Neoadjuvant and adjuvant treatment strategies for hepatocellular carcinoma
2019-08-12CliffordAkatehSylvesterBlackLanlaContehEricMillerAnneNoonanEricElliottTimothyPawlikAllanTsungJordanCloyd
Clifford Akateh, Sylvester M Black, Lanla Conteh, Eric D Miller, Anne Noonan, Eric Elliott, Timothy M Pawlik,Allan Tsung, Jordan M Cloyd
AbstractHepatocellular carcinoma (HCC) is the most common liver malignancy worldwide and a major cause of cancer-related mortality for which liver resection is an important curative-intent treatment option. However, many patients present with advanced disease and with underlying chronic liver disease and/or cirrhosis, limiting the proportion of patients who are surgical candidates. In addition, the development of recurrent or de novo cancers following surgical resection is common. These issues have led investigators to evaluate the benefit of neoadjuvant and adjuvant treatment strategies aimed at improving resectability rates and decreasing recurrence rates. While high-level evidence to guide treatment decision making is lacking, recent advances in locoregional and systemic therapies, including antiviral treatment and immunotherapy, raise the Peer-review started: March 19, 2019 First decision: April 30, 2019 Revised: June 13, 2019 Accepted: June 22, 2019
Key words: Hepatocellular carcinoma; Neoadjuvant therapy; Adjuvant therapy; Neoplasm recurrence; Hepatectomy; Liver cirrhosis
INTRODUCTION
Hepatocellular carcinoma (HCC) is a major cause of cancer-related mortality worldwide, representing the third most common malignancy in men and seventh in women[1,2]. In the United States, there was an estimated 42220 new cases of HCC and an estimated 30200 HCC-related deaths in 2018[3]. Unfortunately, the incidence of HCC is rising and, unlike most other cancers, this increased incidence affects all major demographic groups and populations[4,5]. This increase is particularly higher in men,who have a four-fold increased risk of developing HCC[6,7]. The vast majority of HCC occurs in the setting of chronic liver disease (CLD) with or without cirrhosis most often secondary to chronic hepatitis B (HBV) and hepatitis C (HCV) infections,although there is also a rising incidence of non-alcoholic steatohepatitis (NASH) and non-alcoholic fatty liver disease (NAFLD) related cirrhosis[8-11]. Other major causes include alcoholic cirrhosis, as well as diabetes, hemochromatosis, Alpha 1-antitrypsin deficiency, Wilson’s disease, hemophilia, and Aflatoxin[12-15]. Thus, patients with HCC have two simultaneous challenges: the malignancy itself and the underlying liver disease which can both complicate treatment and predispose to the development of recurrent or de novo cancers.
While numerous systemic and locoregional treatments exist for patients with HCC,curative-intent options mainly include liver transplantation (LT), surgical resection,and ablative therapies. Although LT is appealing in that it treats both the cancer and the underlying CLD, a major challenge is the deficiency of available organs in the United States and around the world. Furthermore, many patients with relatively preserved liver function (e.g., chronic HBV) or those outside Milan criteria (e.g., large solitary tumor or macrovascular invasion) are not eligible for LT. Ablation is a reasonable treatment option, but its outcomes are optimized in patients with small tumors[16]. Therefore, surgical resection remains an important primary treatment option for HCC which, in appropriately selected patients and when performed by experienced surgical teams, is associated with excellent results. Unfortunately, a minority of patients are surgical candidates due to either advanced disease or inadequate liver function to safely undergo hepatectomy. Furthermore, recurrence rates among those patients who are able to undergo surgical resection is relatively common[17].
The last few decades have led to significant advances in both the treatment of viral hepatitis as well as systemic and locoregional treatment options for HCC. Whether these strategies can be used prior to or following surgical resection in order to increase the number of patients who are surgical candidates or to reduce the risk of tumor recurrence following resection remains poorly understood. Indeed, relatively little research has been conducted on the optimal multimodality therapy for patients with surgically resected HCC. In this review paper, we sought to evaluate the available literature on neoadjuvant and adjuvant treatment strategies for patients with resectable HCC.
NEOADJUVANT STRATEGIES FOR HCC
While neoadjuvant therapies are commonly used for patients with other solid-organ malignancies in order to downstage advanced disease, ensure appropriate patient selection, and assess tumor response to treatment prior to resection, the role of neoadjuvant therapies in the management of HCC is less well defined. Indeed,relatively little research exists to support the concept of neoadjuvant therapy, and current guidelines do not recommend this strategy for patients with otherwise potentially resectable cancers. On the other hand, the unique characteristics of HCC,including its relatively aggressive biology, frequent diagnosis at late stages, and the need to preserve normal liver function at the time of surgery given underlying CLD,suggest a neoadjuvant approach may be appropriate.
Transarterial chemoembolization
Transarterial chemoembolization (TACE) combines transarterial embolization (TAE)with chemotherapy infusion. Unlike normal liver tissue, which derives most of its blood supply from the portal venous system, HCCs derive most of their blood supply from the hepatic artery system. As such, embolization of the arterial system results in ischemia and tissue necrosis[18]while allowing for the concentrated delivery of chemotherapy agents. Embolization also prevents the chemotherapeutic agent from being washed out, allowing for a longer duration of action.
TACE was originally developed for management of advanced unresectable disease,but its role in the neoadjuvant treatment of potentially resectable disease has also been explored. One of the earliest experiences with neoadjuvant TACE was reported by Monden et al[19]. The investigators compared 71 patients treated preoperatively with TACE to 21 patients resected without TACE. Although they did not find any differences in survival, histopathologic review demonstrated that tumors from most of the patients who underwent TACE procedure were necrotized. In 2000, Zhang et al[20]conducted a retrospective review of 1457 patients who underwent hepatic resection for HCC at their hospital, including 120 patients treated preoperatively with TACE. Compared to those resected without TACE, patients who underwent preoperative TACE had significantly improved 5year diseasefree survival (DFS). In addition, patients who had more than two preoperative TACE treatments had longer recurrence-free survival (RFS) compared to patients who only had one session.However, a different institutional study from China comparing 183 neoadjuvant TACE to 405 resection-only cases found no difference in 1-, 3-, and 5-year overall survival (OS). Instead, repeated TACE use was associated with significantly higher hospital cost[21]. However, results from a meta-analysis by Qi et al[22]provided some insight into the discordant results. In their analysis of 32 randomized and nonrandomized studies evaluating preoperative TACE to resection-only, they found that preoperative TACE did not improve DFS or OS. However, a subgroup analysis of the results suggested that the outcomes of neoadjuvant TACE followed by resection were influenced by the response to TACE. When patients had complete tumor necrosis following TACE, preoperative TACE had significantly better DFS and OS compared to resection alone. However, when patients had incomplete or no tumor necrosis, the OS did not differ between the two groups.
In addition to its prognostic impact, investigators have shown interest in the ability to downstage previously unresectable patients with neoadjuvant TACE[23-25]. Zhang et al[26]reviewed the results from 831 patients over 10 years treated with TACE. Of these,82 patients were successfully downstaged, and 43 underwent salvage surgery.Compared to those who refused a salvage resection, those who underwent resection had a longer median OS (49 mo vs 31 mo, Ρ = 0.027). However, there was no difference in survival based on the receipt of surgery among patients who experienced a complete response (50 mo vs 54 mo, Ρ = 0.699) compared to patients with only a partial response (49 mo vs 24 mo, Ρ < 0.001). These findings suggest a critical role for resection following downstaging with TACE in patients with a partial response.
Preoperative TACE has also been investigated in the management of recurrent but resectable disease. Tao et al[27]showed that for patients with recurrent but resectable disease, preoperative TACE did not improve survival. On the other hand, it was associated with increased preoperative time and increased blood loss. For patients undergoing extended resections who require preoperative portal vein embolization(PVE) to stimulate compensatory hypertrophy of the future liver remnant[28],preoperative TACE has been investigated as a means of controlling tumor growth.Some speculate that, in the absence of TACE, PVE can result in increased ipsilateral hepatic artery flow, and as such, increased tumor growth. Indeed, some studies have demonstrated improved RFS and OS among patients undergoing TACE+PVE compared to PVE alone[29,30].
In conclusion, the role of TACE in neoadjuvant therapy continues to evolve. While some studies suggest an opportunity to downstage some patients to resection as well as improved long-term outcomes among patients who develop a radiographic response, these therapies currently apply to a minority of patients with HCC, and there is insufficient evidence to predict which patients will respond. Therefore, while TACE is commonly used as a bridging therapy prior to LT[31], its role in the neoadjuvant setting prior to resection remains unclear and is not routinely recommended.
Transarterial radioembolization
Transarterial radioembolization (TARE) is increasingly being performed as an alternative to TACE for patients with HCC. TARE uses90Yttrium (Y-90) loaded microspheres and delivers these radioactive microspheres via arterial cannulation of the feeding vessel[32]. While there is no overwhelming evidence to suggest superiority over TACE, TARE offers several advantages, including a lower side effect profile (i.e.,less post-embolization syndrome)[33]. In addition, for potentially resectable patients,TARE leads to hypertrophy of the contralateral future liver remnant (FLR) in addition to its cytotoxic effects on the tumor[34-36]. This characteristic of TARE has given rise to the concept of radiation lobectomy (RL), a technique which induces hypertrophy to equal or higher levels than PVE[34]. In 2013, Vouche et al[35]reported on 67 patients with HCC subjected to Y-90 radioembolization. 37% of the patients had a greater than 35%increase in the FLR, of whom 3 patients went on to have a successful right hepatectomy, and 6 were transplanted. In 2016, they reported on another group of 10 patients with HCC and insufficient or borderline FLR who underwent Y-90 RL prior to resection. Following RL, the median FLR increased from about 33% (pre-RL) to about 43% (post-RL). Additionally, they reported > 50% necrosis in greater than 92%of the resected tumors[37]. TARE has also been combined with PVE to successfully downstage patients for resection when additional FLR hypertrophy is required[38].
Another advantage of TARE is its application in patients with portal vein thrombosis. Patients with malignant lobar portal vein thrombosis are typically not considered candidates for TACE. Therefore, TARE offers an alternate and preferable approach in the neoadjuvant management of such patients. In a previously reported non-randomized trial comparing TARE to TACE, TARE resulted in a better response than TACE (61% vs 37% partial response) and resulted in more patients being downstaged from UNOS T3 to T2, which could be critical for patients awaiting transplantation[39]. So, while most guidelines do not recommend one modality over the other for downstaging[40-43], an expert consensus group recommend TARE over TACE as a bridging/downstaging therapy for patients with portal venous thrombosis[44].Therefore, while further research is needed, including prospective clinical trials,neoadjuvant TARE with Y-90 may be appropriate for patients with advanced HCC who require downstaging for resection.
Systemic therapy
Until recently, there have been few effective systemic therapy options for patients with HCC. In 2007, the tyrosine kinase inhibitor (TKI) Sorafenib was approved for use in patients with Childs A cirrhosis and unresectable or metastatic HCC[45-47]. Sorafenib has activity against several tyrosine kinases including Raf, as well as vascular endothelial growth factor (VEGF) and platelet-derived growth factor (PDGF)[48,49].Despite its poor side effect profile and OS improvement of < 3 mo, it quickly gained favor given the lack of other systemic therapy options available until 2017. While interest in Sorafenib as a bridging therapy to transplant was abundant[50,51], it was not felt to be an effective downstaging agent to facilitate surgical resection given the minimal response rate observed. Nevertheless, interest in the use of Sorafenib as a neoadjuvant chemotherapeutic agent remained[52,53]but its use has largely been limited by toxicity[54-57].
Over the past several years, there has been an explosion of newer agents approved for use or currently in clinical trials for advanced or metastatic HCC. For example,other TKIs and VEGF inhibitors including Lenvatinib[58], cabozantinib[59],
regorafenib[60], and ramucirumab[61]have all demonstrated efficacy against HCC.However, none have been investigated in the neoadjuvant setting. Some of the most exciting progress has been made in immunotherapy. Nivolumab[62,63], pembrolizumab[64], tremelimumab[65], atezolizumab[66]and chimeric antigen receptor (CAR)T cells[67]have all been investigated or are under investigation for management of advanced HCC. Investigations of these immunotherapeutic agents in the neoadjuvant setting are ongoing at this time[68-70].
Anti-viral therapy
Among patients with HBV or HCV related HCC, a primary contributor to late recurrence is the de novo formation of new tumors related to underlying chronic viral hepatitis[71,72]. Therefore, antiviral therapy prior to resection of HCC should be considered as part of the multidisciplinary treatment of these patients. Indeed,preoperative antiviral therapy has been associated with lower vascular invasion,decreased recurrence, decreased morbidity and faster recovery of liver function in HBV-related HCC[73,74]. On the other hand, antiviral therapy has been more commonly used in the adjuvant setting, which will be described later in this review.
ADJUVANT STRATEGIES FOR HCC
While neoadjuvant strategies are often employed to facilitate downstaging of unresectable patients to potentially resectable candidates, immediate surgical resection remains the recommended treatment for patients with resectable tumors and appropriate liver function. However, recurrence is relatively common even among patients who undergo surgical resection. The purpose of adjuvant therapy, therefore,is to help decrease the incidence of HCC recurrence among those who undergo surgical resection. In general, recurrences following resection of HCC occur in two patterns: early and late. Early recurrences are typically thought to be related to negative prognostic factors (e.g., margin positivity, vascular invasion, etc.) associated with the primary tumor while late recurrences are more likely related to underlying CLD and the development of de novo tumors. Adjuvant strategies that address both of these factors may be most effective.
Antiviral therapy
It has been long known that antiviral therapy following resection of HBV or HCV related HCC may improve outcomes[75-77]. In a 2013 matched control study by Hsu et al[78], researchers compared patients treated with pegylated interferon (PEG-IFN) plus ribavirin to a matched anti-viral naïve cohort and found significantly lower rates of HCC recurrence at 5 years in patients treated with interferon (52.1% vs 63.9%, Ρ =0.001). Lee et al[79], also, reported on a prospective trial of PEG-IFN following surgical resection, which included 93 patients (31 treated and 62 controls). They reported significantly lower recurrence rates at 1 and 2 years treated with interferon (7% vs 24%and 14% vs 34% respectively, Ρ=0.029). These findings were confirmed in a recent meta-analysis by Wu et al[80]. They reviewed 1 RCT and 4 cohort studies, totaling 1356 patients (345 PEG-IFN and 1011 control group) and reported a significant reduction in 3-year and 5-year recurrence rates with PEG-IFN treatment.
The development of direct-acting antiviral drugs (DAA) against HCV has increased the emphasis on adjuvant anti-viral therapy[81]. These interferon-free antivirals directly target the specific nonstructural proteins of the viral replication cycle, limiting replication and infectivity. The current classes include nonstructural proteins 3/4A(NS3/4A) protease inhibitors (PIs), NS5A complex inhibitors, NS5B nucleotide polymerase inhibitors (NPIs), and NS5B non-nucleotide polymerase inhibitors(NNPIs). Combining two or more of these agents has been associated with up to 90%HCV clearance[82]. While adequate surgical resection is necessary for disease control and to improve long-term survival, postoperative control of the chronic viral infection, and maintenance of a sustained virologic response remain critical in preventing recurrence and in ensuring a favorable outcome[83]. While an initial report by Reig et al[84]initially suggested an increase in recurrence rates among patients treated with DAA drugs, this findings was refuted by a large multicenter by Singal et al[85]. In this large retrospective analysis of 783 patients, 304 (38.3%) of whom received DAA agents, there was no significant difference in overall or early HCC recurrence.Indeed, there might be evidence to the contrary. Using propensity-score matching,Cabibbo et al[86]compared 102 patients with BCLC stage O or A HCC treated with DAA agents following curative resection or ablation to 102 matched controls. The researchers reported a significantly higher OS in the DAA group compared to the non-DAA group (HR = 0.39 Ρ = 0.03). In addition, patients in the DAA group who achieved a sustained virologic response had a better OS, lower HCC recurrence rate and decreased incidence of hepatic decompensation. However, there was no difference in HCC recurrence between the DAA and non-DAA groups. As noted by Nault et al[87], despite these encouraging findings, more studies are needed to resolve this controversy.
The use of nucleoside analogs for adjuvant HBV treatment in HBV related HCC has also shown promise (Table 1). Indeed, multiple studies have demonstrated the efficacy of adjuvant antiviral therapy both for decreasing recurrence and for improving outcomes[72,88-91]. Huang et al[72]evaluated the role of nucleoside analogs on HCC recurrence in two separate RCTs. In 2015, the investigators randomized 200 patients with chronic HBV and no previous antiviral therapy, who had undergone an R0 resection of HCC to either postoperative antiviral therapy or no treatment. These authors reported a significant improvement in both OS and RFS. However, all patients in the study had high preoperative HBV-DNA levels (> 2000 IU/mL). More recently, they reported on a separate cohort, all with low (< 2000 IU/mL) preoperative viral levels. Following resection, patients were randomized to receive telbivudine daily or no treatment. The patients in the adjuvant antiviral treatment group had a significantly better 5-year OS (64% vs 44%) and RFS (52% vs 32%). Additionally, the treated patients had lower HBV reactivation rates[92]. Based on these studies, it may be appropriate to treat all patients with chronic HBV or HCV with antiviral therapy following resection, regardless of viral load. Current NCCN guidelines recommend consideration of such an approach[43,94].
Systemic therapy
Although systemic chemotherapy with is commonly used for patients with advanced and metastatic HCC, its use following curative resection is controversial. While early studies suggested that adjuvant systemic chemotherapy might be associated with decreased recurrence and prolonged RFS, other studies have found no benefit[95-97]. In contrast, some studies have shown that adjuvant chemotherapy may be associated with worse outcomes[96,98], which suggest that outcomes may be largely driven by the specific chemotherapeutic regimen and patient population. The STORM trial was a randomized phase 3, double-blind, placebo-controlled trial designed to evaluate the efficacy of sorafenib as adjuvant therapy in patients with resected HCC (although it included some patients with local ablation only). It included about 900 patients (450 in each arm) across 202 sites and in 28 countries who had undergone curative resection with evidence complete disease removal on radiography. After a median duration of about 12.5 months of treatment, there was no difference in RFS between the two groups. Instead, sorafenib treatment was associated with increased adverse effects,including four deaths[99].
The advent of immunotherapy and promising results of immunotherapeutic agents in advanced HCC has renewed interest in adjuvant systemic therapy following resection of HCC. One of the earliest uses of immunotherapy in the adjuvant setting was by Takayama et al[100]. From 1992-1995, the researchers randomized 150 patients to either lymphocyte infusions (termed adoptive immunotherapy) or no adjuvant treatment. Patients in the adoptive immunotherapy arm had a 41% decreased risk of recurrence and significantly longer RFS; however, OS was not different. Tumordirected vaccines also showed moderate success as adjuvant therapy, decreasing tumor recurrence rates and increasing recurrence-free and OS[101,102]. In 2009, Hui et al[103]published on the results of adjuvant immunotherapy with cytokine-induced killer cells following HCC resection. While there was no difference in survival,patients in the immunotherapy group experienced a decrease in the rate of metastasis formation. However, in a 2015 study by Lee et al[104]using autologous cytokine induce killer T-cells and NK cells, the researchers reported increased RFS and OS in the immunotherapy group compared to no adjuvant therapy, though a minority of patients underwent surgery in the study.
The recent discovery of PD-1 and PD-L1 upregulation in tumor infiltrating lymphocytes in HCC and HCC-associated Kupffer cells[105-107], as well as promising results in patients with advanced HCC, has renewed interest in the use of these checkpoint inhibitors as adjuvant therapy following resection[62,65,108]. Unfortunately,there are no published randomized trials evaluating this approach and most of the current trials are evaluating the outcomes of patients with advanced disease[68-70,109].However, the CheckMate 9DX is an ongoing trial evaluating the use of adjuvant Nivolumab in patients with HCC who are at high risk of recurrence after curative resection or ablation[110,111]. The findings of this trial are greatly anticipated.
Hepatic artery infusion pump
While hepatic arterial infusion (HAI) therapy is more commonly used in the management of colorectal liver metastases[112], its role in HCC remains limited[113].Nevertheless, at least one study has evaluated the role of HAI as adjuvant therapy
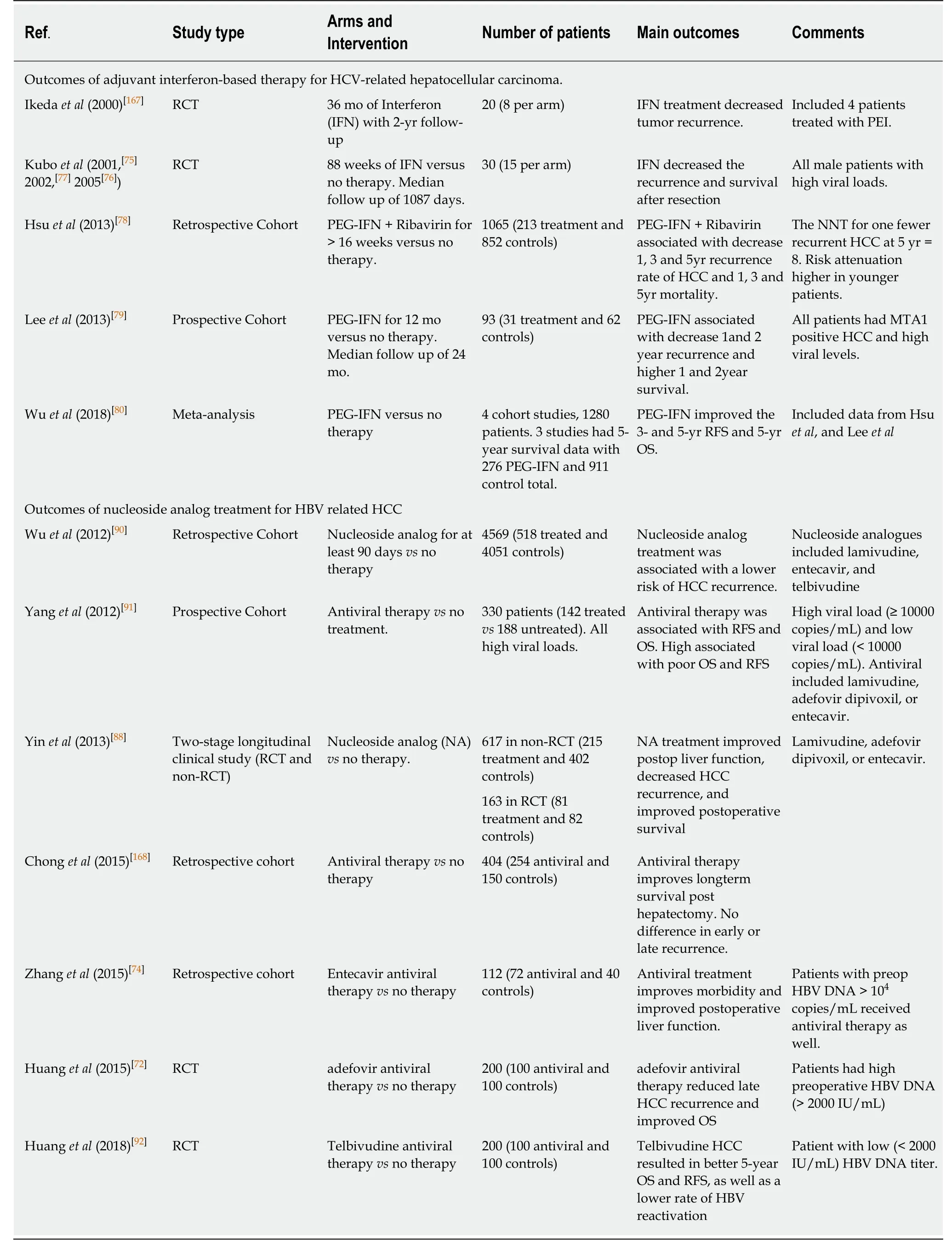
Table 1 Selected studies on the use of adjuvant antiviral therapy
following HCC resection. In this retrospective study (42 patients in each group), the investigators compared patients who received HAI chemotherapy to those who received no adjuvant treatment following curative resection for HCC and reported decreased intrahepatic recurrence, decreased RFS and OS at 5 years in patients who received HAI pump chemotherapy. The chemotherapeutic regimen: 5-fluorouracil(1000 mg/m2), oxaliplatin (85 mg/m2), and mitomycin-C (6 mg/m2) was used in this trial, and started within 3 wk of surgery[114]. As noted above, this treatment option is rarely used in clinical practice for HCC management.
TACEWhile TACE is primarily used in the neoadjuvant setting and for patients with unresectable disease, it has also been evaluated as an adjuvant regimen following resection, though with mixed results. Wang et al reported a phase III RCT of 280 highrisk patients with HBV-related HCC who were randomized to TACE or surveillance following curative hepatectomy. Patients in the TACE arm had significantly less recurrence and longer RFS and OS[115]. However, another trial involving low-risk patients was unable to reproduce these findings[116]. Multiple large single-institution studies have also found a benefit for TACE in patients with risk factors for recurrence[117-119]. Additionally, data from various meta-analyses and systematic reviews of the randomized studies in adjuvant TACE treatment suggest that this regimen may be of benefit in high-risk patients (tumor > 5 cm or vascular invasion).However, there does not appear to be any benefit in low-risk patients (see table below)[22,120-123]. While randomized controlled trials are rare, the findings from the current studies suggest adjuvant TACE treatment might be of benefit in resected patients at high risk for recurrence (Table 2).
Radiolabeled lipiodolLipiodol, derived from poppy seed, has been used as a radiotracer and contrast dye since the 1920s, including in the imaging of hepatic cancers[124]. In 1979, Nakakuma et al[125]reported on the ability of lipiodol to accumulate in HCC relative to normal liver.Injection of the molecule into the hepatic artery resulted in tumor necrosis, and therefore it was investigated as a treatment for HCC with promising results[126,127].Further use of radiolabeled lipiodol has also been investigated in the adjuvant setting.In 2000, Partensky et al[128]conducted a phase 2 study evaluating the role of lipiodol in the adjuvant setting, confirming its safety and potential benefits. A prospective randomized trial by Lau et al[129,130]found that adjuvant radiolabeled lipiodol (131ILipiodol) following resection of HCC was associated with improved DFS and OS.These findings have been replicated in other retrospective studies and metaanalyses[131-133]. Overall, the current data strongly favor the use of intra-arterial radiolabeled lipiodol as adjuvant therapy for HCC. However, this approach is not routinely used in clinical practice and requires further validation from large RCTs in order to be incorporated into practice.
AblationTumor ablation is a form of local-regional directed therapy in patients with nonmetastatic disease. Local ablation can include radiofrequency ablation (RFA)[134,135],percutaneous ethanol injection (PEI)[136-139], microwave ablation[140-142]or irreversible electroporation[143-145]. There is insufficient evidence supporting the use of one approach over the other[140,146], and as such the choice of local ablation therapy is primarily driven by institutional expertise. Ablation is typically used as either definitive therapy or as a bridging therapy to LT, enabling patients to either remain within the Milan criteria[16,147-149]or be downstaged to meet criteria[150,151]. As an adjuvant regimen, it can allow for the extension of the resection margin following tumor resection or debulking[152-155]. In some cases, it can be combined with resection, where the majority of the tumor is resected, and satellite nodules are ablated[156,157]. This combination has been associated with decreased recurrence and improved long-term survival.
Radiation therapyWhile radiation therapy (RT) had traditionally been avoided in the liver due to the risk of radiation-induced liver disease and limited response, advances in technology and understanding of dose-volume effects has allowed for the use stereotactic body radiation (SBRT) in the management of HCC, primarily in patients with no other standard options but also as an adjunct to other therapeutic therapies[158-160]. While there is limited data on its use as a neoadjuvant or adjuvant therapy to surgical resection, it might be of benefit in patients where adequate margins are not attainable[161]. Some studies have suggested that adjuvant RT might be better than TACE with respect to RFS and OS[162]. However, a recent retrospective analysis of the SEER database showed preoperative radiation therapy had better outcomes (OS)compared to adjuvant RT[163]. Overall, while there is great interest in the use of SBRT as a bridge to transplantation[164-166], its use as an adjunct to surgical resection remains underexplored.
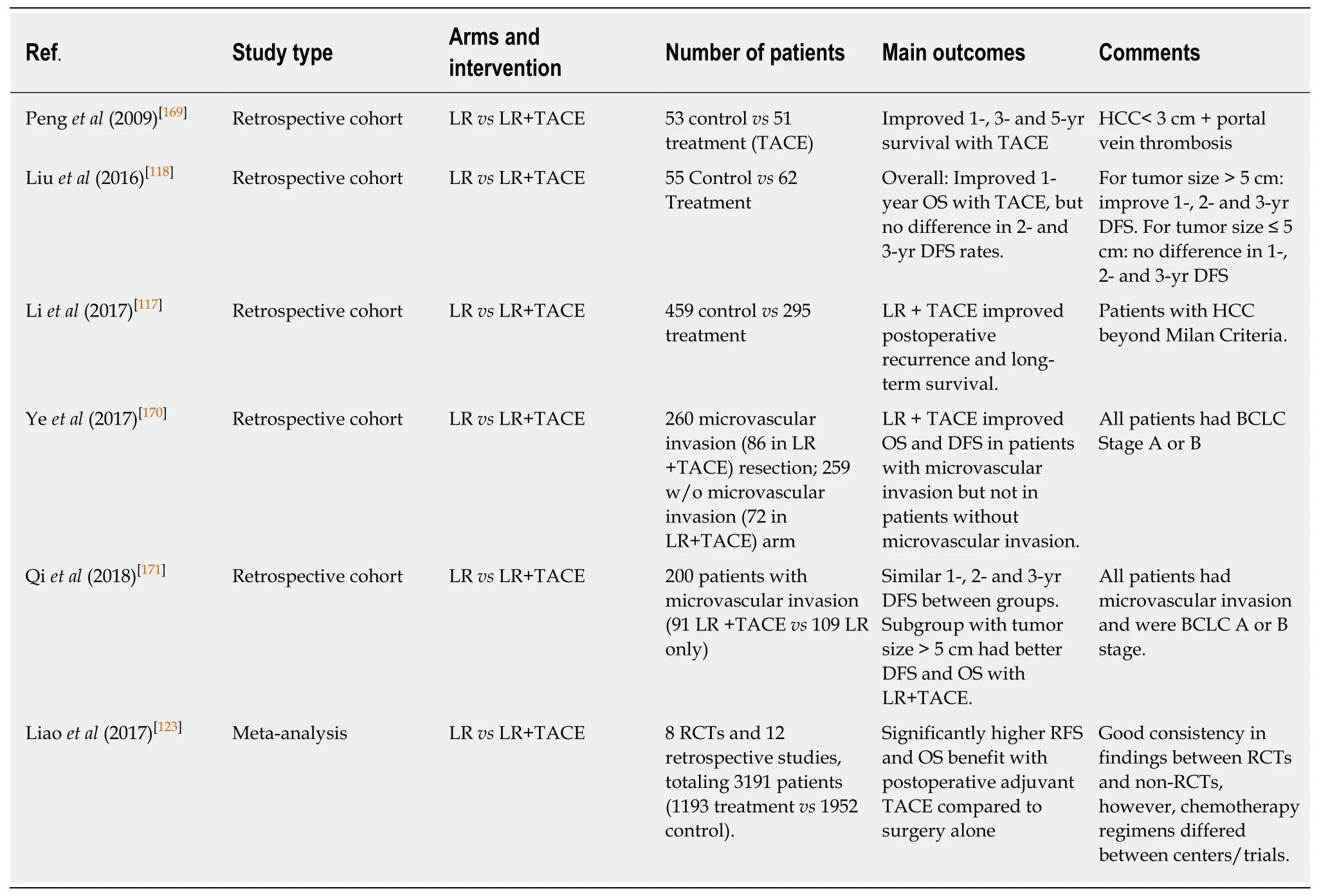
Table 2 Selected studies on the use of adjuvant transarterial chemotherapy
CONCLUSION
HCC remains a challenging disease, with an increasing global incidence and high associated mortality. Resection remains an important curative-intent treatment that should be pursued for patients with resectable disease and appropriate liver function.Multimodality therapy is increasingly being explored in order to increase the number of patients who are surgical candidates as well as decrease the incidence of disease recurrence. Unfortunately, due to the paucity of conclusive literature on the subject,current NCCN guidelines do not recommend for or against the routine use of(neo)adjuvant strategies in HCC resection.
While more commonly used as a bridging therapy prior to LT[94], neoadjuvant transarterial therapies can successfully downstage some patients with advanced tumors to resection. There is insufficient evidence to directly compare preoperative TACE versus TARE though radioembolization with Y-90 has the advantage of stimulating contralateral hypertrophy of the FLR without the need for PVE. Longterm outcomes are improved among those patients who experience a response to neoadjuvant therapy. A major challenge in performing research on neoadjuvant treatments is defining the intent of therapy (e.g., definitive, downstaging, or bridge to transplantation) a priori. Future research would benefit from well-designed prospective studies that clearly define goals of treatment and carefully measure shortand long-term outcomes. Following resection, based on a large phase III RCT,adjuvant Sorafenib is not recommended, but there is insufficient evidence to support the use of other adjuvant therapies. For those patients with HCC in the setting of viral hepatitis, aggressive treatment with antivirals, before or after resection, improves outcomes and should be pursued. The outcomes of these therapies reflect the different mechanisms of HCC recurrence following surgical resection: multicentric carcinogenesis vs intrahepatic metastasis. While antiviral therapies are effective in decreasing neocarcinogenesis, they have less impact on intrahepatic metastases.Cytotoxic therapies aim to decrease recurrence from intrahepatic metastases, but to date have demonstrated limited effectiveness.. The development of more effective targeted and immune-based therapies will hopefully lead to significant advances in recurrence rates.
In conclusion, the optimal multidisciplinary management of HCC continues to rapidly evolve. While surgical resection remains an important treatment option for patients with HCC, the addition of neoadjuvant and/or adjuvant treatment strategies may increase the proportion of patients who are surgical candidates and improve the long-term outcomes of those who undergo surgery. With exciting advances in locoregional and systemic therapies, including developments in immunotherapy,future research will be needed to identify the optimal components of multimodality therapy.
杂志排行
World Journal of Gastroenterology的其它文章
- Role of sodium-glucose co-transporter-2 inhibitors in the management of nonalcoholic fatty liver disease
- lmportance of fatigue and its measurement in chronic liver disease
- Acute kidney injury spectrum in patients with chronic liver disease:Where do we stand?
- Surgical techniques and postoperative management to prevent postoperative pancreatic fistula after pancreatic surgery
- Current approaches to the management of patients with cirrhotic ascites
- Pyrrolizidine alkaloids-induced hepatic sinusoidal obstruction syndrome: Pathogenesis, clinical manifestations, diagnosis,treatment, and outcomes
