Nanothermite colloids: A new prospective for enhanced performance
2019-07-16GerZkyAhmedAdllRkeshShuIshwrPuri
M. Ger Zky , Ahmed M. Adll , Rkesh P. Shu , Ishwr K. Puri ,,
Mostafa Radwan d, Sherif Elbasuney a,*
a School of Chemical Engineering, Military Technical College, Kobry Elkoba, Cairo, Egypt
b Department of Engineering Physics and Department of Mechanical Engineering,McMaster University,1280 Main Street West,Hamilton,Ontario,L8S 4L7,Canada
c Department of Mechanical Engineering, McMaster University,1280 Main Street West, Hamilton, Ontario, L8S 4L7, Canada
d British University in Egypt, Elshorouk City, Cairo, Egypt
Keywords:Hydrothermal synthesis Nanoparticles Carbon nanotubes Nanothermites Energetic materials
A B S T R A C T Nanothermites (metal oxide/metal) can offer tremendously exothermic self sustained reactions. CuO is one of the most effective oxidizers for naonothermite applications. This study reports on two prospectives for the manufacture of CuO nanoparticles. Colloidal CuO particles of 15 nm particle size were developed using hydrothermal synthesis technique. Multiwalled carbon nanotubes (MWCNTs) with surface are 700 m2/g was employed as a substrate for synthesis of CuO-coated MWCNTs using electroless plating. On the other hand, aluminium particles with combustion heat of 32000 J/g is of interest as high energy density material. The impact of stoichiometric nanothermite particles (CuO/Al & Cuo-coated MWCNTs/Al) on shock wave strength of Al/TNT nanocomposite was evaluated using ballistic mortar test.While CuO-coated MWCNTs decreased the shock wave strength by 15%;colloidal CuO enhanced the shock wave strength by 30%.The superior performance of colloidal CuO particles was correlated to their steric stabilization with employed organic solvent. This is the first time ever to report on fabrication,isolation, and integration of stablilized colloidal nanothermite particles into energetic matrix where intimate mixing between oxidizer and metal fuel could be achieved.
1. Introduction
Thermite particles have attracted increasing attention as they can offer massive heat output and act as high energey density material[1-5].They can offer tremendous volumetric heat output compared with common energetic materials (Fig.1) [6,7].
Fig. 1 demontrated that CuO/Al is one of the most effective thermite mixtures in terms of combustion temperature, heat output, and gasous products [9,10]. CuO/Al can offer an adiabatic flame temperature of 5718 K with heat of reaction of about 4100 J/g,and aresonable amount of gaseous products (0.345 g gas/g thermite). Furthermore the formed Cu metal (resulted from thermite reaction) can be transformed into gas due to its low boiling point(1000°C) [11].
The application of thermites in an explosive composition was demonstrated in US Patent 3, 297, 503. It has been reported that when certain thermites (Al/Fe2O3, Zr/B2O3, Li/MoO3, Li/WO3, Al/Mn3O4, Al/V2O5) were incorporated in an explosive formulations based on TNT; they offered good positive afterburning and extended fireballing [12].
The reaction rate of conventional thermite particles is limited by particle diffusion and is comparatively low [13]. Nanothermite particles can offer high interfacial surface area and reactivity [14].Consequently they can offer particles and interparticle distance in the nanoscale [15,16]. These features can offer enhanced reaction rate[17,18].Till now,there have been no publications about the use of nano-thermites in high explosive formulations for enhancedblast. This could be due to difficulties associated with the manufacture and processing of the nano-thermites in cast explosive formulations [12].
The sustainable fabrication of thermite particles at the nanoscale with consistent product quality is a major concern for advanced highly energetic systems i. e incediary devices, blasting caps, environmentally friendly ammunition primers, electric igniters, and metalized explosive formations [10].
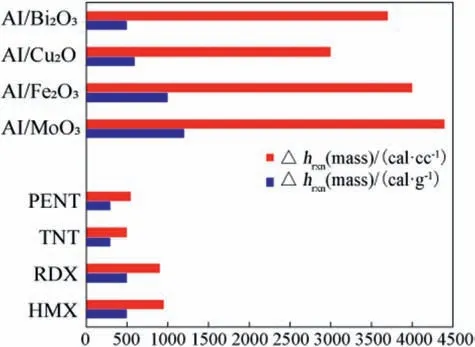
Fig.1. Gravimetric and volumetric energy density of example thermites compared to common explosives [8].
Several techniques can be employed to develop nanothermite particles; for instance, mechanical mixing [19], arrested reactive milling [20], and sol-gel technology [21]. However mechanical mixing requires nanoscale starting materials[15];arrested reactive milling could cause complete oxidation of metal fuel [20]; sol-gel technique includes drying and sintering process that could induce extensive aggregation with significant decrease in surface area and reactivity[21,22].
1.1. Hydrothermal synthesis
There is a great advantages for any approach that can offer sustainable fabrication of mono-dispersed particles that can be effectively isolated, and employed in subsequent nanocomposite manufacture [23]. Continuous hydrothermal processing is a promising technology that has been employed for the production of potentially valuable metal oxide nanoparticles [24-26]. The common employed fluid for hydrothermal processing is supercritical water (ScW). ScW requires extreme conditions; above the critical point the phase boundary disappear and homogenous supercritical phase exists (Fig. 2).
Hydrothermal processing comprises instant mixing of ScW with metal salt; nanoparticles are formed at the interface of two fluids inside the reactor in a continous manner [23,26]. The controlled hydrothermal synthesis conditions including temperature, pressure, and flow rates can allow the fabrication of highly crystalline mono-dispersed oxides with high crystallinity and controlled morphology for nanothermite applications.
1.2. Electroless plating
Electroless plating is an attractive technology as it can offer the deposition of matal particles on the surface of different nanocarbon materials. Multi-walled carbon nanotubes (MWCNTs) can offer large surface area up to 700 m2/g,as well as high catalyzing ability[28-30].MWCNTs could be the ideal substrate to deposit different reactive metal particles;the deposited reactive matal particles can be subsequently annealed to the corresponding matal oxide for nanothermite applications. Electroless plating approach offers efficient low cost facile technology compared with other techniques [28]. Electroless plating allows the deposition of various elements such as copper [31], nickel [32], silver, gold [33], and cobalt on the surface of different of carbon nanomaterials [34].
Electroless plating process includes three main stages as follow:(1) Sensitization
This process includes the attack of MWCNTs structure defects using stannous chloride and strong acid to generate a surface catalyst.
(2) Activation
The adsorbed tin ions on MWCNTs walls are used to reduce palladium ions to palladium which acts as an active site to reduce metal ions [35].
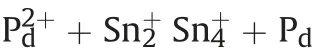
(3) Deposition
The bonded palladium atoms are ideal catalyst for metal salt reduction, consequently metal deposition can be achieved on MWCNTs. The controlled active site density as well as electroless deposition rate plays an important role for enhancing MWCNTs substrate coating [36,37].
In this study CuO particles with an average particle size of 15 nm was developed using hydrothermal processing. CuO-coated MWCNTs were developed by electroless plating. A stociometric binary mixture of CuO/Al and CuO-coated MWCNTs/Al were dispersed into isopropyl alcohol (IPA) using ultraonic probe homogenizer. The impact of developed nanothemite palrticels on shock wave strength of Al/TNT nanocomposite was evaluated using ballsitic mortatr test. While CuO-coated MWCNTs decreased the shock wave strength by 15%; colloidal CuO enhanced the shock wave strength by 30%.
Fig. 2. Phase change of fluid with pressure and temperature [27].
The enhanced performance of CuO particles was ascribed to their stabilization into the employed organic solvent. This novel finding offered nano-thermite particles where particle size and interparticle distance in the nanometer range. This novel finding can extended the applications of nano-thermite particles into different energetic systems.
2. Experimental
2.1. Materials and reagents
Copper acetate(Aldrich)was employed as a metal salt precursor for hydrothermal synthesis of CuO particles in a continous manner.MWCNTs (surface area 700 m2/g) produced by CVD (95% purity)were purchased from US Research Nanomaterials, Incorporation;MWCNTs were employed as a substrate for copper deposition.Copper (II) sulfate penta-hydrate (CuSO4⋅5H2O, Sigma-Aldrich)were employed as metal salt precursor. Sodium citrate(C6H5Na3O7⋅2H2O, Bio shop) was employed as non-complexing agent. Sodium hydroxide (NaOH, CALEDON Laboratory Chemicals), formaldehyde (HCHO, Caledon Laboratory Chemicals), Hydrochloric acid(HCl,CALEDON),Iso propyl alcohol(Sigma-Aldrich),stannous chloride dihydrate(SnCl2⋅2H2O,98%,Caledon),palladium(II)chloride(PdCl2,100%,Artcraft Chemicals Inc.).All reagents were used as received without further purification.
2.2. Hydrothermal synthesis of CuO nanoparticles
Hydrothermal processing can offer the sustainable fabrication of CuO particles for thermite application in a continuous manner. A schematic for continuous hydrothermal synthesis is demonstrated in Fig. 3.
Flow(A)was ScW at 400°C and 240 bar(20 ml/min).The metal salt precursor(B)was an aqueous solution of 0.05 M cupper acetate(10 ml/min) at 25°C and 240 bar [22]. The synthesized colloidal CuO particles demonstrated dark colour and the particles flocculated within 30 min.
2.3. Electroless plating of MWCNTs with CuO
2.3.1. Catalyzation of MWCNTs
Metallization of MWCNTs with a continual Cu layer requires, a surface pre-treatment includes sensitization using stannous chloride/strong acid. Activation with palladium is essential for increasing the catalytic sites on MWCNTs to improve bonding between nanotube and the deposited metal (Fig. 3) [38-40]. The process to obtain the MWCNTs covered with a continual Cu layer acquires a pre-treatment procedure comprised of acid pre-clean,sensitization, and activation to purify MWCNTs and to secure the catalytic sites on MWCNTs for subsequent metallization, which is followed by complete annealing at(250οC)to develop CuO-coated MWCNTs. Complete Cu-metallization of MWCNTs and subsequent annealing is demonstrated in Fig. 4 [38-40].
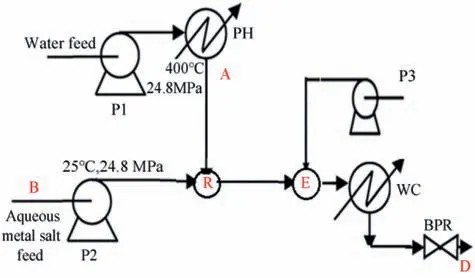
Fig.3. Flow diagram of the continuous hydrothermal processing system employed for sustainable fabrication of colloidal CuO particles. Key:P1, P2,and P3 are HPLC pumps,PH-preheater, R-continuous reactor, E capping point, WC-water cooling, BPR-back pressure regulator [22].
2.3.2. Metallization of MWCNTs with Cu
Electroless plating of activated MWCNTs was conducted by depositing Cu nanocrystals under stoichiometric conditions. The chemical reduction process was designed to obtain γ=16 (w/w)(Cu: MWCNTs). Annealing was conducted to guarantee complete oxidation of Cu to corresponding oxide (CuO) (Fig. 4) [38,41,42].
2.4. Characterization of CuO nanoparticles and CuO-coated MWCNTs
The size and shape of synthesized CuO nanoparticles and CuOcoated MWCNTs were investigated by using TEM (JEM-2010F by Joel Corporation). The crystalline phase was performed with X-ray diffraction (XRD) analysis of powder samples using a Bruker D8 Discover instrument comprising a Davinci diffractometer operating at 35 kV and 45 mA using Co-Kα radiation (λavg=1.79026 Å). The dry powder size and shape was investigated with SEM,ZEISS SEM EVO 10 MA,with three types of detectors secondary electrons(SE),back scattered electron (BSE), and energy dispersive X-ray spectrometer (EDX) Bruker Quantax 200.
2.5. Integration of nanothermite particles into energetic matrix
It has been reported that the integration of colloidal particles into energetic matrix can offer enhanced dispersion to the molecular level[22].A stoichometric binary mixture of CuO/Al and CuOcoated MWCNTs/Al were effectively dispersed in isopropyl alcohol(IPA)using ultrasonic probe homogenizer in an attempt to achieve uniform distribution and to break down any aggregates. These colloidal nanothermite particles were integrated into the energetic matrix (TNT) and the resulting shock wave upon initiation was evaluated using a ballistic mortar test to that of TNT and Al/TNT nanocomposite.
The days we can t spend together physically1, we can still take time to remember them fondly… making phone calls, sending cards or letters helps both us and our loved ones.
2.6. Workability evaluation using ballistic mortar
Ballistic mortar is the main test to evaluate the shock wave strength resulted upon initiation of different energetic materials.Ballistic mortar composed of massive steel mortar suspended from the pendulum axis by a long pendulum arm (Fig. 5).
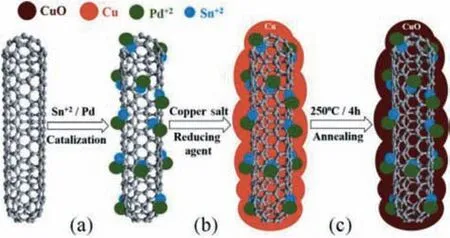
Fig. 4. Schematic of the electroless plating of MWCNTs with copper. (a) MWCNTs are sensitized using SnCl2 and subsequently activated with PdCl2. (b) The plating solution contains a copper salt and a reducing agent, allowing for the deposition of Cu on the activated MWCNTs.(c)Annealing of the Cu-coated MWCNTs at 250°C oxidizes the Cu coating to CuO.
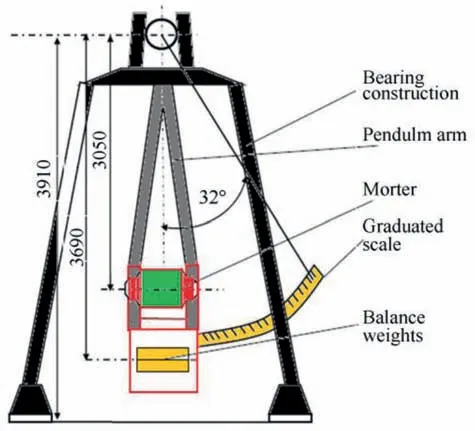
Fig. 5. Schematic diagram of ballistic mortar.
Here, 10 g of the tested energetic matrix formulation are detonated within the mortar cavity, the maximum angular displacement of the mortar was recorded and employed to retrieve the maximum over pressure (using calibration charts). This overpressure value can act as a criteria for the shock wave strength of energetic material [43]. The impact of developed nanothermite particles on shock wave strength was evaluated to that of TNT and Al/TNT nanocomposite.
2.7. Thermal behaviour of nanocomposite energetic matrix
The impact of developed nanotermite particles including CuO and Cuo-coated MWCNTs on thermal behaviour of TNT and Al/TNT nanocomposite was evaluated using DSC in an attempt to evaluate the initiation temperature and total heat released upon detonation.DSC Q-2000 (by TA instruments, USA) was employed for thermal behaviour investigation. The tested sample was heated from 50°C to 350°C at 5.0°C/min heating rate under N2flow rate of 50 ml/min.
3. Results and discussions
3.1. Characterization of CuO/CHS &CuO/MWCNTs
The morphology (size and shape) of synthesized CuO nanoparticles (developed by hydrothermal processing) was visualized using TEM (Fig. 6). Consistent CuO particles with an average particle size of 15 nm was developed; this was ascribed to the fact that nucleation and subsequent particle growth were the same during nanoparticle fabrication [22].
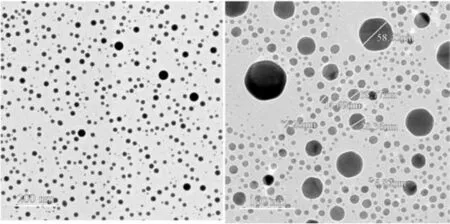
Fig. 6. TEM micrographs of CuO nanoparticles synthesized by hydrothermal processing.
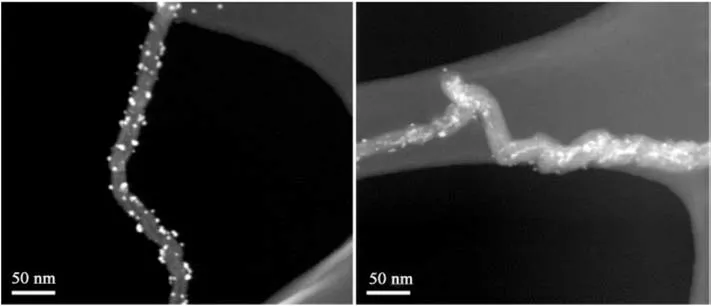
Fig. 7. Dark field STEM micrographs of synthesized CuO-MWNTs hybrid material. The bright spots on the surfaces of the MWNTs are CuO nanoparticles, suggesting uniform metallization and subsequent annealing of Cu nanoparticles to CuO.
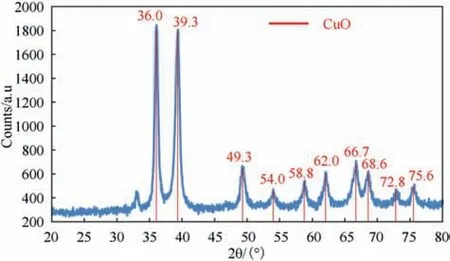
Fig. 8. XRD diffractogram of synthesized CuO.
Electroless plating offered effective coating of MWCNTs with CuO particles, CuO-coated MWCNTs demonstrated effective formation of CuO particles bonded to a substrate of high surface area(Fig. 7).
XRD diffractogram of synthesized CuO nanoparticles and CuOcoated/MWCNTs demonstrated highly crystalline Copper (II) oxide material with 8 characteristic sharp peaks all of them agreed with the Joint Committee on Powder Diffraction Standards(JCPDS)from the International Centre for Diffraction Data(ICDD),indicating the high crystalline and high purity CuO particles (Fig. 8).
The carbon counts in the XRD patterns are low, which is attributed to the high weight ratio of CuO to MWCNTs,i.e.,16:1.The morphology of dry powder for synthesized CuO nanoparticles and CuO-coated MWCNTs was investigated with SEM. SEM micrographs demonstrated a great tendency for dry particles to aggregate and agglomerate with extensive decrease in surface area and reactivity under drying process (Fig. 9).
It is widely accepted that integration of colloidal particles into energetic matrix can offer enhanced dispersion characteristics with intimate mixing between oxidizer and metal fuel [44-46]. Consequently the developed particles were effectively dispersed into IPA with Al nanoparticles for subsequent integration into molten TNT.CuO nanoparticles demonstrated stable colloid as CuO particles could be sterically stabilized with the organic solvent molecules(Fig.10).
It is established that colloidal oxides have a hydrous surface[47,48]. Consequently the hydroxyl group on the surface of CuO particles could undergo strong hydrogen bonding with polar organic solvents such as IPA[49,50].The solvent aliphatic segment could offer a steric barrier keeping the colloidal particles outside the range of van der Waals attractive force. This novel finding can offer intimate mixing between oxide and metal particles.
3.2. Workability evaluation
The impact of developed nanothermite particles on shock wave strength of Al/TNT nanocomposite was evaluated using Ballistic mortar test. The partial replacement of Al particles with CuO or CuO-coated MWNTs was conducted at the same total solid loading level (12 wt %). CuO particles developed by hydrothermal processing demonstrated superior shock wave strength compared with CuO-coated MWCNTs (Fig.11).

Fig. 9. SEM micrographs of synthesized CuO nanoparticles using hydrothermal processing (a), CuO-coated MWCNTs using electroless plating (b).
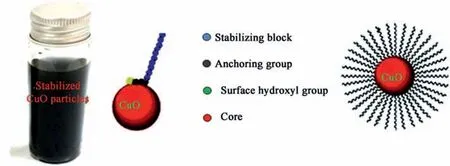
Fig.10. Stabilized CuO particles (a), Stabilization mechanism of CuO with IPA solvent (b).
The superior performance of colloidal CuO particles was ascribed to the fact that ultrafine particles were effectively developed isolated and effectively integrated into the energetic matrix[17]. This approach could fulfil the main requirements of nanothermite particles and secure particle and inter-particle distance in the nanoscale[44,45,51].On the other hand,the synthesis of CuOcoated MWCNTS includes sintering process which could induce extensive aggregation.
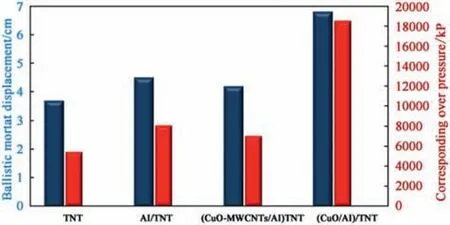
Fig. 11. The impact of developed nanothermite particles on shock wave strength of TNT.
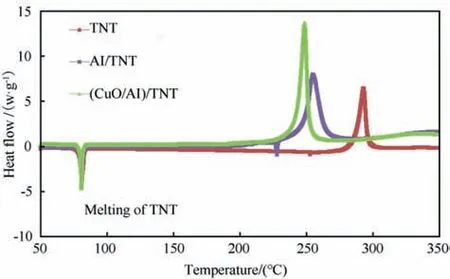
Fig.12. DSC thermograms of TNT and Al/TNT and (CuO/Al)/TNT nanocomposite.
3.3. Thermal behaviour of energetic matrix based on nanothermite
DSC measurements can provide quantitative information about any physical/chemical process that is accompanied with absorption/release of heat.Nanothermite particles could alter the thermal behaviour,and total heat release of TNT as well as Al/TNT.(CuO/Al)/TNT nanocomposite demonstrated thermal behaviour similar to Al/TNT but with further increase in total heat release by 31%. CuO/Al demonstrated a decrease in temperature at maximum heat fow of Al/TNT by 13°C (Fig. 12). This can be ascribed to the enhanced thermal conductivity of(CuO/Al)/TNT nanocomposite as well as the enhanced dispersion characteristics of CuO/Al nanothermite particles.
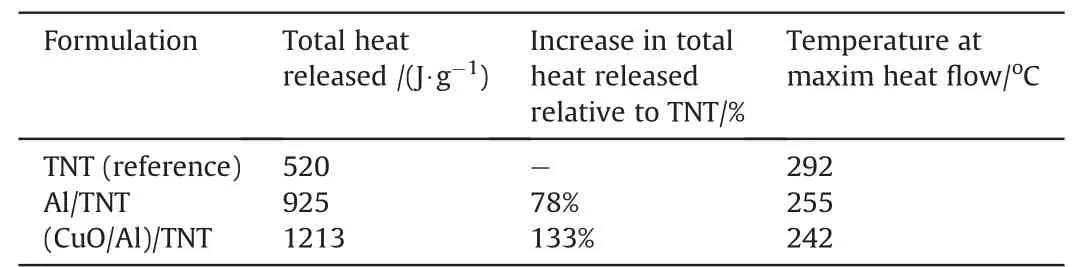
Table 1The impact of colloidal nano-thermite particles(CuO/Al)on thermal characteristics of TNT.
A summary of the main thermal characteristics of(CuO/Al)/TNT nanocomposite to Al/TNT was provided in Table 1.
4. Conclusion
Colloidal CuO particles for nanothermite applications were effectively developed using hydrothermal processing technique.The developed CuO particles were stabilized in organic solvent(IPA) via steric stabilization. The effective integration of colloidal CuO/Al binary nano-thermite mixture offered enhanced shock wave strength by 30% relative to Al/TNT nanocomposite. CuO/Al also offered an increase in total heat release of Al/TNT by 31%using DSC.This superior performance of colloidal CuO particles compared with CuO-coated MWCNTs was ascribed to the fact that one of the most effective thermite reactions was developed in colloidal state and effectively integrated into TNT where particle size and interparticle distance in the nanoscale.This could open the route for the manufacture of different nano-thermite particles for the effective developed of advanced nanocomposite cast metalized formulations.
杂志排行
Defence Technology的其它文章
- Body armour - New materials, new systems Ian G. Crouch*
- Special materials in pyrotechnics VII: Pyrotechnics used in thermal batteries☆
- Real-time calculation of fragment velocity for cylindrical warheads
- Heavy metal free primers: Polymorphism of gadolinium and titanium in the context of GSR glass phase Felice Nunziata
- Mitigation of EDFA transient effects in variable duty cycle pulsed signals
- Ballistic impact properties of woven bamboo- woven E-glassunsaturated polyester hybrid composites
