lmportance of fatigue and its measurement in chronic liver disease
2019-08-12LynnGerberAliWeinsteinRohiniMehtaZobairYounossi
Lynn H Gerber, Ali A Weinstein, Rohini Mehta, Zobair M Younossi
AbstractThe mechanisms of fatigue in the group of people with non-alcoholic fatty liver disease and non-alcoholic steatohepatitis are protean. The liver is central in the pathogenesis of fatigue because it uniquely regulates much of the storage, release and production of substrate for energy generation. It is exquisitely sensitive to the feedback controlling the uptake and release of these energy generation substrates.Metabolic contributors to fatigue, beginning with the uptake of substrate from the gut, the passage through the portal system to hepatic storage and release of energy to target organs (muscle and brain) are central to understanding fatigue in patients with chronic liver disease. Inflammation either causing or resulting from chronic liver disease contributes to fatigue, although inflammation has not been demonstrated to be causal. It is this unique combination of factors, the nexus of metabolic abnormality and the inflammatory burden of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis that creates pathways to different types of fatigue. Many use the terms central and peripheral fatigue. Central fatigue is characterized by a lack of self-motivation and can manifest both in physical and mental activities. Peripheral fatigue is classically manifested by neuromuscular dysfunction and muscle weakness. Therefore, the distinction is often seen as a difference between intention (central fatigue) versus ability (peripheral fatigue).New approaches to measuring fatigue include the use of objective measures as well as patient reported outcomes. These measures have improved the precision with which we are able to describe fatigue. The measures of fatigue severity and its impact on usual daily routines in this population have also been improved,and they are more generally accepted as reliable and sensitive. Several approaches to evaluating fatigue and developing endpoints for treatment have relied of biosignatures associated with fatigue. These have been used singly or in combination and include: physical performance measures, cognitive performance S-Editor: Ma RY L-Editor: Filipodia E-Editor: Zhang YL
Key words: Fatigue; Chronic liver disease; Non-alcoholic fatty liver diseases; Nonalcoholic steatohepatitis; Measurement; Patient-reported outcomes
INTRODUCTION
Fatigue is a critical component of chronic liver disease (CLD)[1]. It is common,complex, confusing and challenging to treat. It is thought to be the hallmark of certain diseases, including autoimmune diseases and chronic congestive heart failure, and is known to accompany many chronic illnesses including cancer, primary biliary cholangitis, sclerosing cholangitis and other cholestatic types of CLD. Relatively recently, investigators have identified that fatigue may also associate with nonalcoholic fatty liver diseases (NAFLD) and non-alcoholic steatohepatitis (NASH)[2].This lag in recognition of an association with NAFLD/NASH is, in the opinion of the authors, in part because NAFLD/NASH have only recently been described as a clinical entity, and it is considered a “silent” disease with low symptom burden.Additionally, the role of the liver in the pathogenesis of fatigue has not been well understood, and it has been attributed to other causes such as autonomic dysfunction,sedentary behavior and sickness behavior/hypothalamic-pituitary axis dysfunction[2,3].
Our point of view, based on our and others’ research with patients with chronic hepatitis C (CHC) and NAFLD/NASH, leads us to a somewhat different perspective.That is, that while the mechanisms of fatigue are protean in the group of people with NAFLD/NASH and CHC, the liver is central in its pathogenesis. It uniquely regulates much of the storage, release and production of substrate for energy generation. It is exquisitely sensitive to the feedback controlling the uptake and release of these energy generation substrates. Metabolic contributors to fatigue, beginning with the uptake of substrate from the gut, the passage through the portal system to hepatic storage and release of energy to target organs (muscle and brain) are central to understanding fatigue in patients with CLD and possibly others.
In addition to energy needs for normal function, the level of inflammation either causing or resulting from CLD contributes to fatigue, although inflammation has not been demonstrated to be causal. It is this unique combination of factors, the nexus of metabolic abnormality and the inflammatory burden of NAFLD/NASH and CHC that creates pathways to different types of fatigue (i.e., central and peripheral fatigue which will be discussed below). These pathways, in our opinion, create guidance for assessment, endpoints for treatments and possible interventions.
Primary fatigue, which is fatigue not associated with an accepted underlying fatigue-causing disease mechanism such as tumor, heart failure, anemia, thyroid dysfunction or medications, is especially difficult to treat. Frequently, depressive symptoms accompany fatigue, and people with CLD are treated for depression or are treated for insomnia. These may be effective in treating primary depression or insomnia but are not shown to be effective for treating fatigue. These observations lead us to support the view that exercise is among the highly specific and effective treatments for fatigue associated with NAFLD/NASH and CHC.
Why are we writing this opinion piece? We are attempting to provide a context in which fatigue is understood and can be clinically evaluated so that it can be distinguished from somnolence, mood disturbance or other co-morbidities often associated with fatigue. New approaches on how to measure fatigue include use of objective measures and patient reported outcomes (PROs). These measures have improved the precision with which we are able to describe fatigue. The measures of fatigue severity and its impact on usual daily routines in this population have also been improved and more generally accepted as reliable and sensitive.
This paper will discuss fatigue in CLD and possible mechanisms, review which treatment approaches may be effective in controlling symptoms and will discuss future opportunities for research that may lead to biosignatures such as performance and serological measures to assess fatigue.
FATIGUE AS A CONSTRUCT
Fatigue is common and experienced by virtually everyone during the course of their lives[4]. However, fatigue is difficult to characterize and define because it encompasses a complex interaction between biological, psychosocial and behavioral processes[5].Therefore, it is important to differentiate it from other related constructs, such as sleepiness, while still creating clear definitions for fatigue[6]. To follow along with this example, sleepiness is simply the propensity to fall asleep, while fatigue can be overall tiredness that is not corrected by sleep. Clear distinctions can be drawn when exact definitions and terminology are utilized. Fatigue needs to be differentiated from symptoms of somnolence (i.e., the quality or state of being drowsy), dyspnea (i.e.,difficult or labored respiration), boredom and weakness.
The most common types of fatigue that are used in the literature are central and peripheral[7]. However, it is important to be aware that these types of fatigue are defined differently across disciplines[8]. Again, clear and exact terminology is important when types of fatigue are discussed. In our research, we have been able to demonstrate clear distinctions between mental (central) and physical (peripheral)fatigue[9]. Central fatigue is characterized by a lack of self-motivation and can manifest both in physical and mental activities. Peripheral fatigue has been classically manifested by neuromuscular dysfunction and muscle weakness[7]. Therefore, the distinction has been about intention (central) versus ability (peripheral). It is important to also consider the types of activities. Fatigue can be experienced differently when performing a physical task versus performing a mental task[10].
For those with CLD, both dimensions of fatigue have been shown to be present[11].However, this one categorization may not be sufficient to provide sensitive assessment of fatigue. In our qualitative work, we were able to show additional dimensions of fatigue that might be useful for treatment and research purposes[12].Capacity across both the central and peripheral domains was an important distinction for patients. Fatigue and energy level were intricately linked and therefore capacity became a way for patients to describe their access to energy (access), their rapid depletion of energy (depletion) and their ability to restore energy once it was used(restoration). We believe that the inclusion of these concepts (access, depletion and restoration) would help to add depth to our understanding of fatigue across the central and peripheral domains. Recently, there have been many reviews of fatigue in the context of liver disease (see Table 1 for a summary of recent reviews). Fatigue has a profound effect on patients’ quality of life[2]. There is a need to increase the depth of our understanding of fatigue in order to be able to better treat it.
FATIGUE IN LIVER DISEASE
Prevalence Estimates of the prevalence of fatigue differ across different studies. However, in the general population it ranges from 5%-7%[13]. For patients within a primary care practice, the prevalence increases to between 10%-25%[13], and in individuals with chronic illness the prevalence ranges widely depending on the illness (from 20%-60%)[14]. In CLD, the prevalence ranges between 50%-85%[11]. Fatigue is the mostcommonly reported symptom in CLD, and it is also the symptom that most often gets individuals to visit their doctors[15]. In addition, the severity of fatigue does not seem to be associated with biochemical or histological parameters of liver disease severity,although the data are mixed on this point[16].
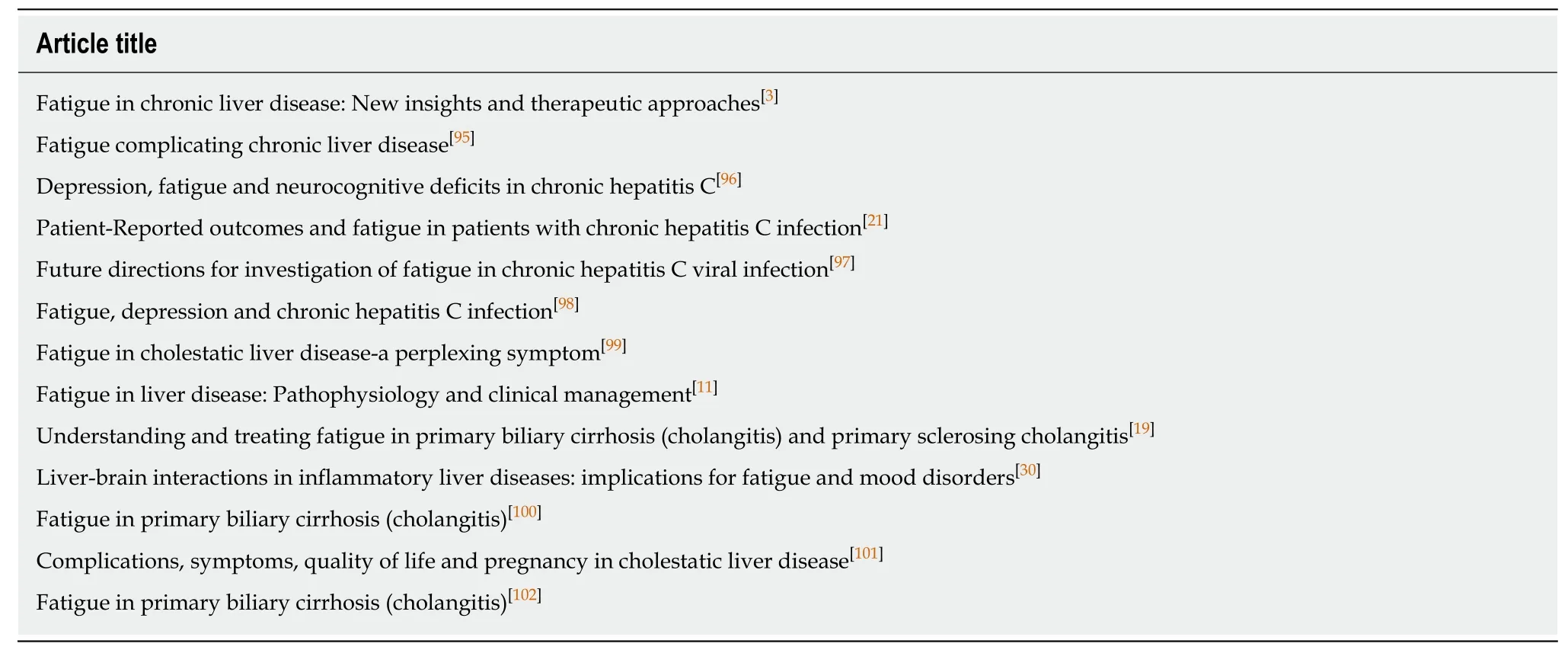
Table 1 Summary of recently published reviews specifically on fatigue in liver disease
Measurement
Although there is a proliferation of measurement tools to assess fatigue, there is no instrument that can provide both specificity and sensitivity for measuring fatigue. The lack of a tool is part of the problem that leads to under diagnosis, under recognition,and under treatment of fatigue in CLD patients. Part of the issue is that the tools that are currently used do not adequately capture the complexity and dimensionality of fatigue[17]. None of the commonly used tools address all aspects of fatigue. Commonly assessed areas include: Descriptions or characterizations of fatigue, feelings of distress associated with fatigue, presumed causes of fatigue and consequences of fatigue[18]. It is important to recognize what components of fatigue are being assessed and what components of fatigue should be assessed. Because there are no tools that address all of these components, it is important for researchers to consider what it is about fatigue that is relevant to the current research or patient and use that to drive the selection of a specific measure[17]. Please see Table 2 for a summary of instruments.
SYMPTOMS OF FATIGUE
Fatigue in liver disease is a well-described syndrome and is recognized as prevalent,persistent and problematic. It is the hallmark of primary biliary cholangitis[19], other forms of cirrhosis[20]and has been associated with CHC[21]. In fact, suggestions have been made that clinically significant fatigue should be an indication for anti-viral therapy[22]. Unlike cancer and myalgic encephalomyelitis/chronic fatigue syndrome(MECFS), there are no specific criteria for a “liver related fatigue” syndrome.However, much of the fatigue literature in hepatology does derive from the excellent work done by the National Cancer Consortium Network in an effort to raise awareness of cancer-related fatigue and to define it[23]. The field has also been influenced by the Centers for Disease Control and Prevention, who has championed the cause of devising criteria for diagnosis of MECFS and the National Institutes of Health, who has spearheaded the need for using common data elements in developing a standard approach to evaluation and performing research into MECFS[24]. These efforts have led to consensus that chronic fatigue is a persistent perception of tiredness that interferes with function, needed and desired activities and is often distressing and difficult to treat[25,26].
One important observation from one of our studies[27]is that the descriptive variables (PRO profiles as well as the serum analytes) differed between people with central fatigue compared with peripheral fatigue. These differences may help in planning treatment.
Chronic fatigue implies fatigue most days for at least a duration of 3 mo.Additionally, it is a multi-dimensional symptom and may be experienced as tirednessin the musculoskeletal system, cognitive decline or fuzzy thinking , muscle fatigue,poor recovery from exercise and decreased motivation for usual activities. See Table 3,which was taken from the International Classification of Disease 10thedition for diagnosis of cancer related fatigue.

Table 2 Commonly used measures of fatigue
The experiential aspects of fatigue may be influenced by age, culture, comorbidities, pain, mood, sleep and affect[28]. In fact, there is a significant interest in the possibility of symptom clusters, such as pain, fatigue, anxiety, depression and insomnia having a common etiology or genetic basis[29]. This is understandable given the overlapping nature of many of the symptoms. This presents a diagnostic and therapeutic dilemma because of the overlap between depressive symptoms and fatigue[30]. In fact, it is believed by some investigators that the word “fatigue” may be used interchangeably or may be a residual sign of depression[31,32].
The relationship between depression and/or depressive symptoms and fatigue suggests additional overlap because of the reported findings of changes in serotonin levels and abnormalities with tryptophan pathway regulation that is common in the depression and fatigue literatures[27,33-37]. Not only does this create diagnostic confusion, but it often leads to treatments for depression, which may not be helpful for reducing fatigue.
Additionally, we rely upon patients and research participants to “fit” their symptoms into standardized evaluations that have specific descriptors about level of intensity. Responses are stereotyped and not personalized, and as a result we get a limited amount of information about what individuals are truly experiencing. Our research group attempted to learn about how people with liver disease are likely to express their symptoms of fatigue (as discussed above)[12]. In this study we provided groups with CHC infection an opportunity to describe their fatigue using any adjective or metaphors they chose. They spoke of the dimensions of the fatigue in terms of intensity, frequency and duration. There were references to having limited capacity to do the things they wished to do. Further, that their energy stores often depleted rapidly without having the restorative power to recharge. Or they were unable to access the energy in order to do things they wished or needed to do. The presentation of their perceptions of fatigue and its impact helped us understand what they were experiencing and how central fatigue influences their functioning and wellbeing. Other investigators have made similar points about how important fatigue is to an individual[38].
Despite the fact that there is no unique signature describing fatigue associated with liver disease, many of the symptoms patients report are consistent with fatigue syndromes previously reported by investigators assessing cancer and MECFS.Interestingly, as in these other diagnoses, fatigue may associate with other symptoms in clusters of pain, anxiety, depression and insomnia. But with recent advances, thereare published data supporting the constructs of central and peripheral fatigue, whose symptoms and impact are very different. Data are also pointing to serological measures (pro- and anti-inflammatory cytokines and growth factors) that are linked to symptoms of fatigue that can be distinguished using self-reports[9]. A summary of associated symptoms is provided in Table 4.
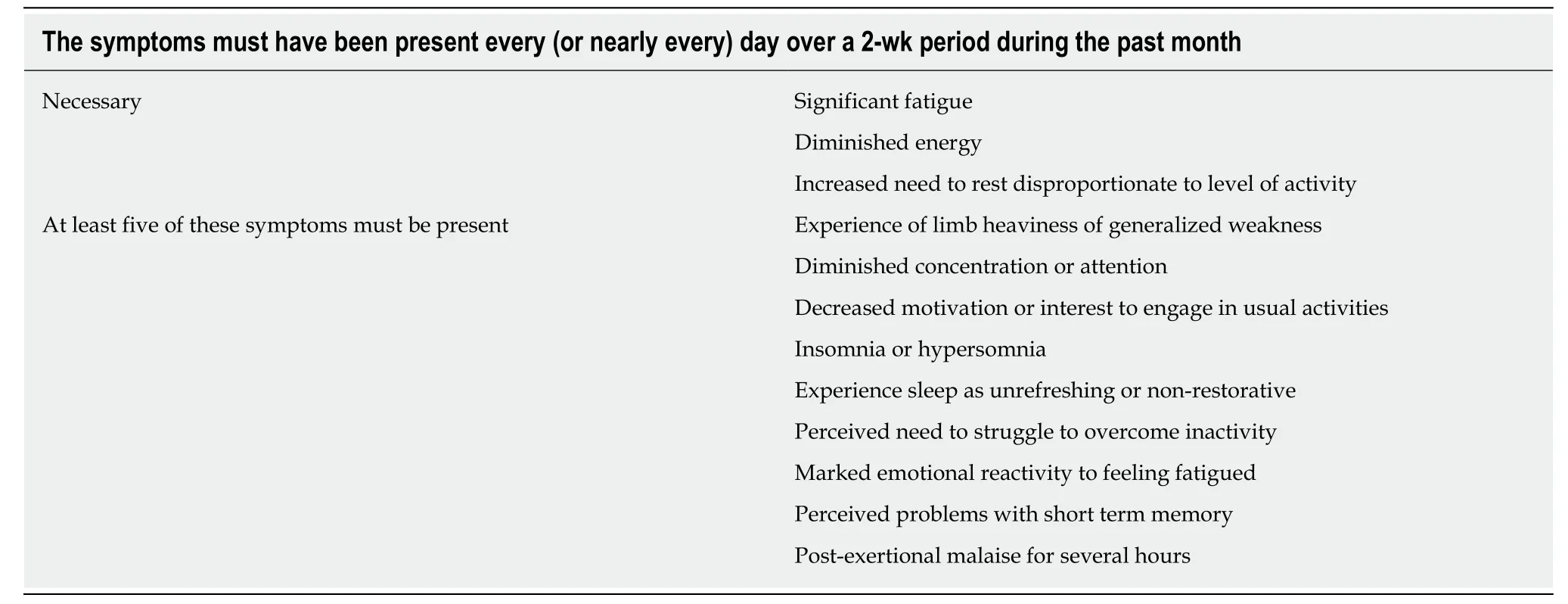
Table 3 Fatigue symptoms for diagnosing pathological fatigue
MECHANISMS OF FATIGUE
Fatigue may be attributed to a mechanism such as neuromotor dysfunction associated with muscle weakness, an organ specific explanation such as hypothyroid state or congestive heart failure. More often, fatigue is used as a non-specific term by patients,and many health care professionals treat it as such without producing a differential diagnosis or seeking a cause for it. Therefore, making the investigation of potential underlying mechanisms of fatigue is an important area.
Central and peripheral fatigue are experienced and measured differently and may be indicators of how the underlying mechanisms of fatigue differ as well. Central fatigue, is mediated by the central nervous system and is characterized by a failure to transmit motor impulses or perform voluntary activities[39], or the inability or reduced ability to perform attentional tasks. Peripheral fatigue, in comparison is a reduction in the ability to exert muscular force after exercise[40]and maintain a maximal force because of muscular limitations[8]. This implies that the source of the fatigue is independent of the muscular apparatus and originates above the neuromuscular junction[41]. A theoretical case can be made for a role for the autonomic nervous system as well[42]. Nonetheless, fatigue has been linked to many specific conditions including:anemia, cancer, cardiac, pulmonary, renal, liver disease, hypothyroid states,nutritional status and medication (Table 4). The assumption is that a deficit or disorder is the cause of the fatigue and correcting the deficit or disorder is likely to reverse the fatigue. When evaluating patients with chronic or “pathological” fatigue,it is advantageous to obtain a full work up to identify possible causative factors of fatigue and/or comorbidities that may contribute to its persistence.
However, there are many possible contributions the liver specifically makes in the pathophysiology of chronic fatigue. One recent review discussed the central role of the liver in metabolism and generation of energy[43]. It creates substrates for the production of ATP responsive to two conditions: (1) When eating and carbohydrate is available, the liver metabolizes glucose into glycogen and fatty acid; and (2) In the fasting state, when it produces energy by metabolizing glycogen via glycogenolysis or via gluconeogenesis. The liver can also metabolize fatty acid into ketone bodies for energy, but this is less efficient and occurs when glycogen is depleted from the liver[44].
The data supporting the central role of glucose to fatigue has been the result of studies in people with diabetes. This group of patients were studied to assess the relationship between blood glucose level and fatigue as well as the fluctuation in blood sugar levels over time[45,46]. This is an important observation because it supports the view that metabolic homeostasis is likely to be important for sustained physical and cognitive activity and because of the highly correlated conditions of type 2 diabetes and CLD (NAFLD/NASH).
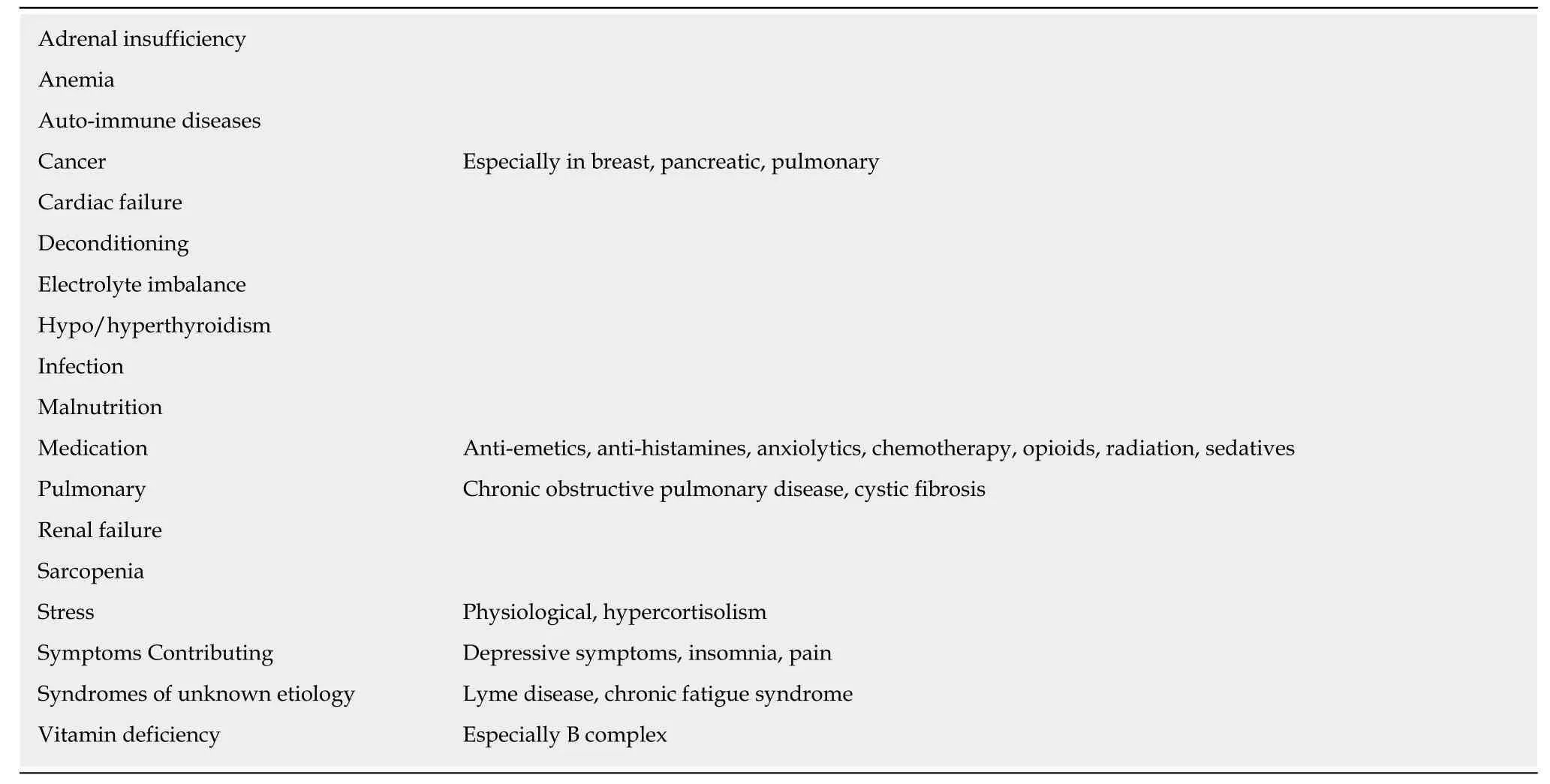
Table 4 Established associations among physical findings, diagnoses and fatigue
The liver is closely connected to extra-hepatic tissues in order to signal energy needs (skeletal muscle, brain), storage (adipose tissue) and substrate (gut). These responses are regulated through hormonal and neuronal networks. The hormonal signaling results from insulin, which stimulates glycolysis and lipogenesis. It suppresses gluconeogenesis and glucagon inhibits the effects of insulin. With respect to the nervous system, both the sympathetic and parasympathetic nervous system are important. The former stimulates and the latter inhibits gluconeogenesis.
In addition, control of liver metabolic processes depends upon several key transcription factors (FOXO1, PGC-1a and others) that control enzyme expression,which in turn controls hepatic metabolic processes[43]. The disruption of energy production and utilization has a profound impact on insulin sensitivity, development of type 2 diabetes and fatty liver. These changes in metabolic status are likely to be related to fatigue.
As mentioned above, the liver is in continual communication with extra-hepatic tissue, and with respect to fatigue it communicates through neuronal and hormonal networks. There are important gastrointestinal hormones that influence hepatic glucose production. Glucagon-like peptide is one that stimulates insulin secretion,and serotonin found in the gut stimulates gluconeogenesis in hepatocytes in the fasting state. Absorption of food and possibly microbiota release substrate through the gastrointestinal tract that send signals to the central nervous system (CNS) via the vagus nerve[47-49]. The sympathetic nervous system and parasympathetic nervous system both work through the CNS (hypothalamus) to regulate hepatic glucose production. Sympathetic nervous system activity increases glucose production and mobilizes substrate to extra-hepatic tissue (e.g., muscle, brain) and parasympathetic nervous system inhibits it[50]. Insulin signaling has an effect on the hypothalamus to stimulate interleukin (IL)-6 production, which suppresses gluconeogenesis[51]. The role of this pro-inflammatory cytokine is also thought to contribute to the progression of steatosis to steatohepatitis[52]. IL-6 is involved in inflammatory and metabolic changes that may stimulate synthesis of other cytokines that induce cell migration and initiate healing processes, including fibrosis development of steatohepatitis[53]. Skeletal muscle has endocrine properties and has been shown to be able to secrete myokines, which are inflammatory peptides. Myokines are involved in the inflammatory response, and physical activity plays a key role in down-regulating their release[54].
Many peripheral factors at the gut, liver and skeletal muscle level, central factors involving a variety of hormones including leptin and growth hormone regulate gluconeogenesis and insulin resistance. The latter is critical to the development of NAFLD and/or type 2 diabetes. Both conditions are associated with metabolic imbalances, metabolic stress and energy production inefficiencies (all of which promote insulin resistance in the liver)[55]. The CNS plays a key role in the perception of fatigue. It is likely that changes in neuronal signaling within the brain gives rise to changes in perceptions of fatigue and influences behavior. Swain et al[3]suggested in a recent review that there are several possible peripheral pathways by which liver inflammation can relay information to the brain that enhances fatigue perception.Signals include inflammation of: (1) The neural pathways via vagal nerve afferents; (2)Direct effect via transport through the circulation of pro-inflammatory cytokines; and(3) Via immune cells in the liver (Kupffer cells, stellate cells, natural killer cells) and recruited neutrophils, monocytes and macrophages[56]. They further suggested that there is evidence linking the basal ganglia to central fatigue[3]. Others identify a critical role for the hypothalamic pituitary adrenal axis (HPA axis). A recent review of its potential mechanisms that contribute to fatigue in cholestasis is available[57].
Because the HPA axis controls many functions of the liver through neuroendocrine pathways as well as mediating inflammation, it is thought to influence cellular and molecular processes in the liver. Fatigue, asthenia and muscular weakness, which can get worse during stress and infection[58], have been correlated with an impaired stress response due to HPA axis dysfunction. Interactions of the HPA axis with the liver also stimulate release of pro-inflammatory cytokines that stimulate release of glucocorticoids by the adrenals and block bile acid efflux impairing glucocorticoid metabolism[59]. In chronic inflammation, the HPA axis function is suppressed. Some investigators suggest that the common symptoms reported by people with CLD, such as fatigue, asthenia, lack of motivation and depressive symptoms are similar to symptoms associated with chronic fatigue syndrome and are suggestive of suppressed HPA axis[60].
Recent data[33]suggest that the monoamine transmitters are elevated in patients with CHC and persistent fatigue. Specifically, in patients taking direct acting antiviral agents, serotonin levels were significantly decreased at post treatment week 4 compared with baseline. Compared with baseline, there were significant decreases in IL-10 levels at end of treatment and 4 wk post-treatment. Changes in dopamine and tryptophan levels at the end of treatment correlated with increasing emotional health scores. Changes in monocyte chemoattractant protein-1 at end of treatment and IL-8 at 4 wk post-treatment correlated with increasing mental health scores. These data support the view that cytokines are involved in the well-being of patients with CHC.Others have reported significant roles for neurotransmitters, including the tryptophan pathway[34,61].
Borrowing from the literature[25,26]and using our own patient base with CHC and NAFLD/NASH, we have observed that patients display some similar symptoms.These include post-exertional malaise and an aversion to physical exercise/activity.They experience mental fatigue, sleep disruption, mood changes consistent with anxiety, depressive symptoms and decreased quality of life[62,63]. Some have difficulty concentrating and processing information. Most of this resolves with viral eradication shortly after completion of anti-viral therapy[11,27]. However, these symptoms persist in 23%-26% of those who achieve sustained viral eradication (SVR)[27]. When evaluating who within the group with CHC continued to have fatigue after achieving SVR, it was the group that had higher baseline depressive and other affective symptoms[27]and who had a higher number of comorbidities. Additionally, the change in cytokine profile after achieving SVR may be clinically meaningful. High baseline serum levels of interferon-g were associated with fatigue. Reductions in levels of chemokine (C-C motif) ligand 2 were associated with persistent fatigue after 12 wks of SVR. With respect to predictors of fatigue, there are no predictors of central fatigue at baseline if one controls for the diagnosis of depression. However, with respect to peripheral fatigue the best predictors at baseline for peripheral fatigue are IL-10, IL-8 and TNFα.TNFα continues to remain a strong predictor of persistent moderate/severe peripheral fatigue after treatment[27]. The contribution of tryptophan pathways and serotonin to fatigue[27,35]and recently to cognitive deficits[64]demonstrate that there are dynamic changes in the central nervous system within the hypothalamushippocampal circuit that cause central fatigue. These changes are associated with increased tryptophan-kynurenic acid pathway activity that causes reduced cognitive function, impaired spatial cognitive memory accuracy and increased hyperactivity and impulsivity[64].
POSSIBLE FATIGUE BIOMARKERS/BIOMARKER SIGNATURES
Current clinical and translational research has led to discussions about possible endpoints for treatment trials and clinical outcomes in managing fatigue. There is interest in the research community to develop objective measures, biomarkers or biomarker signatures for self-reports. According to the National Institutes of Health,“a biomarker is a defined characteristic that is measured as an indicator of normal biological processes, pathogenic processes or responses to an exposure or intervention, including therapeutic interventions. A biomarker signature is a combination of multiple variables to yield a patient-specific indicator of normal biological processes or responses to an exposure or intervention including therapeutic interventions. Biomarker modalities are diverse, and can include genetic, protein,cellular, metabolomics, imaging, behavioral, and physiologic endpoints”[65].
Fatigue is a symptom or state that is multi-dimensional. Hence measures of and outcomes for treating fatigue would benefit from the use of a multidimensional construct, such as the World Health Organization’s International Classification of Functioning, Disability and Health (https://www.who.int/classifications/icf/en/).This provides a framework where one can identify potential contributors to fatigue.For example, anatomic/physiological abnormalities, function, activity and participation in life activities may need to be assessed to thoroughly evaluate fatigue.Potential biomarkers or biomarker signatures for fatigue have emerged with a better understanding of the (1) Fatigue construct; (2) Distinction of central and peripheral fatigue; (3) Potential mechanisms underlying peripheral and central fatigue; and (4)Significant improvement in use of PROs for measuring function and patient experience.
Potential biomarkers/biosignatures for fatigue include: (1) Physical performance:measures such as 6-minute walk times for ambulatory tolerance, up-and-go test for physical mobility, measures of exercise tolerance including gas exchange and strength and local muscle endurance testing; (2) Cognitive performance: measures offer an objective measure of memory, recall, executive functioning and visuospatial processing; (3) Mood/behavioral: measures for depressive symptoms, anxiety, pain and insomnia; and (4) Brain imaging: imaging studies have provided some new insights into brain metabolic activity, but there is no consensus about its meaning with respect to function. Some suggest that functional magnetic resonance imaging is useful in measuring cognitive fatigue[6,40]. These data provide direct support for the Chaudhuri and Behan model of “central” fatigue that suggests these are non-motor functions of the basal ganglia. Some claim there are no associations between fatigue and attention, cognitive performance and brain structure[66]. Others have shown correlations between brain volume[67]and brain health[68]. Despite these differences,imaging is very likely to serve as a biomarker for brain health and possible cognitive function and fatigue in the future[6].
Evidence exists for the role of pro-inflammatory cytokines in CLD. TNFα, IL-1β and IL-6 are elevated during the viremic phase of CHC and decrease after achieving SVR.This observation is temporally related to improved fatigue symptoms[27]. The literature on IL-1 is noteworthy, despite lack of data specifically for NAFLD/NASH and CHC. There are data for type 2 diabetes and because people with NAFLD are often diabetic, the findings may have significant relevance. Cavelti-Weder et al[69]assessed the efficacy of a monoclonal anti-IL-1β antibody compared to placebo in 30 type 2 diabetes patients. Fatigue was reported by 53% of patients and significantly correlated to diabetes duration but not to age. After treatment for 1 mo, fatigue decreased in the groups treated with moderate- and high-dose anti-IL-1β but not in the placebo group.
It is likely that a combination of these measures will need to be configured in order to identify endpoints for clinical trials of fatigue and may serve as treatment targets to better manage the symptom.
FATIGUE SPECIFIC TREATMENTS
Non-pharmacological approachesA significant amount of literature has been written about the treatment of fatigue in MECFS and cancer related fatigue[70-72]. These reviews discuss a variety of nonpharmacological approaches to fatigue management including weight loss, exercise,dietary supplements, acupuncture, insomnia treatment and cognitive and behavioral interventions. These have helped guide treatment for fatigue in CLD.
With respect to CLD however, there are far fewer disease specific interventions that have been tested and shown to be promising. Starting with an approach to this problem is the TrACE model discussed by Swain[3]. This useful approach includes treating the treatable causes of fatigue (i.e., anemia, other comorbidities), ameliorating the modifiable symptoms (i.e., reduce symptom burden of sleepiness, depressive symptoms), coping and empathizing.
There is very little doubt on the effectiveness of exercise and diet/weight loss alone or in combination for treatment of CLD related fatigue[73-77]; and experts have indicated that this type of intervention is worth the effort[78]. Exercise and dietary interventions appear to be effective by mobilizing fat from the liver, increasing insulin sensitivity,improving endothelial function, reducing oxidative stress and decreasing inflammation[54].
Several mechanisms have been postulated. One is that training increases peroxisome proliferator-activated receptor gamma coactivator 1-alpha expression,improves mitochondrial function and leads to reduced hepatic steatosis and inflammation[79]. An excellent review of mechanisms of action of exercise in NAFLD is available[79]. Further, exercise and to some degree increased activity improve all-cause and cardiovascular mortality[80-83]. There is ongoing research to determine the comparative effectiveness of aerobic training versus anaerobic training (e.g., resistance training) in NAFLD/NASH. As of now, both are recommended[84].
The mechanisms by which exercise works is beginning to emerge and includes direct effects on metabolic regulation and increased cardiovascular resilience.Recently, the effects of exercise on the tryptophan clearance by activation of kynurenine pathway of tryptophan metabolism (Figure 1), which has been shown to mitigate fatigue[85]were reported. Tryptophan is the substrate for kynurenine(kynurenine pathway) as well as serotonin (serotonin pathway). Kynurenine and serotonin can cross the blood brain barrier and influence mood, cognition and fatigue[86]. Thus, peripheral tissues have a large impact on metabolism of kynurenine and serotonin and their availability to the CNS. Exercise stimulates not only the catabolism of tryptophan but also the clearance of kynurenine as kynurenic acid thereby reducing availability of kynurenine for transport across the blood brain barrier[87]. There is also a general improvement in insomnia, hypertension and mood.
In our experience, people who are sedentary, overweight, working, managing families and often feeling overwhelmed find it hard to commit to an active lifestyle and/or a specific exercise regimen. Self-efficacy and illness understanding are major determinants of lifestyle-modification among NAFLD patients. This information can assist clinicians in improving compliance with lifestyle changes among these patients[88].
Frith et al[89]reported that patients with NAFLD have significant fear of failing to meet expectations and lack confidence to proceed with an exercise program, which are factors that are modifiable. A recent study suggested that patients with NAFLD,supported by a Web-based approach, can increase the VO2peakto a similar extent as inperson interventions[90]. They noted that patients with low body fat and low VO2peakbenefited the most.
The published literature on predictors for or factors promoting adherence to longterm exercise does not lead to a consensus of how to achieve this. A very good review[91]identified many factors and cited conflicting findings including: poorer health (trending towards increased adherence), depression (trending toward decreased adherence) and life stresses (trending toward decreased adherence). One fairly consistent factor influencing adherence included enabling patients to self-select their exercise programs and have flexibility in the types, duration and locations in which they are implemented[91]. Most of the published literature comes from the cardiovascular, cancer and geriatric populations.
Pharmacological agentsMuch of the literature on the pharmacological treatment of fatigue in NAFLD is preclinical and is based on metabolism of tryptophan[92]. In the clinical setting, altered serotonergic neurotransmission has been reported in hepatitis C patients with fatigue,and treatment with serotonin receptor antagonists have been linked with improvements in fatigue as documented in patients with hepatitis C that were treated with ondansetron, a 5-HT3 receptor antagonist[93]. Additionally, s-adenosylmethionine(a methyl donor) is thought to work through the dopamine pathway and has been shown to mitigate symptoms of depression. Clinically, the level of evidence of effectiveness is low, although some therapeutic benefits have been reported in terms of fatigue reduction in people with intrahepatic cholestasis[3,94].
CONCLUSION
Fatigue is prevalent and persistent in people with NAFLD/NASH. Fatigue is a multidomain construct whose deconstruction into central and peripheral fatigue enables us to better evaluate the condition and identify potential causes and/or correlates. Liver is central to the pathogenesis of peripheral and central fatigue, which in our view is dependent upon energy regulation and crosstalk between the gut, liver, muscle and brain. Measurement of fatigue has improved such that performance (objective) and PROs can effectively be used to identify potential causal factors, treatments and endpoints for treatment. Although further work is needed to provide even more specificity to the fatigue construct and its measurement. Biosignatures for fatigue are being tested and validated that reflect metabolic and inflammatory pathways of relevance. Non-pharmacological treatments have been explored and shown to be effective in NAFLD, NASH, and CHC. These include weight loss and aerobic and resistance exercise. Pharmacological agents to date have not been shown to have a significant, reliable effect in reducing fatigue.

Figure 1 Tryptophan metabolism and the physiological role of its metabolites.
杂志排行
World Journal of Gastroenterology的其它文章
- Role of sodium-glucose co-transporter-2 inhibitors in the management of nonalcoholic fatty liver disease
- Acute kidney injury spectrum in patients with chronic liver disease:Where do we stand?
- Neoadjuvant and adjuvant treatment strategies for hepatocellular carcinoma
- Surgical techniques and postoperative management to prevent postoperative pancreatic fistula after pancreatic surgery
- Current approaches to the management of patients with cirrhotic ascites
- Pyrrolizidine alkaloids-induced hepatic sinusoidal obstruction syndrome: Pathogenesis, clinical manifestations, diagnosis,treatment, and outcomes
