Pyrrolizidine alkaloids-induced hepatic sinusoidal obstruction syndrome: Pathogenesis, clinical manifestations, diagnosis,treatment, and outcomes
2019-08-12XiaoQianYangJinYeXinLiQianLiYuHuSong
Xiao-Qian Yang, Jin Ye, Xin Li, Qian Li, Yu-Hu Song
Abstract Hepatic sinusoidal obstruction syndrome (HSOS) can be caused by the intake of pyrrolizidine alkaloids (PAs). To date, PAs-induced HSOS has not been extensively studied. In view of the difference in etiology of HSOS between the West and China, clinical profiles, imaging findings, treatment, and outcomes of HSOS associated with hematopoietic stem cell transplantation or oxaliplatin might be hardly extrapolated to PAs-induced HSOS. Reactive metabolites derived from PAs form pyrrole-protein adducts that result in toxic destruction of hepatic sinusoidal endothelial cells. PAs-induced HSOS typically manifests as painful hepatomegaly, ascites, and jaundice. Laboratory tests revealed abnormal liver function tests were observed in most of the patients with PAs-induced HSOS. In addition, contrast computed tomography and magnetic resonance imaging scan show that patients with PAs-induced HSOS have distinct imaging features, which reveal that radiological imaging provides an effective noninvasive method for the diagnosis of PAs-induced HSOS. Liver biopsy and histological examination showed that PAs-induced HSOS displayed distinct features in acute and chronic stages. Therapeutic strategies for PAs-induced HSOS include rigorous fluid management, anticoagulant therapy,glucocorticoids, transjugular intrahepatic portosystemic shunt, liver transplantation, etc. The aim of this review is to describe the pathogenesis, clinical profiles, diagnostic criteria, treatment, and outcomes of PAs-induced HSOS.Key words: Hepatic sinusoidal obstruction syndrome; Pyrrolizidine alkaloids; Hepatic sinusoidal endothelial cells; Pyrrole-protein adducts; Diagnostic criteria; Symptomatic
INTRODUCTION
Hepatic sinusoidal obstruction syndrome (HSOS), previously known as hepatic venoocclusive disease (HVOD), is an obliterative venulitis of the terminal hepatic venules[1-5]. HSOS is typically manifested as a classical triad of weight gain, painful hepatomegaly, and jaundice. The etiologies of HSOS include cytoreductive therapy prior to hematopoietic stem cell transplantation (HSCT)[2,6-8]; oxaliplatin-containing adjuvant chemotherapy[9-15]; intake of pyrrolizidine alkaloids (PAs)-containing plants[16-21]; use of tacrolimus in liver transplantation[22]; autosomal recessive condition of veno-occlusive disease with immunodeficiency, etc. In developed countries, HSOS usually occurs in patients who have received cytoreductive therapy prior to HSCT or oxaliplatin-containing chemotherapy for colorectal carcinoma[8,23-25]. In China, the primary cause of HSOS is the ingestion of PAs-containing herbals or dietary supplements[16,20,26]. Most clinical studies have focused on HSCT-related HSOS and oxaliplatin-induced HSOS in recent decades. Given the difference in etiologies of HSOS between western countries and China, clinical profiles, imaging findings,treatment, and outcome of HSOS associated with HSCT or oxaliplatin might be hardly extrapolated to PAs-induced HSOS.
The purposes of this review were: (1) To elucidate the pathogenesis of PAs-induced HSOS; (2) To describe clinical manifestations; (3) To describe imaging features; (4) To define the criteria for diagnosis; and (5) To evaluate treatment and outcome.
PATHOGENESIS
HSOS is characterized by toxic injury to small hepatic vessels, particularly the sinusoidal endothelium in zone 3 of the liver acinus[27]. Damaged sinusoids lead to sloughing and downstream occlusion of terminal hepatic venules[27]. A core pathogenic event of HSOS is toxic destruction of hepatic sinusoidal endothelial cells(HSECs)[1]. Injured HSECs and central venous endothelial cells can be replaced by progenitor cells derived from bone marrow, and toxicity of the drugs and the plants to bone marrow progenitors impairs the replacement of the injured endothelial cells[28,29]. All these indicated toxic injury to sinusoidal/central venous endothelial cells and bone marrow progenitors contributes to the pathogenesis of HSOS.
PAs-containing plants are widely distributed in the world. More than 300 PAs have been identified in over 6000 plants[5]. Although the precise mechanism of PAs-induced HSOS remains unknown, it is well accepted that the initial event in PAs-induced HSOS is toxic destruction of HSECs in zone 3 of the liver acinus by toxic metabolites of PAs[16,17]. Firstly, water-soluble PAs salts are readily absorbed from the gastrointestinal tract and then transported to the liver through the portal venous system when individuals ingest herbal remedies or herbal teas containing PAs. Then, in liver,PAs are metabolized through cytochrome-P450-mediated activation to produce dehydropyrrolizidine alkaloids (DHPAs) and dehydroretronecine (DHR). DHPAs and DHR further interact with glutathione (GSH) or proteins to generate pyrroleglutathione conjugates[30]or pyrrole-protein adducts (PPAs)[31], respectively. PPAs are believed to be the primary cause of PAs-induced HSOS[32,33]. GSH conjugation of DHPAs and DHR is a main detoxification mechanism in metabolism-mediated PAs intoxication[18,34]. HSECs have a low basal GSH level, thus HSEC is susceptible to the reactive metabolites with severe GSH depletion and PPAs formation[34]. PPAs covalently bind to the F-actin cytoskeleton of the HSECs, which results in depolymerization of F-actin. Depolymerization of F-actin triggers the release of matrix metalloproteinase (MMP)-9. The combination of F-actin depolymerization, which allows HSECs to round up, and degradation of the subcellular extracellular matrix by MMP-9, which loosens HSECs tethering to the space of Disse, creates gaps within and between HSECs[35]. Then, erythrocytes, leukocytes, and cellular debris penetrate the gaps in the endothelium into the space of Disse, and the sinusoidal lining consisting of HSECs, Kupffer cells, and hepatic stellate cells is dissected. Emboli of dead sinusoidal lining cells obstruct the sinusoidal flow. With progressively narrowing venous lumen and reduced sinusoidal venous outflow, post-sinusoidal portal hypertension occurred[23]. In addition, the occlusion of the small centrilobular veins leads to hepatic congestion and subsequent hemorrhagic parenchymal necrosis. This pathophysiological process gives rise to the presence of the clinical syndrome of HSOS,including weight gain, ascites, painful hepatomegaly, and jaundice[23](Figure 1).
CLINICAL MANIFESTATION AND LABORATORY TESTS
The clinical presentation of HSOS includes jaundice, right upper quadrant pain,tender hepatomegaly, ascites, and weight gain. In western countries, HSOS occurs most commonly in cytoreductive therapy prior to HSCT or oxaliplatin-containing chemotherapy. For HSCT-related HSOS, the patients present with a wide spectrum of severity. Severe HSOS is typically associated with multiorgan failure. For oxaliplatininduced HSOS, clinical manifestations of the patients appear to be mild or absent[9,13,36].This indicates that clinical presentation of HSOS correlates with the etiology of HSOS.To determine the clinical presentation of the patients with PAs-induced HSOS, clinical profiles of patients with PAs-induced HSOS have been analyzed. The clinical presentation of the patients is summarized in Table 1. The most common clinical features were ascites (98%-100%), hepatomegaly (65.1%-92%), jaundice (39.8%-57.8%),and abdominal distention (98.3%-99.1%); whereas, a small proportion of PAs-induced HSOS patients had weight gain (16%-18%), edema (37.3%-39.5%), and right upper quadrant pain (19.7%-36.4%). In addition, splenomegaly (27%-34%) and gastroesophageal varices (18.3%-36.8%) were observed in a small proportion of patients with PAs-induced HSOS[17,26,37-39].
In addition, laboratory tests have been analyzed in patients with PAs-induced HSOS. The results of laboratory tests are summarized in Table 1. Routine blood tests showed that levels of erythrocytes, leukocytes, and platelets were within the normal range in most patients with PAs-induced HSOS. Abnormal liver function was observed in most PAs-induced HSOS patients. Elevation of serum bilirubin, alanine aminotransferase, aspartate aminotransferase, and γ-glutamyl transpeptidase was observed in patients with PAs-induced HSOS; moreover, albumin level was abnormal(Table 1). Ascites is the most common clinical presentation of PAs-induced HSOS, and laboratory investigation showed that patients had a serum ascites albumin gradient >11 g/L, which indicated portal hypertensive ascites[16,39]. A wide spectrum of disease severity was observed in patients with PAs-induced HSOS. Mild HSOS is considered when it meets diagnostic criteria and has a self-limiting course. Patients with severe HSOS developed multiorgan failure and had a high risk of mortality.
IMAGING FEATURES
The use of imaging techniques to evaluate liver lesions has been confirmed in clinical practice. Ultrasonography, computed tomography (CT), and magnetic resonance imaging (MRI) have been widely used in diagnosing liver diseases. Recently, several researchers have investigated the imaging characteristics of PAs-induced HSOS and determined the diagnostic value of radiological imaging. Imaging techniques provide valuable imaging signs as well as an effective method for diagnosing PAs-induced HSOS.

Figure 1 Pathogenesis of pyrrolizidine alkaloids-induced hepatic sinusoidal obstruction syndrome. A: Oral PAs are transported into the liver, metabolites bind with glutathione, resulting in detoxification, or combine with protein to generate PPAs; B: In HSECs, PPAs cause depolymerization of F-actin and release of MMP-9,which damages HSECs; C: Injured HSECs round up, MMP-9 triggers degradation of extracellular matrix, then gaps between HSECs appear, blood cells penetrate the gaps into the space of Disse, and sinusoidal lining cells obstruct the sinusoidal flow, resulting in postsinusoidal portal hypertension. PAs: Pyrrolizidine alkaloids; GSH:Glutathione; PPAs: Protein-pyrrole adducts; HSECs: Hepatic sinusoidal endothelial cells; MMP-9: Matrix metalloproteinase-9; ECM: Extracellular matrix; DHPAs:Dehydropyrrolizidine alkaloids; DHR: Dehydroretronecine; HSOS: Hepatic sinusoidal obstruction syndrome.
Liver ultrasonography is a cost-effective method in the diagnosis of liver diseases.A recent study demonstrated that ultrasonography is useful for diagnosis of PAsinduced HSOS. Doppler ultrasound examination of patients with PAs-induced HSOS includes hepatomegaly, decreased portal vein flow velocity, and hepatic vein stenosis[17]. In addition, typical ultrasonic features include heterogeneous enhancement of the arterial phase, slow portal vein filling, and an extended transit time between the hepatic artery and vein[16,40].
Several studies have described radiological features of PAs-induced HSOS. Early studies described the imaging features of PAs-induced HSOS based on small samples.They found that ascites, patchy liver enhancement, narrowing of the right hepatic vein, hepatomegaly, and gallbladder wall thickening are common features of CT(Figure 2)[19,41]. Recently, our group[39]and Zhuge et al[17]confirmed the above findings in large sample sizes. Beside this, heterogeneous hypoattenuation, pleural effusion,obscure or invisible hepatic veins, and stenosis of the hepatic segmental inferior vena cava were observed in most patients (Figure 2). Splenomegaly and portosystemic collateral circulation were observed in a small proportion of patients with PAsinduced HSOS[16,17,39]. Importantly, we found that patchy liver enhancement and heterogeneous hypoattenuation were valuable signs of PAs-induced HSOS[39].Quantitative analysis of CT images revealed that the ratio of hepatic lesion volume to liver volume in patients with PAs-induced HSOS is associated with clinical course and outcome[42].
MRI is a good choice of imaging technique because it yields different information and does not have the hazards of X-rays compared with CT. Recent studies demonstrated MRI is useful in detecting oxaliplatin-induced HSOS in patients with metastatic colon cancer[11,15,36,43]. Our previous work demonstrated that the common signs of PAs-induced HSOS include ascites, heterogeneous hypointensity, narrowing of the inferior vena cava, periportal edema, gallbladder wall thickening,hepatomegaly, abnormal hepatic vein (narrowing or invisibility of right hepatic vein),and pleural effusion (Figure 3). We have also found inhomogeneous enhancement around the hepatic veins (“claw” type enhancement) in the portal venous phase that results from differential blood perfusion in the peripheral and central region of the liver parenchyma as well as hypointensity in susceptibility-weighted imaging (SWI)and T2*-weighted imaging (T2*WI)[44](Figure 3). Hemosiderin derived from extravasated erythrocytes in the space of Disse is detected by T2*WI and SWI, which results in the imaging sign of hypointensity. We have also investigated the imaging features of PAs-induced HSOS on gadoxetic acid-enhanced MRI[45]. Heterogeneous hypointensity of liver parenchyma in hepatobiliary phase (HBP) is an imaging sign of PAs-induced HSOS (Figure 3). The severity of heterogeneous hypointensity scored by volume fraction in HBP of gadoxetic acid-enhanced MRI is positively correlated with prothrombin time and international normalized ratio, and the severity of

Table 1 Demographic information, clinical manifestation, and laboratory tests of pyrrolizidine alkaloids-induced hepatic sinusoidal obstruction syndrome
hypointensity in HBP is a mortality risk factor. In summary, these studies have demonstrated that heterogeneous hypoattenuation/hypointensity and irregular enhancement are valuable imaging features of CT and MRI.
PATHOLOGY
Liver biopsy and histological examination are gold standards for diagnosis of HSOS.PAs-induced HSOS exhibits acute and subacute/chronic features. In the early stage,the first recognizable histological change is widening of the subendothelial zone between the basement membrane and the adventitia of central veins and sublobular veins[46,47]. In acute disease, swelling, damage, and shedding of HSECs are observed in acinar zone 3 (Figure 4). Significant dilation and congestion of hepatic sinusoids with centrilobular hepatocellular necrosis, dissection of erythrocytes into the space of Disse, thickening of the walls of small intrahepatic veins with narrowing and occlusion of the lumen are frequently observed[16,38]. In the later stage, deposition of extracellular matrix in subendothelial spaces and sinusoids and extensive collagenization of sinusoids and venules were characteristic histological features[38]. In addition, our studies also found complete loss of pericentral hepatocytes as well as sinusoidal dilatation in subacute or chronic stages (Figure 4)[38]. However, percutaneous liver biopsy is difficult to perform in patients with PAs-induced HSOS due to extensive ascites, coagulation disorders, and thrombocytopenia. Furthermore, the uneven distribution of HSOS decreases histopathological credibility. Transjugular liver biopsies have been performed in PAs-induced HSOS patients with extensive ascites, coagulation disorders, and thrombocytopenia. More importantly, transjugular liver biopsy with hepatic venous pressure gradient (HVPG) provides valuable information. HVPG > 10 mmHg has > 90% specificity and > 85% positive predictive value for the diagnosis of HSOS following HSCT[48]. In a retrospective study, seven patients with PAs-induced HSOS underwent HVPG measurement, and they all had a significant increase in HVPG (mean value 23.50 ± 3.02 mmHg)[17]. However, this technique has not been widely used in many hospitals because it requires a dedicated setting and is expensive.

Figure 2 A 53-year-old man with pyrrolizidine alkaloids-induced hepatic sinusoidal obstruction syndrome who received contrast-enhanced computed tomography. A: Image of plain phase. The imaging signs included hepatomegaly, ascites (white star), heterogeneous hypodensity (white arrow); B and C: Image of equilibrium phase. Gallbladder wall thickening (white arrow, B), patchy liver enhancement, “claw-shaped” enhancement surrounding hepatic veins (white arrow, C),obscuring of hepatic main veins; D: Sagittal images. Stenosis of hepatic segmental inferior vena cava (white arrow) was indicated.
CRITERIA FOR DIAGNOSIS OF PAs-INDUCED HSOS
In clinical practice, the combination of history, clinical manifestation, laboratory data,imaging, biomarkers, and pathological findings has established diagnosis of diseases.Diagnosis of HSOS relies on history, clinical symptoms, laboratory examination,imaging, biomarkers, and liver histopathology. To date, most of the studies on diagnostic criteria of HSOS have focused on HSCT-related HSOS. The diagnosis of HSCT-related HSOS is largely based on history and the classical triad of weight gain,painful hepatomegaly, and jaundice. Symptoms of HSCT-related HSOS occur within 10 d after HSCT. Established clinical criteria, including modified Seattle[49]and Baltimore[50]criteria, require that patients must be within 21 d after HSCT to establish the diagnosis (Table 2). Cytoreductive therapy prior to HSCT is essential for diagnosis of HSOS. However, late onset HSOS/HVOD beyond day 21 has been reported. The researchers thus recommended including late onset HSOS/HVOD (beyond day 21) in HSCT-related HSOS[6].
Since clinical manifestations of the PAs-induced HSOS and HSCT-related HSOS are similar, some of the diagnostic criteria for PAs-induced HSOS have been developed according to those for HSCT-related HSOS (Table 2). History of PAs exposure is essential to establish the diagnosis. However, PAs exposure is always obscure, due to variability of the plant components, storage conditions for the plant products,mislabeling or misidentification of the plant, and outright contamination[5].Fortunately, a serum biomarker derived from toxic PAs metabolites has been identified in patients with PAs-induced HSOS. PPAs are used as biomarkers of toxic PAs exposure. PPAs measured by ultra-performance liquid chromatography-tandem mass spectrometry are highly sensitive and specific for diagnosis of PAs-induced HSOS. Thus, the measurement of PPAs should be used together with the conventional HSOS clinical criteria for definitive diagnosis of PAs-induced HSOS. Given this, a history of PAs exposure, biomarkers, and clinical symptoms provide important evidence for the diagnosis of PAs-induced HSOS. Thus, the diagnostic criteria proposed by Gao et al[51]were as follows: (1) Meeting the criteria for drug-induced liver injury, Roussel Uclaf Causality Assessment Method score > 3; (2) Meeting the modified Seattle criteria for HSOS; and (3) A history of PAs exposure and detection of PPAs. These criteria (Table 2) have been used in some studies[37,45]. However, blood PPA concentrations decrease after 40 d exposure to PAs. Moreover, blood PPA concentration is related to the severity of HSOS, and PAs metabolites bind with multiple serum proteins that affect blood PPA concentration. All these confined the diagnostic value of PPA determination. More importantly, this assay is not performed in most hospitals.
Recently, some studies have described the imaging signs of PAs-induced HSOS and demonstrated the diagnostic value of the imaging techniques[39,42,44,45]. Histopathological findings provide confirmative evidence of PAs-induced HSOS. Thus,imaging techniques and liver biopsy were incorporated into the Nanjing Criteria[16](proposed by the Chinese Society of Gastroenterology Committee of Hepatobiliary Disease). However, prospective data on performance of Nanjing Criteria have not been provided until now, and further studies should be performed to evaluate their diagnostic performance in large sample sizes. Another important issue is the classification of staging and severity. Unfortunately, the natural course and severity of PAs-induced HSOS are still unknown, and relevant data are not available.

Figure 3 A 53-year-old man with pyrrolizidine alkaloids-induced hepatic sinusoidal obstruction syndrome (same patient) received gadoxetic acidenhanced magnetic resonance imaging scan. A: T1-weighted imaging. Ascites (white star), heterogeneous hypointensity (white arrow) were shown; B and C: T2-weighted imaging. Imaging findings included ascites (black star, B), gallbladder wall thickening (white arrow, B), periportal edema (arrowhead, C), heterogeneoushypointensity (white arrow, C); D and E: T2*WI (D) and SWI (E). Heterogeneous hypointensity (white arrow) was observed, and distribution of hypointensity in SWIand T2*WI was similar; F: Image of portal venous phase. Patchy liver enhancement, “claw-shaped” enhancement surrounding hepatic veins (white arrow), stenosis of right hepatic vein (arrowhead) and inferior vena cava (black arrow); G: Image of hepatobiliary phase. Heterogeneous hypointensity (white arrow) was shown. SWI:Susceptibility-weighted imaging; T2*WI: T2*-weighted imaging.
TREATMENT
Current management of PAs-induced HSOS is a challenge for hepatologists.Unfortunately, no definitive treatment for PAs intoxication is available. The therapeutic strategies for PAs-induced HSOS consists of termination of PAs exposure,symptomatic treatment, anticoagulant therapy, transjugular intrahepatic portosystemic shunt (TIPS), liver transplantation, etc. Importantly, different strategies should be performed in patients with PAs-induced HSOS according to disease severity and stage. In mild cases as spontaneous recovery occurs, symptomatic treatment is needed[52]. In severe cases, symptomatic treatment, anticoagulant therapy,TIPS, or liver transplantation should be performed.
Symptomatic treatment is important for PAs-induced HSOS and includes discontinued exposure to PAs, liver protection, and management of ascites. The latter consists of restriction of water and sodium supply, use of diuretics, albumin infusion,serial paracentesis, and TIPS. Oral furosemide and spirolactone are preferentially used for diuretic therapy. Albumin infusion is beneficial in patients with hypoalbuminemia. Serial paracentesis is recommended in patients who have poor response to diuretics. TIPS may be considered to control refractory ascites.Hemodialysis and mechanical airway protection may be required in HSOS patients with multiorgan failure.
Recent studies have demonstrated the beneficial effect of anticoagulant therapy in prevention of HSCT-related HSOS; however, a systematic review and meta-analysis showed negative results[53]. Recently, some studies have been performed to determine the effect of anticoagulant therapy on PAs-induced HSOS. In a retrospective study reported by Nanjing Drum Tower hospital, anticoagulant therapy (low molecular weight heparin combined with warfarin) significantly improved response rate of patients with PAs-induced HSOS compared with the non-anticoagulant group (60%vs 27%)[17]. A retrospective study from the first Affiliated Hospital of Zhengzhou University confirmed these results[37]. In addition, some of the studies demonstrated the effectiveness of anticoagulant therapy in Chinese journals[54-56]. Thus, Chinese guidelines recommend that anticoagulant therapy should be started as soon as possible in patients with acute/subacute HSOS after ruling out contraindications.Low molecular weight heparin is the anticoagulant of choice, either combined with or followed by oral administration of warfarin. The recommended dose of low molecular weight heparin is 100 IU/kg, every 12 h, subcutaneous injection. Warfarin is the preferred oral anticoagulant for long-term therapy, and the recommended international normalized ratio is 2.0-3.0, which may satisfy both the anticoagulant effect and safety. To date, prospective multicenter studies have not been performed to determine the effectiveness of anticoagulant therapy, thus large randomized control trials (RCTs) should be performed in future.

Figure 4 Pathology of pyrrolizidine alkaloids-induced hepatic sinusoidal obstruction syndrome. A: Acutestage (200 ×). Dilation and congestion of hepatic sinusoidals, the penetration of erythrocytes into the space of Disse;B: Subacute stage (200 ×). Complete loss of pericentral hepatocytes, sinusoidal dilatation.
Defibrotide is the only approved drug for severe HSCT-related HSOS and HSCTrelated HSOS with renal or pulmonary dysfunction in western countries[57]. Since defibrotide is not approved in China, its effectiveness remains unknown in patients with PAs-induced HSOS. Steroids have also been used in HSOS patients, and beneficial effects of early high-dose steroid therapy have been shown in severe pediatric HSCT-related HSOS[58], but adverse events such as infection should be considered. Although sporadic reports show the effectiveness of steroids in both PAsinduced HSOS patients and mouse models[59-61], the effectiveness of steroids for PAsinduced HSOS remains inconclusive and further RCTs should be performed.
TIPS may be considered when PAs-induced HSOS patients have refractory ascites or severe portal-venous hypertension. In a retrospective study, TIPS improved the prognosis of 29 patients who did not respond to symptomatic treatment plus anticoagulation[17]. While another retrospective study showed that TIPS failed to improve survival[37]. The efficacy of TIPS should be evaluated based on RCTs in the future. Liver transplantation can be considered in severe cases of PAs-induced HSOS with liver failure, and in theory, this should improve survival. Other treatments such as ursodeoxycholic acid, antithrombin III, and recombinant human thrombomodulin have been tried in HSCT-related HSOS, but the evidence in PAs-induced HSOS is lacking.
OUTCOME
The mortality rate of PAs-induced HSOS is reported to vary from 16% to 40%[17,26,37,45,51],and a common cause of death is liver failure[26,37]. A systematic review showed that increased total bilirubin and aspartate transaminase are indicators of poor survival in patients with PAs-induced HSOS[26]. A retrospective study of 117 cases demonstrated that hepatic encephalopathy, serum bilirubin, and albumin levels were major prognostic factors for Gynura segetum-induced HVOD[37]. In addition, Gao et al[51]demonstrated that PPA concentration was related to the severity and clinical outcome of PAs-induced HSOS.
CONCLUSION
The intake of PAs is one of the major etiological causes of HSOS in China. Here, we described the pathogenesis, clinical profiles, diagnostic criteria, treatment, and outcomes of patients with PAs-induced HSOS. Although progress has been made in PAs-induced HSOS, several issues remain to be resolved. A suitable animal model of PAs-induced HSOS should be established through repeated administration of PAs,which resembles the pathological status of PAs-induced HSOS in humans; the Nanjing criteria should be validated in prospective studies; disease severity grading should be developed; large-scale RCTs should be performed to determine the safety and efficacy of therapeutic strategies for PAs-induced HSOS; and prognostic factors should be accurately identified.
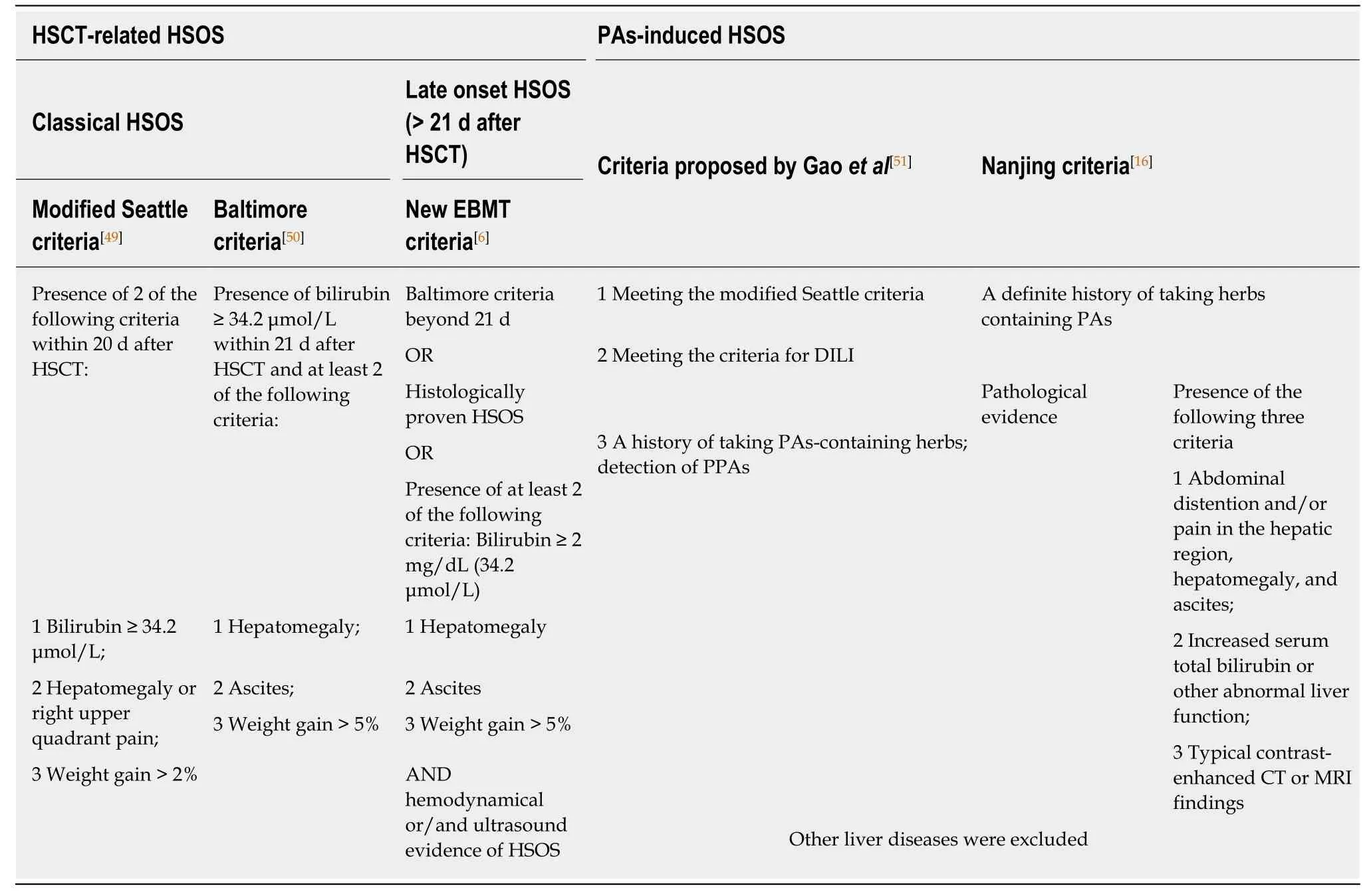
Table 2 Diagnostic criteria for hepatic sinusoidal obstruction syndrome
杂志排行
World Journal of Gastroenterology的其它文章
- Role of sodium-glucose co-transporter-2 inhibitors in the management of nonalcoholic fatty liver disease
- lmportance of fatigue and its measurement in chronic liver disease
- Acute kidney injury spectrum in patients with chronic liver disease:Where do we stand?
- Neoadjuvant and adjuvant treatment strategies for hepatocellular carcinoma
- Surgical techniques and postoperative management to prevent postoperative pancreatic fistula after pancreatic surgery
- Current approaches to the management of patients with cirrhotic ascites
