Heavy metal free primers: Polymorphism of gadolinium and titanium in the context of GSR glass phase Felice Nunziata
2019-07-16ProfessionalMemberoftheCharteredSocietyofForensicScienceItaly
Professional Member of the Chartered Society of Forensic Science, Italy
Keywords:GSR Heavy metal free primers Gadolinium Glass phase EBSD TKD
A B S T R A C T The possibility of identifying gunshot residue (GSR) particles produced by non-toxic primers containing only titanium and zinc is a very difficult task using SEM/EDX analysis employed in the analysis of GSR originating from primers containing lead,barium and antimony.However,Bauer et al.demonstrated that non-toxic (Ti-Zn) primers form a TiZn2O4 spinel crystalline structure using SEM/EDX with EBSD (Electron Back Scatter Diffraction) and TKD(Transmission Kikuchi Diffraction), whereas GSR originating from gadolinium-doped Ti-Zn primers form a non-crystalline glass phase. Here, a possible explanation of these different phenomena is hypothesized.
1. Introduction
Scanning Electron Microscopy interfaced with Energy Dispersive X-Ray spectrometry (SEM/EDX) enables the recognition of characteristic GSR particles formed on the discharge of primer mixes consisting of lead styphnate, antimony sulfide and barium nitrate. The alleged uniqueness of the composition of some particles was the criterion for the identification of the GSR,but over the years, on the basis of always new experiences and experiments,GSR scientists realized that the particles containing lead, barium and antimony also of spheroidal morphology had to be considered no longer as solely attributable to gunshot residues but only compatible with them, since they potentially can came also from anthropogenic sources completely different from firearms. This development of knowledges has had an impact on the complexity of the interpretation of the analytical results obtained by SEM/EDX:in fact the evolution of the interpretative criteria can also be read in the subsequent editions of the technical standard E 1588 or STANDARD GUIDE FOR GUNSHOT RESIDUAL ANALYSIS BY SCANNING ELECTRON MICROSCOPY/ENERGY DISPERSIVE X-RAY SPECTROMETR.In the latest versions it speaks of classifications in terms of particles characteristic of and consistent with.As stated,the term characteristic of must therefore be understood in terms of compatibility and not exclusivity. This change in classification should not be surprising as the development of a scientific method is, by its very nature, constantly evolving. Currently ASTM E 1588-17 also provides for“lead free or no toxic ammunitions”elemental formulations characteristic of and consistent with (see Fig.1).
Non-toxic primers have been developed to mitigate the release of toxic pollutants in closed space shooting environments.Particles produced by the discharge of these primers are not easily detected by SEM/EDX equipped with automated GSR search systems because within the spectral resolution calculated on the Mn Kα line(<150 eV)of the SEM/EDX,barium and titanium fluorescence lines can not be well resolved, await the difference in terms of a few tenths of keV (Ti Kα=4.51 keV versus Ba Lα=4.46 keV - Ti kβ=4.93 keV versus Ba Lβ=4.82 keV). Barium is within the classification algorithms because is present in the composition of traditional primer mixture Sinoxyd type. Furthermore, as titanium and zinc containing particles are common environmental contaminants,analysis of particles having compositions corresponding to GSR derived from this type of ammunition [1] needs to be critically assessed in order to determine whether the particles are GSR or not. Consequently, it is necessary to fully understand the GSR formation processes [2] for this type of primer in order to classify particles found on the collected samples.
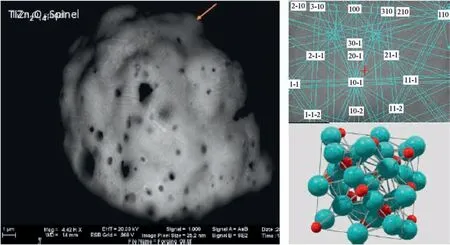
Fig.1. SINTOX®GSR particle-Phase on Surface.(from Frank Bauer Heavy Metal Free(HMF)and Fulminate Containing Ammunition:BasicInvestigations ENFSI Firearms and GSR 22nd Annual Meeting Limassol, Cyprus November3th - 5th, 2015).
2. Background
This paper proposes a theoretical explanation for the findings of Bauer et al. for GSR derived from non-toxic Ti-Zn (SINTOX®)primers and Gadolinium-doped, non-toxic Ti-Zn (SINTOX FORENSIS®) primers using SEM/EDX with EBSD (Electron Back Scatter Diffraction) and TKD (Transmission Kikuchi Diffraction) [3].
SINTOX®primers contain tetrazene, diazodinitrophenol, zinc peroxide Zn(OH)2and titanium (Ti). GSR products from SINTOX®primers were found to form a crystalline spinel,TiZn2O4,phase[3].
The presence of a TiZn2O4spinel phase in a particle may be essential to discriminate non-toxic Ti-Zn GSR from non-GSR particles having the same elemental composition. However, the detection and identification of the TiZn2O4spinel phase requires instrumentation not commonly interfaced with forensic SEM/EDX,involves complex sample preparation and requires a high skill level to interpret EBSD and TKD data.
SINTOX FORENSIS®primers have the same composition as SINTOX®primers with the addition of Gadolinium(GD)as a tracer[3]. Ammunition, incorporating SINTOX FORENSIS®primers, is distributed in some European countries to Defence Forces, Police and/or special units. The Gd tracer provides discrimination of SINTOX FORENSIS®GSR from other forms of GSR in cases of forensic interest.
As opposed to GSR from SINTOX®, GSR products fromSINTOX FORENSIS®form a non-crystalline glass phase. Expected products resulting from the initiation of SINTOX®are ZnO,ZnTiO3,Zn2Ti3O8,TiO2, TiZn2O4etc [4].
Yang and Swisher's study of the phase diagramof ZnO-TiO2[5]suggests that the initiation products from the SINTOX®primer could be possible phases of TiZn2O4(cubic), ZnTiO3(hexagonal) and Zn2Ti3O8(cubic).
TiZn2O4spinel formation can occur by solid state reaction between ZnO and TiO2; by oxidation in vapor phase or by the oxidation reaction of Zn and TiO2in powder form [6].
It is, therefore, possible that, starting from precursors such as Zn(OH)2and Ti (~40 μm grain size) [7], that ZnO and TiO2metaproducts[8],which are the basis of SINTOX®derived GSR,can form through oxidation reactions.
3. Discussion
The explanation of these the empirical observations, namely glass phase properties of Ti-Zn-Gd GSR produced by the initiation of SINTOX FORENSIS®primer,by a physico-chemical point of view,could probably be attributed to three competing factors:
⇒Ti and Gd polymorphism;
⇒in theTi-Gd system immiscibility of the phases occurs where the phases are either Gd-rich orTi-rich,between 1200°C and 1600°C [9];
⇒in theTi-Zn system (three) peritectic and (one) eutectic
points occur between 400°C and 500°C [10] (see Fig. 2).
Zinc has a hexagonal crystalline structure and is not polymorphic. Titanium and gadolinium, on the other hand, are polymorphic metals.The low temperature modification of both involves a hexagonal lattice (Fig. 3), while the high temperature modification involves a body-centered lattice (Fig. 4), each with distinct physical properties.
High temperature forms of polymorphic metals, due to the tendency to decrease the total energy of the crystalline arrangement,always involve simple crystalline lattices(with respect to the low temperature form).However,it involves a cantered,crystalline structure which involves maximum plastic deformation with respect to shape and dimensional size of the crystal structure but without any fracturing. With an increase in temperature of a polymorphic metal,the proportion of metallic bonds increases due to the destruction of the covalent electron bridge due to thermal vibrations of the atoms.
In SINTOX®mixtures, the solubility of Ti in Zn is low and, as stated previously, the Ti-Zn system has three peritectic and one eutectic point in the temperature range between 400°C and 500°C.As the temperature decreases after the primer initiation,one liquid phase develops and two solid phases,α(rich in A)and β(rich in B)at the peritectic points. As the temperature approaches the lower termic point (eutectic), the compositions of the two solid phases change and simultaneously come to equilibrium with the liquid phase.The solid phases could act as nucleating agents if the liquid phase binds to one of the two solid Ti-rich phases and simultaneously aggregates with ZnO, (a reaction product of the primer combustion), thereby inducing the formation of TiZn2O4spinel.
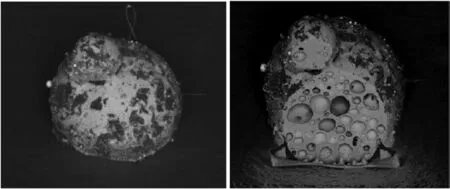
Fig. 2. GSR SINTOX FORENSIS ®, Gd-containing particles (glassy phase non-crystalline) (from Frank Bauer Heavy Metal Free (HMF) and Fulminate Containing Ammunition: Basic Investigations ENFSI Firearms and GSR 22nd Annual Meeting Limassol, Cyprus November3th - 5th, 2015).
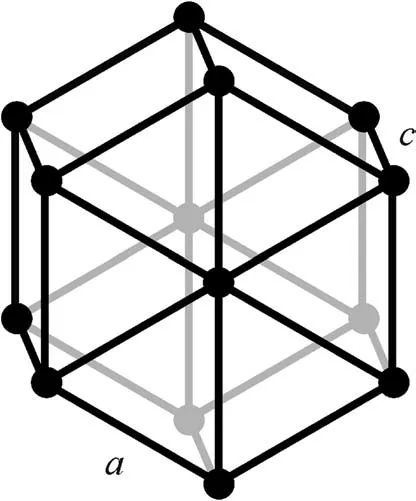
Fig. 3. Hexagonal lattice.
However, the reaction products formed on the initiation of the SINTOX FORENSIS®primer is determined by the chemical and physical properties of Gd and the Ti-Gdeutectic system, in particular, the formation of the separate Gd-rich and Ti-rich phases that form between 1200°C and 1600°C, Gd (as with Zn) demonstrates an affinity to react with oxygen. Gd has poor solubility in Ti but reacts with oxygen from the Ti-rich matrix to form insoluble microoxides which promote grain refinement.Gd is also a surface active element and migrates to the liquid-solid interface or be absorbed onto the growth front. Therefore, it can modify both solid-liquid interfacial energy and crystal surface energy, slowing down the phases of growth and causing further grain refinement.It may also favor discontinuous phases located along the edges of the grain by limiting growth.
If we remind during the particles nucleation process Gibbs function in term of undercooling ΔT (solidification temperature at equilibrium minus the temperature of molten matter) then free energy changes on nucleation are due to bulk free energy reduction+increase in surface energy+increase in strain energy.A mechanism for spontaneous grain refinement comes from the breaking of dendrites on mergers growths occurring during highly undercooled fusions. The processes of atomic rearrangement in solid-liquid interfaces,may be of the collision-limited or diffused category. Grain refinement mechanisms cause a strong restriction effect of the growth front, leading to heterogeneous nucleation of α-Gd (the hexagonal, close-packed form). In fact grain refinement contrasts with rearrangement of close-packed array of anions after primer initiation: spinel ordered structure cannot forms because was changed distance between the ions and hence lattice enthalpy is not favored. Consequently, the presence of Gd inhibits the formation of Ti-Zn-O,spinel, structures.
A very similar glass phase could be obtained by doping a SINTOX®primer mixture with terbium (Tb) [11] because it possesses very similar chemical and physical properties as Gd. It is polymorphic within the temperature range of Gd polymorphism and its electronic configuration favors oxygen sequestration. Experimentation with Tb-doped triggers could contribute to validating this theoretical model.
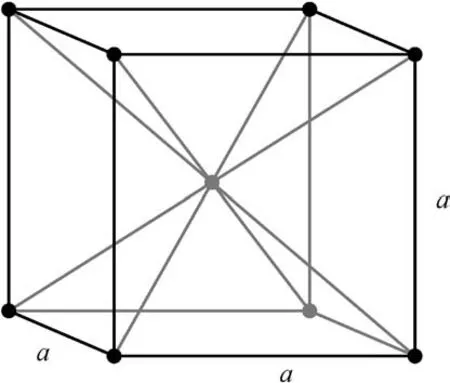
Fig. 4. Body-centered lattice.
4. Conclusion
Reasons for the differences in the atomic structures of GSR formed by SINTOX®and SINTOX FORENSIS®has been postulated on the basis of the chemical and atomic properties of gadolinium in SINTOX FORENSIS®.
GSR formed on the initiation of SINTOX®is crystalline with a spinel-like composition. SINTOX FORENSIS®has the same composition as SINTOX®but with the addition of gadolinium dopant.GSR formed on the initiation of SINTOX FORENSIS®is glass-like with no coherent crystallinity.
Gadolinium because of poor solubility in Ti and of ability to react with oxygen of the Ti-rich matrix,on the basis of the Fick's laws,can likely migrate to the liquid-solid interface and/or be absorbed on the growth front. These circumstances, not contradictory with the empirical observations and thermodynamically related to bulk free energy reduction,increase of surface energy and increase of strain energy,inhibits the formation of a crystalline structure,principally because can to modify both solid-liquid interfacial energy and crystal surface energy, slowing down the phases of growth and it may also favor discontinuous phases located along the edges of the grain by limiting growth.Finally polymorphism of gadolinium and titanium implies,with an increase in temperature during explosive phase,a destruction of the covalent electron bridge due to thermal vibrations of the atoms and hence the proportion of metallic bonds increases and it involves a centered, crystalline structure which involves maximum plastic deformation with respect to shape and dimensional size of the crystal structure but without any fracturing.Consequently, the presence of Gd inhibits the formation of Ti-Zn-O,spinel structures.
杂志排行
Defence Technology的其它文章
- Body armour - New materials, new systems Ian G. Crouch*
- Special materials in pyrotechnics VII: Pyrotechnics used in thermal batteries☆
- Real-time calculation of fragment velocity for cylindrical warheads
- Mitigation of EDFA transient effects in variable duty cycle pulsed signals
- Ballistic impact properties of woven bamboo- woven E-glassunsaturated polyester hybrid composites
- Experimental investigations on wear properties of Palm kernel reinforced composites for brake pad applications
