Thoracic aorta thickness and histological changes with aging: an experimental rat model
2019-08-19GiulioCesarGequelimDjaniraAparecidadaLuzVeronezGustavoLenciMarquesCamilaHarumiTabushiRonaldodaRochaLouresBueno
Giulio Cesar Gequelim, Djanira Aparecida da Luz Veronez, Gustavo Lenci Marques, Camila Harumi Tabushi, Ronaldo da Rocha Loures Bueno
1Master's Degree Student in Postgraduate Program of Internal Medicine, Federal University of Paraná (UFPR), Medical Resident in Cardiology in Hospital de Clínicas UFPR, Curitiba-PR, Brazil
2Department of Anatomy, School of Medicine, UFPR, Curitiba-PR, Brazil
3Master's Degree in Internal Medicine, Adjunct Professor, Department of Internal Medicine, School of Medicine, UFPR, Curitiba-PR, Brazil
4Medical Student, Department of Internal Medicine, School of Medicine, UFPR, Curitiba-PR, Brazil
5Department of Internal Medicine, School of Medicine, UFPR, Curitiba-PR, Brazil
Keywords: Aging; Collagen; Elastic fibers; Smooth muscle cells
The three main components of the media layer of the aorta are elastic fibers, collagen fibers and smooth muscle cells (SMC).[1]This layer's elastic properties are major determinants of its biomechanics in health and disease states.[2,3]Age-related changes in such elastic properties are associated with altered hemodynamic parameters, such as systolic hypertension,[4,5]and end-organ damage,[6,7]and are thought to result from changes of its main components.[8]
Most elastic fibers in the media layer are incorporated into concentric layers called lamellae, while collagen, proteoglycans, SMC and other elastic fibers are contained in the interlamellar spaces.[9]Since elastin synthesis is negligible after the neonatal period in mammals,[10]elastic fibers are lifelong exposed to the factors associated with aging process. Media layer collagen, on the other side, is synthesized by fibroblasts, and its content increases with age.[11]Parallel to such changes in extracellular matrix, age-related media layer smooth muscle cell senescence has been recognized,[12]along with a reduction in media layer SMC count.[13]
This animal-based study has been conceived as a rat biological model of normal aortic arterial wall aging. It has been focused in the histologic phenomena of aging, to allow for a simultaneous morphoquantitative evaluation of all aforementioned major components of the arterial media layer thought to be involved in age-related changes in vascular structure and function, that is, elastic and collagen fibers and SMC, along with a morphometric evaluation of arterial wall thickness, throughout the aging process.
Our research was approved by Ethics Committee for Research with Animals of Federal University of Paraná (protocol number 23075.031142/2013-73, certificate number 732). Study animals were 60 male Wistar rats (Rattus norvegicus albinus), maintained under a light-dark cycle of 12 h and controlled temperature (22°C) and receiving water and food (standard pellet diet; Nuvilab-Nuvital®, Curitiba, Brazil) ad libitum until they were sacrificed. Rats were divided in six even groups, kept in even conditions until the age each group was ascribed for the time of the sacrifice for the study (Table 1).
Animals underwent euthanasia by intraperitoneal injection of a solution of Ketamine and Xylazine. Thereafter, a 1 cm long segment was dissected from the descending part of the thoracic aorta immediately below the aortic arch. Segments' dimensions were assessed using a digital caliper rule (Digimess®).
Next, aortic segments were fixated in Bouin's solution (picric acid, 40% formaldehyde and acetic acid) for 18 h, and then dehydrated in a decrescendo sequence of xylene and ethanol. Such material was then included in Paraplast®, and each rat provided material for three histological laminae, each with 10 slices 5 μm thick. The three laminae were stained, respectively, with Hematoxilin & Eosin (HE), Masson's trichrome, and Weigert's elastin stain.

Table 1. Number of rats per group and life time.
For histological analysis, an Olympus®BX50 microscope with acquisition camera DP71 3CCD pro series was used. Images were exported to a Sony Trinitron®colored screen and scanned by an Oculus TCX (Coreco®) scanner. The software for image acquisition was MetaSystems®VSViewer 2.1.102.
The same software was employed for morphometric analysis. Intima, media and adventitia layer thicknesses were measured in laminae stained by HE, five measurements per layer per rat on alternate slices, and the average thickness of each layer of each rat was registered in micrometers.
For morphoquantitative analysis, media layer snapshots were taken as follows: for smooth muscle cell count, five snapshots with 0.0080 mm² each, in the same relative position of alternate slices, in each HE stained lamina; for collagen quantification, five snapshots with 0.0100 mm² each, in the same relative positions of alternate slices, in each Masson's trichrome stained lamina; for elastic fiber quantification, 10 snapshots with 0.0042 mm² each, in 10 standard positions around media layer's circumference, in each Weigert stained lamina.
Morphoquantitative data were obtained from each snapshot as follows. For SMC, visual count of nuclei was performed, and result registered as number of nuclei per 0.0080 mm2area. For elastic and collagen fiber quantification, one parted from the principle that elastin is stained purple by Weigert, while collagen is stained blue by Masson's trichrome, therefore the proportion of the area of each snapshot occupied by these colors was determined using the software Media Cybernetics®Image-Pro Plus 6.0 for Windows®. Such color area quantification was performed using HSI histogram based color segmentation, after color equalization by the same software. Elastin's purple color on Weigert stained laminae was empirically determined to be in the HSI range (0-255, 255-255, 0-255), while collagen's blue color on Masson's trichrome stained laminae was empirically defined to be in the HSI range (60-180, 70-255, 65-160), and empty spaces in the HSI range (10-255, 0-40, 220-255). Colors falling out of these ranges corresponded to the remaining components of the vessel's media layer.
The areas occupied by each of these color ranges were measured by the software, and the quantification of collagen and elastic fibers was expressed by the percentage of the field area stained for each of them: elastic fibers = 100 × elastic fibers area/(elastic fibers area + remaining components area) and collagen = 100 × collagen area/(collagen area + remaining components area). This procedure was performed for each snapshot, and the average of each animal was considered for statistical analysis.
Images with artifacts were excluded from the analysis. If the entire lamina contained artifacts, the rat was not evaluated for the variable correspondent to that lamina, but could be evaluated for other variables if images exempt from artifacts were present in other laminae.
Data were analyzed with the software IBM®SPSS Statistics v.20. Results of the studied variables were described by mean, median, minimum and maximum values and standard deviations. For comparisons between all study groups, ANOVA model with one factor was used, and for multiple post-hoc comparisons the LSD test was used. Normal distribution of the variables was determined using the Kolmogorov-Smirnov test. Statistical significance was indicated by P-values < 0.05.
All study animals remained alive for the lifetime ascribed for each of them. Two animals from group 18 months were found to have tumors by the time of the tissue harvest, not affecting any aspects of the research's methodology. Number of subjects (n) per group differed from 12 in individual analyses due to the presence of artifacts compromising the analysis of histological specimens. Notably, the entire 18 months group was excluded from elastic fiber analysis, and the entire 24 months group from collagen analysis for this reason.
Media layer thickness had a statistically significant increase with age (Table 2), and comparisons between individual groups are presented on a separate table (Table 3).
SMC count and elastic fiber concentration decreased, whereas collagen concentration had a tendency to increase that did not reach statistical significance (Tables 4 and 5).
Our study has built a model of aortic arterial wall aging in rats that allowed us to both describe the increment in media layer thickness that occurs during normal aging, and quantitatively assess the changes in elastic and collagen fibers and SMC, namely a decrease in elastic fibers and SMC content. As relative elastic fiber content decreased while media layer thickness increased, such thickening is supposed to have been at the expense of increased collagen fiber content (i.e., fibrosis), a trend that was observed in our results, though without reaching statistical significance.

Table 2. Media layer thickness in micrometers.

Table 3. Comparison of media layer thickness between individual groups.
From the quantitative point of view, although the total content of elastic fibers in elastic lamellae remain constant throughout life,[14]its concentration reduces, as a result of increased collagen synthesis between lamellae,[14]and almost complete substitution of elastic fibers by collagen and proteoglycans in the interlamellar space.[15]From a qualitative standpoint, phenomena as altered aminoacid crosslink,[16]glyco-oxidative reaction,[17,18]fragmentation,[19-21]increase matrix metalloproteinases (MMP) activity,[15,19]and calcification have been observed.[22]
Media layer elastic module has two components: elastic fibers account for the first component, physiological, with a flatter stress-strain relationship, while collagen fibers account for the second component, whose stress-strain relationship is steeper.[23]Age-related elastic fiber changes[17,18]are thought to account for loss of its elastic properties[16]and, as a consequence, transmitting of hemodynamic forces to underlying, stiffer,[24]media layer collagen fibers,[25]resulting in increased blood vessel stiffness.[11,26,27]
Collagen fiber staining involved more steps than other employed staining, thus increasing the possibility of artifact insertion, such that more laminae had to be excluded from the analysis due to presence of artifacts, thus possibly lowering the sample power to detect a significant change in collagen fiber content between groups. Moreover, Masson's trichromic, the stain we utilized in our analysis, is less specific, for quantitative purposes, than Picrosirius' red stain,[28-30]which has been successfully employed in other study[31]to quantitatively assess collagen content in aortic media layer and make comparisons between groups.
Recent data propose a possible link between loss of elastin concentration and integrity to smooth cell growth signaling.[32,33]This link has been suggested based on findings that normal elastin interacts with growth factors in the paracellular level in the aortic media layer.[34-36]Others researchers have found that aortic media layer SMC themselves become stiffer with aging, raising the possibility of a direct contribution of the cellular component of the media layer to arterial stiffening besides stiffening of the extracellular matrix.[12]Media layer cellularity, which is made up predominantly by SMC, was found to decrease with age[13]though the mechanism for this remains to be elucidated. Importantly, SMC dysfunction has a potential pathogenic role in atherosclerosis and aneurysm formation.[37]
In the present study, rats have been raised up to the age of 24 months, which has been shown to parallel human 60-year age.[38]Therefore, although our data can be regarded as reflecting the aging process through adulthood to old age, but not necessarily its continuation from that point on. On the other side, since we found statistical significance, our results may signal to possible future studies that that age is enough time to wait for aging changes in rats.
The present study described age-related histological alterations in the thoracic aorta in an experimental model with rats. Extracellular matrix increases were observed, leading to layer thickening. This increase seems to be driven by the collagen content, resulting in decreased elastic fiber concentration and smooth muscle cell count.

Table 4. Results of SMC count, elastic fiber and collagen quantitative analysis.
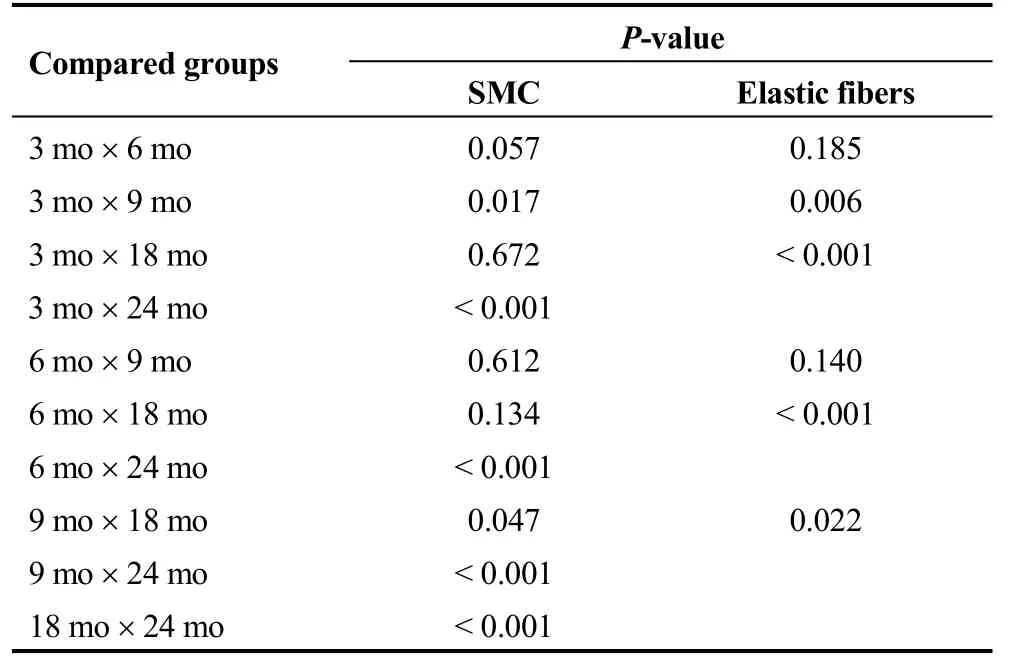
Table 5. Comparisons of SMC count and elastic fiber proportion between individual groups.
Acknowledgments
All resources for this research were provided by the Animal Facility Services of Angelina Caron Hospital and by the Biological Sciences Sector of Federal University of Paraná. The authors confirm that this article content has no conflict of interest.
杂志排行
Journal of Geriatric Cardiology的其它文章
- The utility of coronary computed tomography angiography in elderly patients
- Predictors of non-response to cardiac resynchronization therapy implantation in patients with class I indications: the markedly dilated left ventricular end-diastolic dimension and the presence of fragmented QRS
- The relevance of serum albumin among elderly patients with acute decompensated heart failure
- The value of serum metabolomics analysis in predicting the response to cardiac resynchronization therapy
- Effects of febuxostat on atrial remodeling in a rabbit model of atrial fibrillation induced by rapid atrial pacing
- Adverse reactions of Amiodarone
