Toll-like receptor 4 promotes migration and invasion abilities of human non-small-cell lung cancer cells*
2019-07-30YANGPingShangruiLIWenlingZHANGYuqinPANQiXUMengyaoYANGBoHUYa
YANG Ping, LÜ Shang-rui, LI Wen-ling, ZHANG Yu-qin, PAN Qi, XU Meng-yao, YANG Bo, HU Ya-e△
(1Department of Pathophysiology, 2Department of Clinical Medicine, The Medical School of Nantong University, 3Department of Clinical Pediatrics, 4Department of General Medicine, Nantong University Xinglin College, Nantong 226001, China. E-mail: huli0120@163.com)
[ABSTRACT]AIM: To evaluate the expression and biological role of Toll-like receptor 4 (TLR4) in human non-small-cell lung cancer (NSCLC) cells. METHODS: The mRNA and protein levels of TLR4 in NSCLC tissue were exa-mined by RT-qPCR, Western blot, and immunohistochemistry. After treating the A549 cells and SPC-A-1 cells with TLR4 stimulator lipopolysaccharide (LPS) and inhibitor TAK-242, RT-qPCR, Western blot and flow cytometry were performed to detect the expression of TLR4. The migration and invasion abilities were detected by Transwell assay, and the mRNA expression of matrix metalloproteinase (MMP)-2, MMP-9, and vascular endothelial growth factor (VEGF) was also detected. RESULTS: The mRNA and protein levels of TLR4 were higher in the NSCLC tissue than those in the noncancerous tissue (P<0.01). LPS stimulation significantly increased the mRNA and protein expression levels of TLR4 in the NSCLC cell lines A549 and SPC-A-1 (P< 0.01). The LPS-induced TLR4 activation enhanced the migration and invasion abilities of A549 cells and SPC-A-1 cells (P<0.01). LPS increased the expression levels of MMP-2, MMP-9 and VEGF in the A549 cells and SPC-A-1 cells (P<0.01). Moreover, the expression levels of TLR4, MMP-2, MMP-9 and VEGF, as well as the migration and invasion abilities of the cells were blocked by TAK-242 (P<0.01). CONCLUSION: TLR4 might be involved in the migration and invasion of NSCLC cells, and TLR4 inhibition might be considered as a therapeutic target for treatment of NSCLC.
[KEY WORDS]Cell invasion; Cell migration; Non-small-cell lung cancer; Toll-like receptor 4
Lung cancer is the most common cause of cancer-related mortality in the world[1]. Non-small-cell lung cancer (NSCLC) accounts for approximately 80% of all lung malignancies. Despite continuous improvement in the diagnosis and treatment of lung cancer, the 5-year survival rate of lung cancer is only 15%[2]. Tumor invasion and metastasis greatly limit treatment options and account for 90% of cancer-related deaths. Tumor invasion and metastasis are the main malignant biological behaviors, which lead to poor treatment outcomes and poor prognosis in the patients with malignant tumors[3]. However, the mechanism of invasion and metastasis of lung cancer is complex and not fully clarified. Therefore, further studies on the mechanism of invasion and metastasis of lung cancer and the search for new targets for anti-lung cancer therapy have become increasingly important.
The effect of inflammation on the tumor has been a concern recently. About 15%~20% of the tumor is associated with chronic infection and inflammation. Stu-dies have showed that chronic inflammatory diseases of the lungs are important acquired factors for the development of lung cancer[4]. Lipopolysaccharide (LPS) from Gram-negative bacteria is important in chronic inflammatory response and also promotes the development of tumors. It is specifically recognized by the cell membrane receptor Toll-like receptor 4 (TLR4), which is expressed on not only the immune cells but also the tumor cells[5]. Furthermore, TLR4 is related to the development of a variety of tumors, such as ovarian can-cer, prostate cancer, breast cancer, and head and neck squamous-cell carcinoma[6-9]. The TLR4 signaling transduction pathway is important in tumor metastasis. LPS enhances the invasion and metastasis of tumor cells, such as colon cancer, prostate cancer, and breast cancer. Its interference with the expression of TLR4 in tumor cells significantly inhibits the distant metastasis[10-11]. However, studies on LPS-activated TLR4 signaling in lung cancer cell lines are limited. The relationship between TLR4 activation and the invasion and metastasis of lung cancer cells or its molecular mechanism is not clear. The present study investigated the effect of TLR4 signaling on the migration and invasion abilities of both NSCLC cell lines A549 and SPC-A-1. The results might provide new ideas and therapeutic targets for interfering with lung cancer invasion and metastasis.
MATERIALS AND METHODS
1 The data of the patients
A total of 50 patients diagnosed with NSCLC at the Affiliated Hospital of Nantong University from October 2015 to October 2016 were enrolled in this study. After surgical resection, the cancer tissue and adjacent noncancerous tissue were collected. The patients were aged 40~83 years, with an average age of (67.0±12.6) years. None of the patients were treated with chemotherapy before this study. The specimens were collected from necrotic cancer tissue and adjacent noncancerous tissue within 3 cm, frozen at -80 ℃, or fixed and embedded in paraffin. The cancerous samples were diagnosed as NSCLC by pathological examination. The study was approved by the Ethics Committee of Affiliated Hospital of Nantong University. All the patients signed an informed consent form before enrolled in this study.
2 Cell culture
The human NSCLC cell lines A549 and SPC-A-1 (American Type Culture Collection) were cultured in RPMI-1640 medium (HyClone; with 10% fetal bovine serum, 1×105U/L penicillin, and 100 mg/L streptomycin) in an incubator with a humidified atmosphere (5% CO2, 37 ℃). When the cells grew to 80% confluence, they were detached using a solution containing 0.25% trypsin and 0.002% EDTA (Gibco) and collected for subsequent experiments.
3 The experimental procedures
3.1TreatmentofthecellsA549 cells and SPC-A-1 cells were seeded in 6-well culture plates (5×105cells/well) and incubated overnight. After removing cell culture supernatants, the cells were stimulated with LPS (Sigma) at various concentrations (0, 5, 10, and 20 mg/L) for 24 h. The A549 cells and SPC-A-1 cells were pretreated with TLR4 antagonist TAK-242 (100 nmol/L, MedChem Express) for 1 h to observe the effect of TAK-242 on the LPS-induced expression of TLR4. Then, the cells were treated with LPS (10 mg/L) for 24 h. For all the experiments, TAK-242 was dissolved inN,N-dimethylformamide, diluted with an appropriate medium, and added to the cells just before the stimulation.
3.2RT-qPCRRT-qPCR was used to test the mRNA expression levels of TLR4, matrix metalloproteinase (MMP)-2, MMP-9, and vascular endothelial growth factor (VEGF). Total RNA was isolated using the TRIzol reagent, and 2 μg of total RNA was reversely transcribed into cDNA according to the reverse transcription protocol. The primers were designed using the Primer Premier 6.0 software (Premier Co.). The sequences of all primers were showed in Table 1.
A PCR with a total volume of 20 μL, containing 10 μL SYBR Green Master Mix (Life Technologies Corporation), 1 μL of template cDNA, 0.4 μL of both forward and reverse primers, and 8.2 μL of nuclease-free water, was performed. The reactions were run using the following parameters: initial denaturation of one cycle at 95 ℃ for 10 min, followed by 40 cycles of denaturation at 95 ℃ for 15 s, annealing at 60 ℃ for 30 s, and extension at 72 ℃ for 30 s. All experiments were carried out in triplicate and repeated 3 times. All samples were normalized to the GAPDH level. The specificity of the amplified PCR products was valued using the melting curve analysis, and the relative gene expression level was calculated by the 2-ΔΔCtmethod.
3.3WesternblotanalysisThe fresh tumor and adjacent noncancerous tissues or cells were washed twice using cold phosphate-buffered saline (PBS), lysed in lysis buffer (with protease inhibitor) at 4 ℃ for 30 min, and centrifuged at 12 000×gfor 10 min. Subsequently, 50 μg of protein was separated by 10% so-dium dodecyl sulfate-polyacrylamide gel electrophoresis and electroblotted to a polyvinylidene difluoride membrane using a Mini-PROTEAN transmembrane system under constant current (200 mA) for 120 min. The membranes were blocked for 1 h and then incubated with rabbit polyclonal anti-TLR4 antibody (1∶200, Abcam), rabbit polyclonal anti-MMP2 antibody (1∶1 000, Abcam), rabbit monoclonal anti-MMP9 antibody (1∶1 000, Abcam) and rabbit polyclonal anti-VEGF antibody (1∶1 000, Abcam) overnight, followed by incubation with horseradish peroxidase (HRP)-conjugated secondary antibodies for 1 h. Enhanced chemiluminescence was used for detection. The bands were scanned and quantified using the Scion Image software, and the amount was normalized with GAPDH values in the same lane.
3.4ImmunohistochemicalstainingParaffin-embedded lung cancer and adjacent tissues were cut into 4-μm sections and transferred onto glass slides. After baking for 1 h at 90 ℃, the tissue sections were dewaxed and rehydrated. Antigen retrieval was conducted by heat mediation in sodium citrate buffer (10 mmol/L, pH 6) for 15 min. Endogenous peroxidase was blocked by 0.3% hydrogen peroxide at 37 ℃ for 10 min. The tissue sections were incubated with polyclonal rabbit anti-TLR4 antibody (1∶50, Abcam) overnight at 4 ℃. After rinsing with PBS, undiluted HRP-conjugated goat anti-rabbit immunoglobulin G (IgG) (MXB Biotechno-logies) was added to the tissue sections for 30 min at room temperature. The slides were washed and incubated with 3,3’-diaminobenzidine (MXB Biotechnologies). The nucleus was stained with hematoxylin (Nanjing Jiancheng Bioengineering Institute). The tissue section was covered with a glass coverslip and assessed under a microscope.
The staining results were assessed and scored on the basis of both the proportion of positively stained tumor cells and the intensity of staining by 2 clinical pathologists. The proportion of positive cells was scored as follows: 0, no positive cells; 1, <25% positive cells; 2, 25%~50% positive cells; 3, 50%~75% positive cells; and 4, >75% positive cells. The staining intensity was scored as follows: 0, no staining; 1, light yellow; 2, yellow; 3, yellow-brown; and 4, dark brown. The total score was calculated using the staining intensity score and the proportion of positive cells. The expression level of TLR4 protein was determined using the total score. A total score of 1~4 was defined as low expression and that of 5~8 as high expression.
3.5FlowcytometryanalysisThe A549 cells and SPC-A-1 cells were treated with LPS and TAK-242. The expression level of TLR4 was determined by flow cytometry. The cells were detached using 0.03% EDTA and incubated with the anti-TLR4 antibody (1∶100), followed by anti-rabbit IgG (Alexa Fluor 549-conjugated) secondary antibody (1∶500, Cell Signaling Technology). The cells were analyzed using the FACSCalibur system (BD Biosciences), and the data were analyzed using the FlowJo software (BD Biosciences).
3.6TranswellinvasionandmigrationassaysPolycarbonate membrane culture inserts of 8-μm pore size (Corning) were placed in 24-well culture plates, separating the upper and lower chambers. For cell invasion assay, the polycarbonate membrane was treated with Matrigel, and 3×104cells were seeded into the upper chambers in serum-free RPMI-1640 media, and 600 μL complete media (RPMI-1640 media with 10% fetal bovine serum) was added to the lower chambers. For cell migration assay, 1.5×104cells were seeded into the upper chambers in serum-free media, and 500 μL complete media was added to the lower chambers. The cells were incubated at 37 ℃ overnight. The number of invading and migrating cells was quantified by counting 5 independent visual fields under a microscope (Olympus 600 Autobiochemical Analyzer), and the cell morphological changes were observed by staining using Wright-Giemsa Stain Kit (Nanjing Jiancheng Bioengineering Institute). Each experiment was performed at least 3 times.
4 Statistical analysis
All results were expressed as mean±standard deviation (SD). Statistical analysis was performed using the Stata 8.0 and SPSS 17.0 software. The data were analyzed using Studentttest, one-way analysis of variance (ANOVA) with Scheffe test for multiple comparison, and2test. A value ofP<0.05 was considered statistically significant.
RESULTS
1 TLR4 was over-expressed in the NSCLC tissue
The mRNA and protein expression levels of TLR4 in the NSCLC tissues (T) and adjacent noncancerous tissues (ANT) were examined by RT-qPCR, Western blot, and immunohistochemistry. Both mRNA and protein levels of TLR4 were markedly up-regulated in human NSCLC tissues as compared with paired adjacent noncancerous tissues (Figure 1A and 1B). TLR4 was localized in the cytomembrane and cytoplasm of carcinoma cells, and its expression was stronger in the NSCLC tissues. However, TLR4 was hardly expressed in adjacent noncancerous tissues (Figure 1C). Moreover, the expression level of TLR4 significantly correlated with local lymphatic metastasis (Table 2).
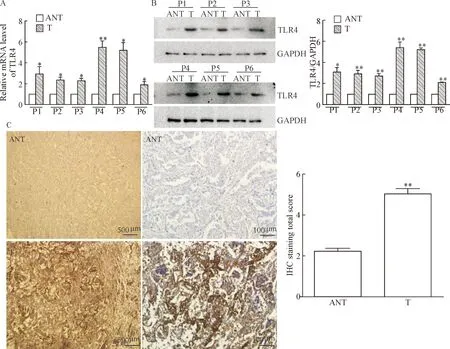
Figure 1.The expression of TLR4 in paired human NSCLC tissues and adjacent noncancerous tissues. A and B: RT-qPCR and Wes-tern blot analysis of TLR4 expression in paired human NSCLC tissues (T) and adjacent noncancerous tissues (ANT); C: representative images of TLR4 immunohistochemical (IHC) staining in human NSCLC tissues and ANT were showed, and the total scores were calculated. Mean±SD.n=3.*P<0.05,**P<0.01vsANT.

Table 2.TLR4 expression levels in the patients with NSCLC
2 LPS increased the expression level of TLR4 in human NSCLC cells
The mRNA and protein expression levels of TLR4 in the A549 cells and SPC-A-1 cells were analyzed by RT-qPCR and Western blot. When cultured A549 cells and SPC-A-1 cells were treated with increasing concentrations of LPS (0, 5, 10 and 20 mg/L) for 24 h, the mRNA and protein expression levels of TLR4 were increased. After stimulated with LPS (10 and 20 mg/L) for 24 h, an approximately triple increase was observed in the mRNA and protein expression levels of TLR4 in the A549 cells and SPC-A-1 cells as compared with the cells in LPS (0 mg/L) group (Figure 2). Based on these results, all subsequent experiments were conducted using LPS at 10 mg/L.
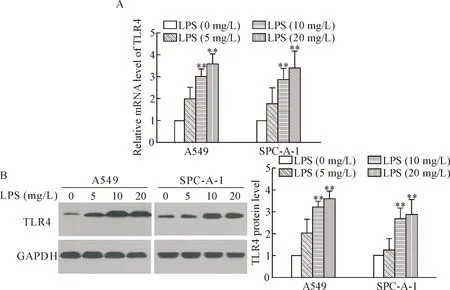
Figure 2.The expression of TLR4 in human NSCLC cells stimulated by LPS. A549 cells and SPC-A-1 cells were treated with LPS at 0, 5, 10 and 20 mg/L for 24 h. A: RT-qPCR analysis of mRNA expression levels of TLR4; B: Western blot analysis of TLR4 protein expression levels. Mean±SD.n=3.**P<0.01vsLPS (0 mg/L) group.
3 TLR4 antagonist TAK-242 inhibited the LPS-induced expression of TLR4 in human NSCLC cells
RT-qPCR revealed that LPS at 10 mg/L enhanced the mRNA expression level of TLR4 in the A549 cells and SPC-A-1 cells, which was almost completely blocked by TLR4 antagonist TAK-242 (Figure 3A). The protein level of TLR4 stimulated by LPS was also reduced in LPS+TAK group (Figure 3B). Flow cytometry was used to detect the protein level on the cell membrane of the A549 cells and SPC-A-1 cells. LPS increased the expression level of TLR4 on the cell membrane of the A549 cells and SPC-A-1 cells, which was also blocked by TAK-242 (Figure 3C). Incubation with TAK-242 alone did not bring significant changes of the mRNA or protein expression levels of TLR4 as compared with control group.

Figure 3.The effect of TLR4 antagonist TAK-242 on LPS-induced expression of TLR4 in human NSCLC cells. A549 cells and SPC-A-1 cells were pretreated with TLR4 antagonist TAK-242 at 100 nmol/L for 1 h, and then the cells were treated with LPS at 10 mg/L for 24 h. A: the mRNA expression level of TLR4 was analyzed by RT-qPCR; B: Western blot analysis of the protein expression level of TLR4; C: flow cytometry analysis of membrane TLR4 protein. Mean±SD.n=3.**P<0.01vscontrol group;##P<0.01vsLPS group.
4 TLR4 antagonist TAK-242 blocked LPS-induced invasion and migration abilities of human NSCLC cells
The results of Transwell invasion and migration assays showed that LPS enhanced the invasion and migration abilities of A549 cells and SPC-A-1 cells. The invasion and migration abilities of the cells in LPS+TAK group were significantly reduced as compared with LPS group. Treatment with TAK-242 alone did not obviously change the invasion and migration abilities of A549 cells and SPC-A-1 cells as compared with control group (Figure 4).
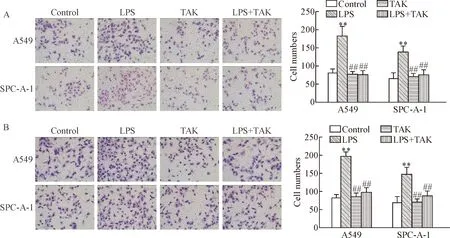
Figure 4.TLR4 antagonist inhibited the invasion and migration abilities induced by LPS in human NSCLC cells. A549 cells and SPC-A-1 cells were pretreated with TLR4 antagonist TAK-242 at 100 nmol/L for 1 h, and then the cells were treated with LPS at 10 mg/L for 24 h. A: the invasion ability of A549 cells and SPC-A-1 cells was detected using Transwell assay (×100); B: the migration ability of A549 cells and SPC-A-1 cells was measured by Transwell assay (×100). Mean±SD.n=3.**P<0.01vscontrol group;##P<0.01vsLPS group.
5 TLR4 antagonist TAK-242 down-regulated the LPS-induced expression levels of MMP-2, MMP-9 and VEGF in human NSCLC cells
The expression levels of the genes related to angiogenesis and invasion were detected in the A549 cells and SPC-A-1 cells. After the stimulation with LPS at 10 mg/L for 24 h, both mRNA and protein expression le-vels of MMP-2, MMP-9 and VEGF were significantly increased in the A549 cells and SPC-A-1 cells as compared with control group. The expression levels of MMP-2, MMP-9 and VEGF were significantly decreased in LPS+TAK group as compared with LPS group. Treatment with TAK-242 alone did not obviously change the expression levels of MMP-2, MMP-9 and VEGF in A549 cells and SPC-A-1 cells as compared with control group (Figure 5).
DISCUSSION
Recently, the role of inflammation in tumor genesis and progression has been of great concern. TLR4 was the first type of TLRs found in 1997, which recognizes pathogen-associated molecular patterns like LPS to induce different immune responses[12]. TLR4 also me-diates MyD88-dependent pathways by interaction with a series of cytokines to promote tumorigenesis. TLR4 is over-expressed in various tumor cell lines and correlated with the tumor growth and metastasis of oral tongue squamous-cell carcinoma, gastric tumors, ovarian can-cer, prostate cancer, and other types of tumor cells[13-15]. In the present study, TLR4 was positively expressed in 70% cases of NSCLC tissues and less expressed in 30% cases of adjacent noncancerous tissue. The positive expression rate was almost consistent with previous studies[16]. TLR4 was expressed more strongly in NSCLC tissues than in ANT, which significantly correlated with the tumor size and local lymphatic metastasis. These results indicated the involvement of TLR4 in the development of NSCLC and provided some clues to link TLR4 and NSCLC metastasis.
Metastasis is the main cause of lung cancer-asso-ciated deaths. The metastatic process consisted of a series of events: cell detachment from the primary tumor mass, migration into and transport along the bloodstream, and finally tumor cell arrest and proliferation within the distant tissue[17]. Invasion through basal membrane is a key metastatic step for cell detachment from primary loci and intrusion into distant organs[18]. In this study, TLR4 ligand LPS and antagonist TAK-242 were used in human NSCLC cell lines A549 and SPC-A-1 to further confirm the role of TLR4 in NSCLC metastasis. The results showed that TLR4 was expressed in both A549 cells and SPC-A-1 cells, and the expression levels of TLR4 in both cell lines were significantly increased after LPS stimulation, which was blocked by TLR4 antagonist. The LPS stimulation enhanced the invasion and migration abilities of the A549 cells and SPC-A-1 cells, and TLR4 antagonist inhibited the invasion and migration abilities of LPS-induced A549 cells and SPC-A-1 cells, detected using invasion and migration Transwell assays. These results indicated that the activation of TLR4 might be important in the invasion and migration abilities of NSCLC cells.
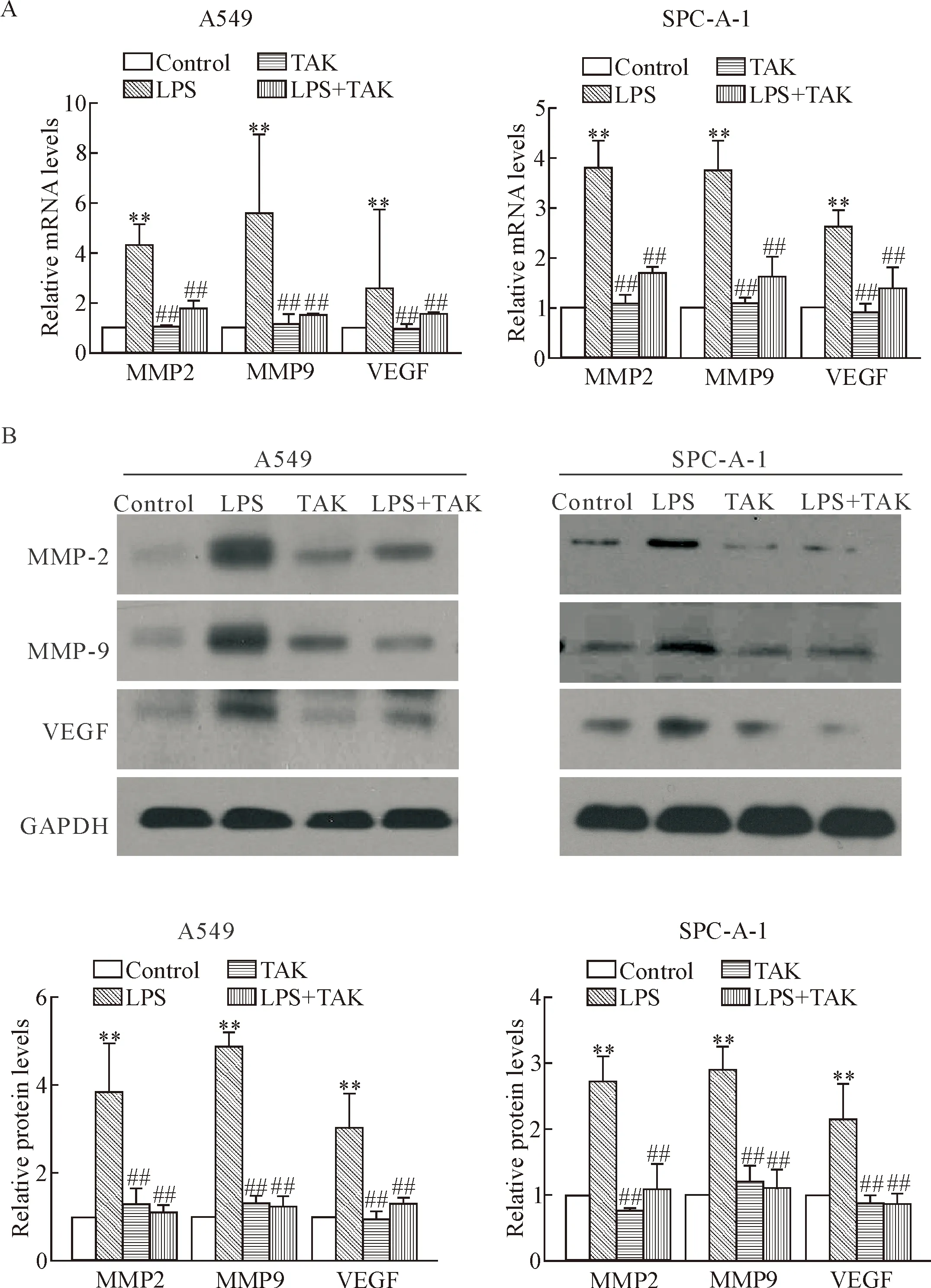
Figure 5.TLR4 antagonist decreased the LPS-induced expression levels of MMP-2, MMP-9 and VEGF in human NSCLC cells. A549 cells and SPC-A-1 cells were pretreated with TLR4 antagonist TAK-242 at 100 nmol/L for 1 h, and then the cells were treated with LPS at 10 mg/L for 24 h. A: the mRNA expression levels of MMP-2, MMP-9 and VEGF were analyzed by RT-qPCR; B: the protein expression levels of MMP-2, MMP-9 and VEGF were detected by Western blot. Data were normalized to GAPDH levels and quantitatively analyzed. Mean±SD.n=3.**P<0.01vscontrol group;##P<0.01vsLPS group.
The MMPs are zinc-dependent proteases involved in the degradation of extracellular matrix. Recent studies have revealed that the expression level of MMPs, especially MMP-2 and MMP-9, is extremely high in lung tumors compared with nonmalignant lung tissue and is associated with cancer progression[19-20]. Moreover, VEGF is a well-known angiogenesis factor involved in many physiological and pathological processes. Its significance has been implicated in promoting tumor growth and metastasis[21]. VEGF and MMPs have been found to exhibit significant impacts on tumor invasion, metastasis, advanced tumor stage, and adverse prognosis[22]. In this study, LPS stimulation significantly increased the expression levels of MMP-2, MMP-9 and VEGF in the human NSCLC cell lines A549 and SPC-A-1, which was blocked by TLR4 antagonist.
In conclusion, TLR4 was found to be strongly expressed in NSCLC tissues, especially in the patients with lymphatic metastasis. Furthermore, the over-expression of TLR4 enhanced the invasion and migration abilities of human NSCLC cell lines by increasing the expression levels of MMP-2, MMP-9, and VEGF. Therefore, TLR4 is involved in the invasion and migration of NSCLC cells, and TLR4 inhibition might be considered as a therapeutic target for treatment of NSCLC.
ACKNOWLEDGEMENTS
We thank Zhu Jun for sample collection, and the laboratory members for helpful suggestions.
