormal lumen is desired. Development of such equipment has not reached clinical trial stage primarily due to power capacity issues and mechanical complexity[35].Future development would depend on enhanced power storage or usage technology[36].
Non-actuated wireless capsules have struggled to completely examine the stomach lumen, owing to its large size impeding full visualization[37]. However, a feasibility study has suggested that with one liter of simethicone-containing swallowed water,good views of the upper GI tract can be obtained[38]. More advanced software that incorporates larger frame rates and artificial intelligence may also potentiate the diagnostic accuracy of this approach. Magnetically guided wireless capsules have been developed to be able to better navigate the device around a fluid distended stomach. There may also be a role for this test in screening for gastric cancer, with provisional feasibility studies of asymptomatic patients showing promise[39].Magnetically driven capsules also help to lower storage needs for power thus potentially allowing space for interventional tools[35]. Progress continues on the development of biopsy models, which have shown promise in in vitro and animal models[40]. Further application in clinical trials is needed before the potential for interventional WCE in healthcare can be realized. Robotic assistance in controlling magnetic wireless capsules has been the subject of some clinical trials, showing superiority of this method of actuation over manual manipulation of magnetically guided WCE on viewing installed targets on an ex-vivo colon model[41]. A multicenter blinded study of patients with upper abdominal complaints examined roboticallyassisted magnetically guided WCE with the gold standard of conventional gastroscopy and concluded that detection of focal lesions in the upper and lower stomach had comparable accuracy[42]. This device has also shown better diagnostic yield than EGD in patients presenting for investigation of iron deficiency anemia[43].The evidence points towards WCE having a greater future role for diagnosis of GI disorders although it will require more time and research, particularly on the costeffectiveness front, to determine which manifestations warrant widespread application[43,44](Figures 2-4).
SCANNING SINGLE FIBER ENDOSCOPY
Newer forms of optical technology have been developed to meet the demands for endoscopic imaging that is of higher resolution than UTE can provide[46](Figure 5).Scanning single fiber endoscopy (SFE) involves narrow bands of light being projected onto tissue and reflecting back onto the fiber, before an image is created one pixel at a time. The resultant image is of a superior quality to those from an ultrathin endoscope of a similar caliber[47]. In gastrointestinal endoscopy, as well as permitting access to poorly accessible areas like the upper biliary tree and pancreas, SFE may have a place as an adjunct to more conventional endoscopes. One example of this could be more complete visualization of a lesion whenever full views are not obtained on a single plane.
Spectrally-encoded scanning fiber endoscopy uses polychromatic light emissions from the endoscopic probe, encoded by wavelength which is then reflected from the surface and decoded outside the body to produce a one-dimensional impression.Rotation of the instrument builds information for a two-dimensional image of the visualized surface[46]. The endoscope can be as thin as 80-250 micrometers in diameter,limited only by the size of the light-emitting fiber and any accessory instruments[48,49].Spectral encoded endoscopy using a single fiber can perform three-dimensional topological analysis and real-time subsurface imaging[50]. This multispectral SFE may be used in combination with white light endoscopy to collect wide field fluorescence images which can permit early detection of dysplasia and cancer[51]. Although research has shown progress in animal models, development of this technology for analyzing human tissue is required.
SFE has been undertaken in limited cases to perform cholangioscopy in patients with pancreaticobiliary strictures. It is a feasible technology to directly view such areas with better resolution than current cholangioscopic tools[52]. A tethered SFE“capsule” for conducting esophagoscopy has been developed, in what could represent an evolution of the tethered wireless capsule endoscope[53]. The patient swallows the device and images are transmitted live up the single fiber into a processor, in contrast with the tethered WCE which stores images for viewing at a later time. With the SFE capsule, real time images mean that pathologies and potential problems are identified at an immediate stage. Research into the application of SFE in real clinical scenarios is required but this has the potential for gastrointestinal endoscopy to be safer, more cost-effective, better tolerated and more advanced than current technology allows[47].The progress of this technology continues at a rapid pace, with prototype devices as thin as a human hair that carry better resolution, being developed[54].
CONCLUSION
Miniature GI endoscopy has many forms and is in many ways a relative term.Through the recognition that endoscopy is an invasive procedure to which patients are prone to experiencing significant discomfort, and that accessibility to areas of the gastrointestinal tract requires development of existing equipment, endoscopes with narrower calibers have been produced. The three main domains in miniature endoscopy currently are ultrathin devices, scanning fiber endoscopes and wireless capsules. Within these domains many products are being developed at a rapid pace.
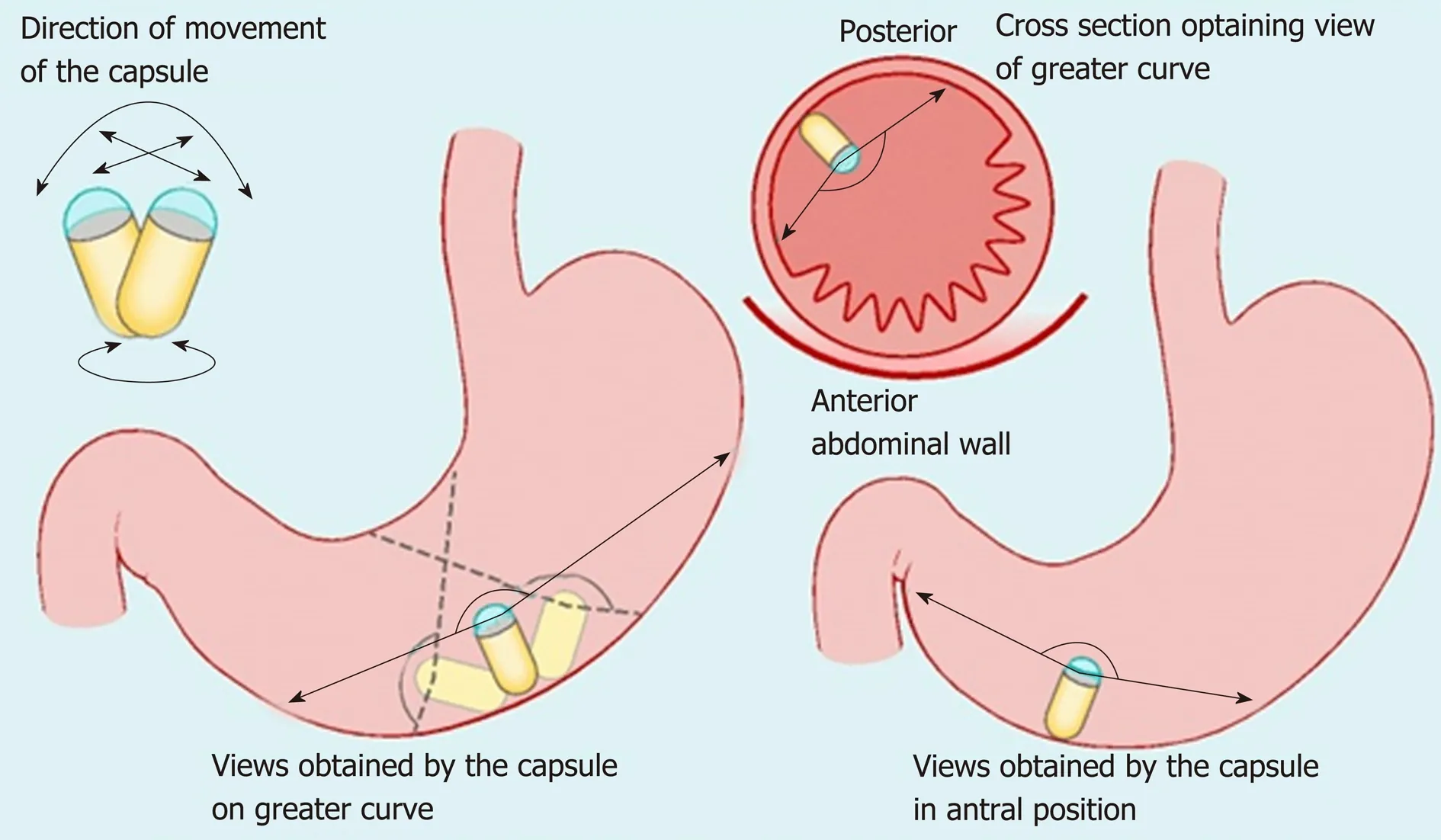
Figure 2 Maneuvers of the magnetically guided wireless capsule endoscopy in the stomach. Reproduced with permission from Ching HL et al[43]. Copyright Thieme Group.
The role of gastrointestinal endoscopy can be generally categorized into two aspects- diagnostic and therapeutic. From a healthcare perspective, it is clear that a suitably accurate means of diagnosing GI diseases, which is better tolerated and eventually more cost-effective than standard endoscopy warrants major consideration for future practice. Screening for various luminal GI diseases, in particular, malignant and pre-malignant conditions is a topical issue. We believe that miniature devices such as ultrathin endoscopes and capsules can bring a high quality screening service that satisfies the needs outlined by Wilson et al[55]. As evidenced above, the diagnostic capabilities of miniature endoscopic devices such as SFE and magnetically guided WCEs enhance today’s practice. Through the enhanced access provided by miniature endoscopy, therapeutic interventions like hemostasis and delivery of medication may be achievable in the future by incorporating robotics and remote controlling systems.

Figure 3 MicroCam Navi equipment (magnetically-assisted capsule endoscopy). Reproduced with permission from Ching HL et al[38]. Copyright Thieme Group.
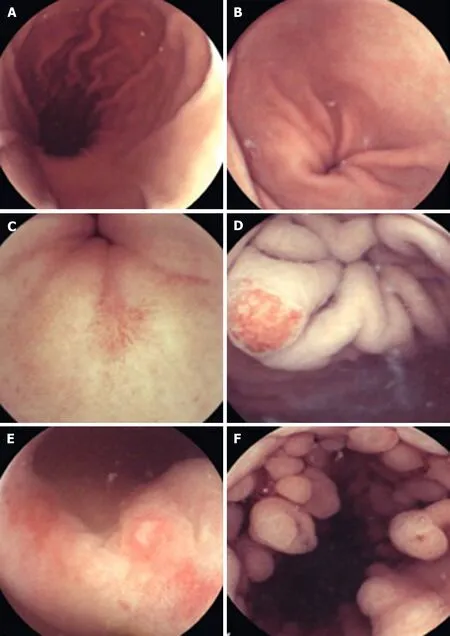
Figure 4 Capsule endoscopy. A: Longitudinal view of the gastric body and lesser curve. B: Gastric antrum. C: Pre-pyloric erosion. D: Angioectasia in the cardia. E:Nonsteroidal anti-inflammatory drug-related erosive gastropathy. F: Fundic gland polyps. Reproduced with permission from Hale MF et al. Copyright Thieme Group.
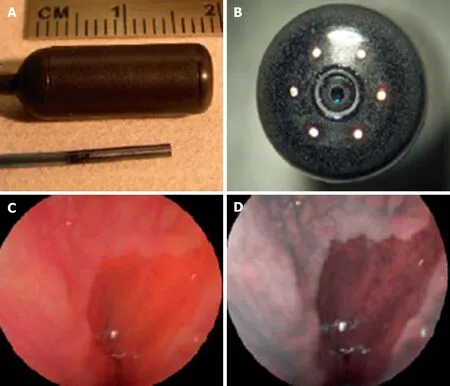
Figure 5 Scanning single fiber endoscopy. A: Scanning fiber endoscopy (SFE) endoscope probes showing 9 mm rigid tip length of 1.2 mm diameter prototype and 18 mm capsule length of 6.4 mm diameter TCE. A front view of the distal end of the TCE is shown in (B) illustrating that the TCE is a standard SFE probe with collection fibers modified for capsule use. The gastroesophageal junction of a human subject is shown in single 500-line RGB image contrast (C) compared to postprocessed ESI contrast of the same SFE image frame (D). The lighter esophageal tissue is more clearly differentiated from the red-colored gastric mucosa in the ESI image. Reproduced with permission from Lee CM et al[46]. Copyright John Wiley and Sons, Inc.
杂志排行
World Journal of Gastroenterology的其它文章
- Exhaled breath analysis in hepatology: State-of-the-art and perspectives
- Issues and controversies in esophageal inlet patch
- Role of hepatocyte nuclear factor 4-alpha in gastrointestinal and liver diseases
- G protein-coupled estrogen receptor in colon function, immune regulation and carcinogenesis
- Helicobacter pylori and cytokine gene variants as predictors of premalignant gastric lesions
- Intestinal enteroids/organoids: A novel platform for drug discovery in inflammatory bowel diseases
