Puerarin alleviates radicular pain caused by lumbar disc herniation by inhibiting spinal glial cell activation and inflammatory response*
2019-07-30HUYumingZHULirongZHAOYuanshuXUMingxianZHONGYi
HU Yu-ming, ZHU Li-rong, ZHAO Yuan-shu, XU Ming-xian, ZHONG Yi
(Key Laboratory of Neuroscience, School of Basic Medical Science, Guangzhou Medical University, Guangzhou 511436, China. E-mail: victoria0720@126.com)
[ABSTRACT]AIM: To investigate the role of Chinese medical herb Radix Puerariae extract puerarin in a rat model of radicular pain caused by lumbar disc herniation (RAPLDH) and to explore the possible mechanism involving spinal glial cell activation and inflammatory response. METHODS: The rat model of RAPLDH was induced by autologous nucleus pulposus (NP) implantation. The rats in sham group received the same operation procedure except NP implantation. Puerarin injection at different doses (50, 100 and 150 mg/kg) was delivered intraperitoneally 1 h before surgery, and once daily for 7 d. Mechanical paw withdrawal threshold (PWT) test was employed for assessing pain behaviors. Spinal microglia and astrocyte activation was evaluated by immunofluorescence staining of relevant specific markers. The expression of pro- and anti-inflammatory cytokines in spinal dorsal horn was measured by ELISA. RESULTS: The rats with NP implantation showed long-lasting pain behaviors, characterized by decrease in PWT from day 3 to day 14 after surgery. Compared with vehicle group, puerarin at doses of 100 mg/kg and 150 mg/kg significantly increased PWT of the rats with NP implantation. Puerarin significantly reduced the expression of spinal microglia marker ionized calcium-binding adaptor molecule 1 and astrocyte marker glial fibrillary acidic protein (P<0.01). Puerarin also decreased spinal expression of pro-inflammatory cytokines tumor necrosis factor-α, interleukin-1β and interleukin-6, and increased anti-inflammatory cytokine interleukin-10 (P<0.01). CONCLUSION: Puerarin alleviate RAPLDH by inhibiting spinal glial cell activation and inflammatory response.
[KEY WORDS]Puerarin; Radicular pain; Lumbar disc herniation; Spinal glial cells; Inflammatory response
Lumbar disc herniation (LDH) is the main cause of radicular pain, which is characterized by hyperalgesia, allodynia and spontaneous pain[1]. It impairs individuals’ life quality and aggravates social and economic burden. However, the mechanism is still not clear and the treatment stays unsatisfied.
Spinal glial cells, such as microglia and astrocytes, are involved in radicular pain when activated[2]. Activation of microglia and astrocytes may be determined by increased cell number and increased staining of specific cell markers, such as microglia marker io-nized calcium-binding adaptor molecule 1 (Iba-1) and astrocyte marker glial fibrillary acidic protein (GFAP)[3-4]. Activated glial cells may release pro-inflammatory cytokines, such as tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β) and IL-6[5]. Inflammatory response is one of the well-recognized me-chanisms of radicular pain from LDH[6]. Drugs targeting glia cell activation and inflammatory response may be helpful for alleviating radicular pain.
Puerarin (PU) is an isoflavonoid extracted from the Chinese medical herbRadixPuerariae(kudzu root) and has been traditionally used as vasodilator for the treatment of coronary heart disease, cerebral infarction, retinal vein occlusion and sudden deafness[7]. Recently, clinical and experimental data demonstrate that puerarin may moderate chronic pain induced by nerve injury[8]. However, the therapeutic effect of puerarin on the radicular pain caused by lumbar disc herniation (RAPLDH) is still not known. In the present study, we investigated the role of puerarin in RAPLDH. In particular, we explored the mechanism involving spinal glial cell activation and inflammatory response.
MATERIALS AND METHODS
1 Animals
Male Sprague-Dawley (SD) rats (200~250 g) were provided by Guangdong Laboratory Animal Center. All animal experimental procedures were carried out in accordance with the guideline of the International Association for Study of Pain and China Animal Committee, and were approved by the Animal Care and Use Committee of Guangzhou Medical University.
2 Drugs and reagents
Puerarin injection (Kangenbei Pharmaceutical Limited Corporation, China) was intraperitoneally delivered for 7 d at the doses advised by previous reports, and the RAPLDH rats with puerarin treatment were sacrificed on day 14 for immunofluorescence and ELISA detection. Paraformaldehyde (PFA) and Triton X-100 were purchased from Sigma-Aldrich. ELISA kits for detecting TNF-α, IL-1β, IL-6 and IL-10 were purchased from Boster Biological Technology. Mouse anti-Iba-1 monoclonal antibody (microglia marker, Abcam) and goat anti-GFAP polyclonal antibody (astrocyte marker, Abcam) were used. The second antibodies used were donkey anti-mouse polyclonal fluorescein isothycyanate (FITC)-conjugated IgG and donkey anti-goat polyclonal FITC-conjugated IgG (Jackson ImmunoResearch).
3 Experimental procedures
3.1EstablishmentoftheratmodelofRAPLDHbyautologousnucleuspulposus(NP)implantation[9]Surgery on the rats was performed under anesthesia with sodium pentobarbital (40 mg/kg, ip). The left transverse processes were exposed by dissecting the paraspinous muscles from the spinous processes. Left laminectomy was made in L4/5 segment and the facet joint was carefully removed to expose lumbar nerve roots. NP was harvested from coccygeal intervertebral discs, which were exposed between 2 vertebral bodies ventrally. The outsider annulus fibrosus were severed and the insider NP (about 10 mg) was harvested by smooth forceps. Subsequently the NP was immediately relocated on the exposed lumbar nerve roots without compression. The rats in sham group received the same harvesting procedure, but the NPs were abandoned instead of implanted.
3.2MechanicalallodyniatestThe rats were habituated to the testing environment by placing to the testing chamber for 20 min on 3 separate days before formal testing. Mechanical pain threshold was assessed using a set of von Frey hairs (0.41, 0.70, 1.20, 2.04, 3.63, 5.50, 8.51 and 15.14 g) with an up-down method described previously. The 2.04 g stimulus was applied first. If paw withdrawal was absence, the next stronger stimulus was applied. On the contrary, a weaker stimulus was chosen. The stimuli were applied to the surface of hindpaw, and each lasted 6~8 s with a 5-min interval between stimuli. Brisk withdrawal or lic-king of the paw following stimulus was considered a positive response.
3.3ImmunofluorescencestainingAfter anesthesia, the rats were perfused with saline followed by cold 4% PFA in phosphate buffer (0.1 mol/L, pH 7.4). The L4~L5 spinal cord were removed, post-fixed with 4% PFA solution for 3 h and then dehydrated with 30% sucrose solution for 2 d at 4 ℃. The spinal tissues were transversely sliced into sections with 25 μm thickness by a cryostat (Leica CM1900; -20 ℃) and processed for immunofluorescence according to the methods described previously. Briefly, all sections were blocked with 3% donkey serum in 0.3% Triton X-100 for 1 h at room temperature and incubated with mouse anti-Iba-1 or goat anti-GFAP (1∶500) overnight at 4 ℃, followed by incubation with donkey anti-mouse or donkey anti-goat FITC-conjugated IgG (1∶400) for 1 h at room temperature. The stained sections were examined under a fluorescence microscope (Leica), and images were captured with a CCD spot camera (Leica).
For quantification of the immunofluorescence staining, the area of Iba1- or GFAP-immunoreactivity (IR) per section was measured using a Leica Qwin V3 digital image processing system. The positively stained structure was identified by setting a density threshold above background level. The area occupied by these structures was recognized as positive area. In each animal, 6 sections were selected randomly. An average percentage of area of Iba1- or GFAP-IR relative to the total area of the sections was obtained for each animal, and was normalized to the control values. Six rats were included for each group for quantification of immunofluorescence results.
3.4ELISAAfter anesthesia, the dorsal quadrants of L4/5 spinal dorsal horn was rapidly harvested and homogenized in ice-cold phosphate-buffered saline, followed by centrifugation at 4 ℃ for 15 min at 13 000×g. The supernatants were used to measure the concentrations of TNF-α, IL-1β, IL-6 and IL-10, using corresponding ELISA kits according to the manufacturer’s instructions. The absorbance was detected at 450 nm (A450) and standard curve was delineated based on the absorbance of standards. The cytokine levels were calculated according to the standard curve.
4 Statistical analysis
All data were expressed as mean±standard deviation (mean±SD) and analyzed with SPSS 13.0. The data were analyzed by one-way analysis of variance (ANOVA) followed by Fisher’s protected least significant difference (PLSD) post hoc analysis.P<0.05 was considered statistically significant.
RESULTS
1 Puerarin increased mechanical pain threshold of rats with NP implantation in a dose-responsive manner
Compared with sham group, the rats with NP implantation showed long-lasting mechanical allodynia, characterized by decrease of paw withdrawal threshold (PWT) from day 3 to day 14 after surgery (P<0.01; Figure 1A). Puerarin injection at different doses [low dose (PU-L): 50 mg/kg; middle dose (PU-M): 100 mg/kg; high dose (PU-H): 150 mg/kg] was intrape-ritoneally administered 1 h before surgery, and once daily for 7 d. Compared with vehicle solution (normal saline), puerarin at doses of 100 and 150 mg/kg significantly increased PWT of the rats with NP implantation (P<0.01), but lower dose (50 mg/kg) of puerarin had no significant effect on PWT (Figure 1B). These data showed that puerarin alleviated mechanical pain behaviors induced by NP implantation in a dose-responsive manner.
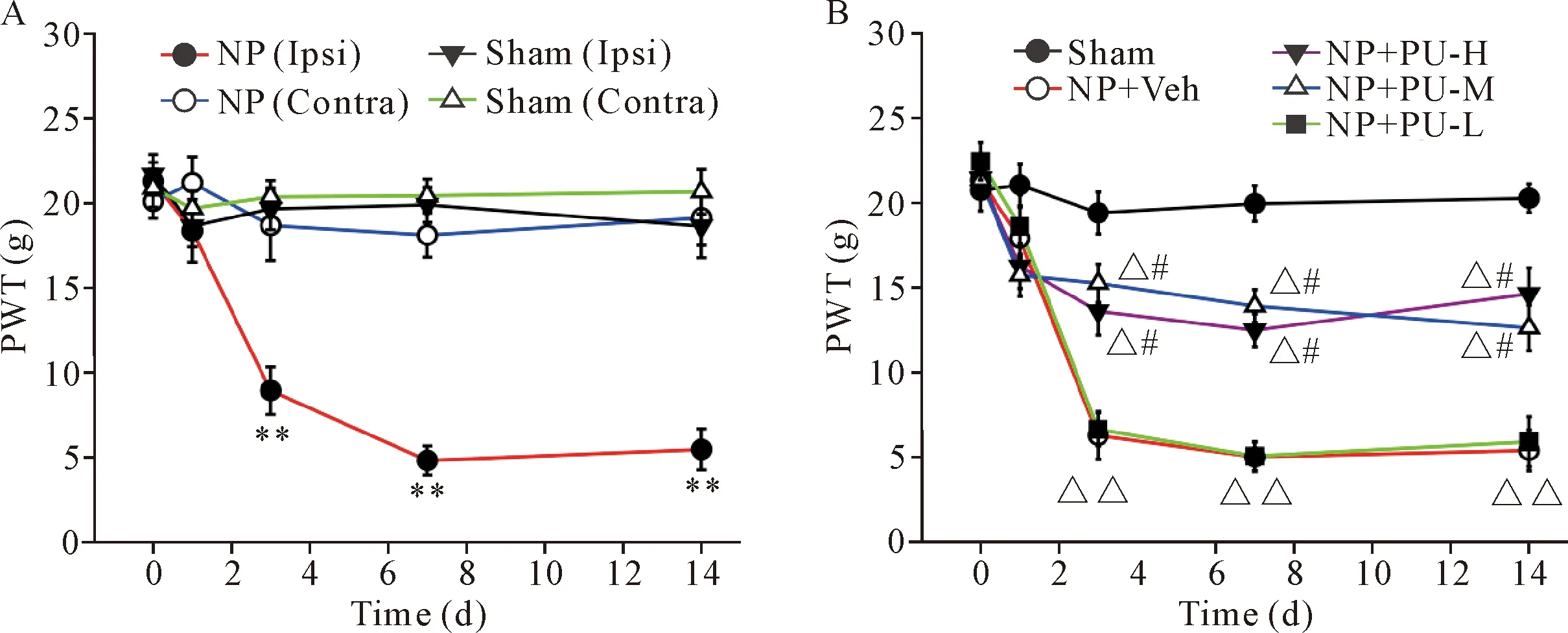
Figure1.Thedecreaseinpawwithdrawalthreshold(PWT)ofipsilateralhindpawsinNPgroupwasreversedbypuerarin(PU)inadose-responsivemanner.A: compared with sham group, PWT of ipsilateral hindpaws in NP group was decreased significantly on days 3, 7 and 14 after surgery; B: compared with vehicle (Veh; normal saline), middle (PU-M) or high (PU-H) doses of PU significantly increased the PWT of ipsilateral hindpaws in NP group. Mean±SD.n=6.**P<0.01vsother groups;△P<0.05,△△P<0.01vssham group;#P<0.05vsNP+Veh group or NP+PU-L group.
2 Puerarin inhibited spinal microglia and astrocyte activation in the rats with NP implantation
As consecutive administration of puerarin at 100 mg/kg had obvious and stable analgesic effect, this dose was employed for the mechanism study. The activation of spinal microglia and astrocytes were determined by calculating the positive areas of specific cell markers Iba-1 and GFAP in immunofluorescence staining of spinal sections. Spinal expression of Iba-1 (P<0.05 orP<0.01) and GFAP (P<0.01) was increased from day 3 to day 14 after NP implantation (Figure 2 and Figure 3). Puerarin significantly decreased the expression of Iba-1 (P<0.01) and GFAP (P<0.01). The microglia and astrocytes in NP group showed typical cell body hypertrophy with thickened and retracted processes which was the main character of their activated state. Puerarin also inhibited the activation of microglia and astrocytes morphologically.

Figure2.Puerarin(PU)significantlyinhibitedspinalmicrogliaactivation.A~E: representative immunofluorescence staining images (×200) showed the expression of Iba-1 in different groups; the figures in big white boxes (A, D and E) showed the morphological changes of microglia (×400). F: the quantification of Iba-1 positive area in different groups indicated that PU significantly decreased Iba-1 expression. Mean±SD.n=6.*P<0.05,**P<0.01vssham group;##P<0.01vsNP for 7 d and 14 d groups.

Figure3.Puerarin(PU)significantlyinhibitedspinalastrocyteactivation.A~E: representative immunofluorescence staining images (×200) showed the expression of GFAP in different groups; the figures in big white boxes (A, D and E) showed the morphological changes of astrocytes (×400). F: the quantification of GFAP positive area in different groups indicated that PU significantly decreased GFAP expression. Mean±SD.n=6.**P<0.01vssham group;##P<0.01vsNP for 7 d and 14 d groups.
3 Puerarin decreased spinal expression of TNF-α, IL-1β and IL-6, but increased spinal expression of IL-10
Puerarin decreased spinal expression of pro-inflammatory cytokines TNF-α, IL-1β and IL-6, but increased anti-inflammatory cytokine IL-10 in the rats with NP implantation (P<0.01; Figure 4).

Figure4.Puerarin(PU)significantlyreducedtheexpressionofTNF-α,IL-1βandIL-6,butincreasedIL-10expressioninspinaldorsalhornoftheratswithNPimplantation.Mean±SD.n=6.**P<0.01vssham group;##P<0.01vsNP group.
DISCUSSION
In this study, the rat model of RAPLDH was induced by autologous NP implantation, which was harvested from coccygeal vertebra and relocated in lumbar 4/5 spinal nerve roots. The rats with NP implantation showed ipsilateral long-lasting mechanical allodynia, increased expression of glia cell markers Iba-1 and GFAP, increased spinal expression of TNF-α, IL-1β and IL-6, and decreased expression of IL-10. The results demonstrated that puerarin may be helpful to alleviating RAPLDH by inhibiting spinal glia cell activation and inflammatory response.
LDH is a common cause of radicular pain characterized by ipsilateral chronic low back pain and pain sensation radiating into leg and foot[1]. It was once thought that mechanical compression of nerve roots from herniated NP may lead to pain sensation. However, clinical reports found that the degree of compression was not in accordance with the severity of pain sensation. Except for conventional analgesics, decompressive discectomy is considered one of the best approaches to alleviate pain symptoms[10]. Unfortunately, more than 10% patients have a chronic intractable pain after undergoing decompressive surgeries to remove the affected disc[11]. Other mechanisms such as neuroinflammation may contribute to radicular pain[2,12].
Substantial evidence has demonstrated that activation of spinal microglia and astrocytes works as important contributor to the pathogenesis of radicular pain[12]. In the present study, we found that spinal microglia and astrocytes were significantly activated after NP implantation, which was manifested as increased expression of specific cell markers (Iba-1 and GFAP, respectively), as well as morphological changes such as cell body hypertrophy with thickened and retracted processes. In cerebral ischemia model, activation of microglia and astrocytes were determined to be the result of ischemia in the early stage and to be due to anti-injury response in the late stage[13]. A recent published paper indicated that microglia was an essential component of the neuro-protective factor after spinal cord injury[14], indicating that activation of microglia and astrocytes may be helpful for tissue recovery in some situations, such as ischemia and injury. On the other hand, activated glial cells produce inflammatory mediators (TNF-α, IL-1β and IL-6) to activate or sensitize nociceptive neurons[15], which is the main cause of chronic pain. Pharmacological inhibition of these cells or cytokines may alleviate pain sensation[16]. Therefore, mo-dulation of neuroinflammation response may be a promising target for radicular pain.
Puerarin is a plant-derived traditional Chinese drug with potent antioxidant and anti-inflammatory effects[7]. Other than its effect on cardiovascular protection, puerarin is demonstrated to improve the learning-memory ability of aging mice[17], and exhibit beneficial effects in Parkinson’s disease[18]. In experimental liver injury, puerarin may effectively regulate the balance between pro-inflammatory cytokines (TNF-α, IL-1β, IL-4, IL-6 and TGF-β1) and anti-inflammatory cytokines (IL-10)[19]. Puerarin prevents LPS-induced acute lung injury via reducing TNF-α, IL-1β and IL-6 expression in lung tissues[20]. These reports strongly supported anti-inflammatory effects of puerarin. Recently, the therapeutic effects of puerarin on neuropathic pain have received more and more attention. It is reported that puerarin alleviated neuropathic pain from nerve injury via modulating the cell signals of P2X3 receptors in dorsal root ganglion neurons[8]. Puerarin may attenuate inflammatory pain through inhibition of inflammatory mediators (TNF-α, IL-1β and IL-6) and enhancing the levels of anti-oxidant enzymes (HO-1 and SOD2)[21]. In the present study, we assessed analgesic role of puerarin in radicular pain in a rat model of lumbar disc herniation. Intraperitoneally delivery of puerarin for 7 d alleviated mechanical pain behaviors in a dose-responsive manner. Puerarin significantly inhibited spinal glial cell activation, characterized by reduced expression of glial cell markers Iba-1 and GFAP. What’s more, the protein levels of TNF-α, IL-1β and IL-6 were decreased after puerarin administration but IL-10 was increased. In conclusion, these data indicated that puerarin may alleviate RAPLDH by inhibiting spinal glial cell activation and inflammatory response.
