Burning rate and other characteristics of strontium titanate (SrTiO3)supplemented AP/HTPB/Al composite propellants
2019-07-16SunilJinGrimGuptDhirendrKshirsgrVrushliKhireBlsurmninKndsurmnin
Sunil Jin , Grim Gupt , Dhirendr R. Kshirsgr , Vrushli H. Khire ,Blsurmnin Kndsurmnin
a HEMRL, Sutarwadi, Pune, India
b DIAT, Girinagar, Pune, India
Keywords:Strontium titanate Ammonium perchlorate Composite propellant Burning rate
A B S T R A C T In a quest of search for a new burning rate modifier for composite propellant,strontium titanate(SrTiO3),a perovskite oxide has been chosen for evaluation in a composite propellant formulation based on its other catalytic applications.Initially,SrTiO3 was characterized for particle size,morphology and material/phase identification(using XRD).By varying SrTiO3 content in a standard composite propellant,different compositions were prepared and their performance and processing parameters like the end of mix(EOM) viscosity, mechanical properties, density, burning rate, pressure exponent (n-value), etc. were measured. The results reveal that 2% SrTiO3 causes more than 12% enhancement in propellant burning rate (at 70 ksc pressure) in comparison to the standard propellant composition. The pressure exponent also increases to 0.46, whereas the standard composition was having its value as 0.35.
1. Introduction
Composite propellant [1,2] containing ammonium perchlorate(AP), Aluminium powder (Al) and hydroxyl-terminated polybutadiene(HTPB)is the workhorse solid rocket propellant for most of the present missiles and rockets.To achieve a higher burning rate of composite propellant,burning rate modifiers are used in almost all propellant compositions. The transition metal oxides (TMOs)and their complexes are found to be the most effective burning rate modifiers for composite propellants. The effect of transition metal oxides on ballistic properties of composite propellant was studied and reviewed by many researchers [3-7]. The incorporation and evaluation of transition metal complexes (ferrocene,catocene, etc.) and grafted complexes (butacene) in composite propellant formulations have also been carried out by a number of researchers [8-10]. Mixed metal oxides are advantageous than single metal oxide in composite propellant formulations due to selectivity of catalytic reactions [11,12]. Various mixed oxides such as chromites [13,14], ferrites [15-17], etc. have been investigated as burning rate modifiers in composite propellant formulations by various researchers. However, only limited references are available for perovskite oxides application in composite propellant formulations.
The perovskite structure is adopted by many oxides that have the chemical formula ABO3[18]. The general crystal structure of perovskite is a primitive cube, with the A-larger cation in the corner, the B-smaller cation in the middle of the cube, and the anion,commonly oxygen,in the centre of the face edges,where A is a monovalent, divalent or trivalent metal and B a pentavalent,tetravalent or trivalent element, respectively. The cation B is generally a transition metal element which can exhibit multiple valances.This may facilitate many intermediate reactions during AP decomposition or composite propellant combustion. Further, the perovskite crystal structure imparts possibility of having active catalytic sites on the surface of particles due to crystal imperfections(mainly substitutional defects).Hence,perovskite oxides may behave as a catalyst for AP decomposition or composite propellant combustion.
Wang et al. have reported the catalytic effect of perovskite oxides LaFeO3,LaCoO3,and LaNiO3on AP decomposition and found a decrease in its second exothermic peak temperature [19]. Yu et al.reported a significant decrease in AP decomposition peak temperature (second exothermic) by NdCrO3[20]. Guan et al. compared catalytic effectiveness of irregular and nano-sheet FeTiO3on AP decomposition[21].From the available literature,it is observed that detailed study using perovskite oxides in propellant compositions have not been carried out so far.
Strontium titanate, an important perovskite oxide, has been found effective in many areas of catalysis.It has been reported as a useful catalyst by J.J.Wu et al.for oxalic acid ozonation[22].Doped SrTiO3has been used as a reforming catalyst to produce hydrogen from dodecene [23]. There are references for application of SrTiO3as a photocatalyst for degradation of furfural [24] and also water splitting for producing hydrogen [25]. By considering the above catalytic applications of SrTiO3, it was hypotheses that it may also act as a good catalyst for burning rate enhancement of composite propellants.
In the present study, strontium titanate was characterized and then systematically evaluated in a standard composite propellant.The processing/performance parameters such as EOM viscosity,thermal, mechanical and ballistic properties of SrTiO3based propellant compositions were then compared with the parameters of standard composition in order to ascertain its suitability as a possible burning rate modifier.
2. Experimental
2.1. Materials
Bi-modal AP (300 μm and 50 μm) were used in propellant compositions. AP-300 μm was purchased from M/s Pandian Chemicals Ltd. (Cuddalore, India). The 50 μm average size fraction AP was prepared by grinding of 300 μm AP fraction in a pin disc mill(ACM-10). HTPB (average molecular weight: 2560 and hydroxyl number:41.8 mg KOH/g)and Al powder(average size 15 μm)were purchased from M/s Anabond Ltd. (Chennai, India) and M/s The Metal Powder Company (Madurai, India), respectively and employed as received. Strontium titanate (purity>99%), dioctyl adipate (DOA), toluene diisocyanate (TDI), N-phenyl-2-naphthylamine (NONOX-D), trimethylolpropane (TMP) and 1, 4-butanediol (n-BD) were purchased from trade and employed as received.
2.2. Characterization
X-ray diffraction (XRD) study of SrTiO3was carried out on a Phillips PANalyticalX'pert pro powder diffractometer employing Cu-Kα radiation. The particle size of SrTiO3was determined by photon cross-correlation spectroscopy (PCCS) based Sympatec NANOPHOX analyser. The surface morphology of SrTiO3was determined by using a ZEISS Sigma FESEM (Field Emission Scanning Electron Microscope).The propellant slurry EOM viscosity was measured using a dial-type Brookfield viscometer (Model HBT). A differential scanning calorimeter (DSC) of TA Instruments make(Model Q20) was used to carry out the thermal decomposition study at a heating rate of 10°C/min. The mechanical properties(tensile strength, E-modulus and percentage elongation) evaluation of the cured propellant samples were performed on a universal testing machine(UTM)of Hounsfield make following ASTM-D-638 type IV standard at 50 mm/min crosshead speed. The thermal transport properties (thermal conductivity, etc.) of propellant samples were measured using laser flash method on Anter Corporation,USA make machine(model flashline-3000)by taking the samples in the form of disks of 1.5 mm thickness and 12.5 mm diameter. The friction and impact sensitivities of the studied propellant compositions were determined using a Bundesanstalt fur Materialprufung(BAM)fall hammer(2 kg drop weight),Model No.BFH-10(OZM,Czech Republic)and a BAM friction apparatus,Model No. FSKM-10 (OZM, Czech Republic), respectively. Parr isoperibol calorimeter(model 6200)was used to measure calorimetric values(cal-val)of the prepared propellant samples under N2atmosphere.The density of cured propellant samples were measured using gas pycnometer. The propellant solid strand burning rate (SSBR) was measured by a stainless steel bomb pressurized with N2using acoustic emission technique.
2.3. Incorporation of SrTiO3 in propellant formulations
The mixing of propellant was carried out in a 1L mixer,following a standard procedure[7].All the liquid ingredients(except TDI)and antioxidant were added to the initially. Then catalyst, Al and AP were added in sequence with intermittent mixing. Before the addition of TDI, vacuum mixing was accomplished for 30 min.maintaining the mix temperature of 45±2°C. At this stage, the required quantity of TDI was incorporated into the mix and mixing was continued for another 40 min.After final mixing,the viscosity of the propellant slurry was measured and the slurry was cast into a 150 mm×150 mm X 40 mm aluminium tray under vacuum. The tray was kept at 50°C for 5 days in a water jacketed oven for propellant curing.
In the present study,the propellant processing and performance parameters were studied for the propellant formulations mentioned in Table 1.
3. Results and discussion
3.1. Strontium titanate characterization
Material/phase identification study of SrTiO3was carried out with the help of a continuous scanning mode powder XRD instrument(at 2°/min scanning rate).Fig.1 shows the obtained XRD peaks pattern which clearly reveals the crystalline nature of SrTiO3as sharp peaks are present.The peaks also match closely with XRD peaks presented in JCPDS-35-734 for cubic SrTiO3.
Photon cross-correlation spectroscopy (PCCS) based analyser NANPHOX was used to measure the particle size of SrTiO3. The analysis revealed the average size of the powder as 0.22 μm.
The surface morphology of SrTiO3was determined by FESEM.The FESEM image(Fig.2)shows irregularly shaped particles having size in the range of 200-400 nm.
3.2. Catalytic effect of SrTiO3 on ammonium perchlorate
Before evaluating SrTiO3in the composite propellants,its effect on AP thermal decomposition was studied. A mixture comprising 1.427% SrTiO3and 98.573% AP (corresponding to 1% SrTiO3in propellant composition)was analysed at a heating rate of 10°C/min by DSC.The results presented in Table 2 and Figs.3 and 4 indicate that the addition of SrTiO3in AP causes a reduction in temperatures of both exothermic peaks. However, the reduction in II exothermic peak temperature is quite significant in comparison to I exothermic peak. These findings infer that SrTiO3has the capability of catalysing AP thermal decomposition.
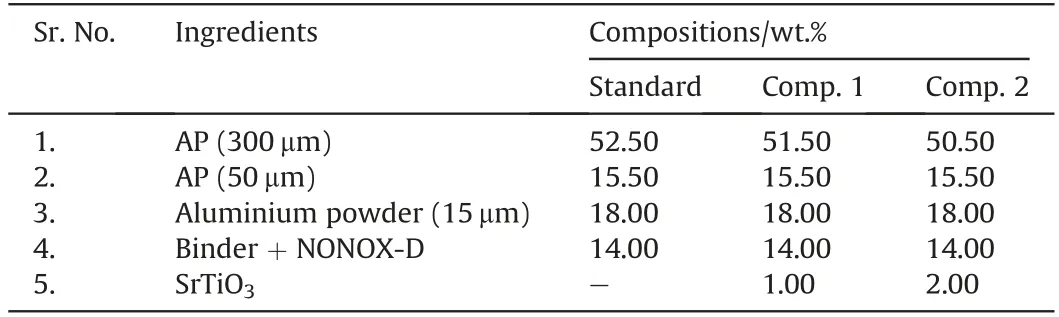
Table 1Composition details of the studied propellants.
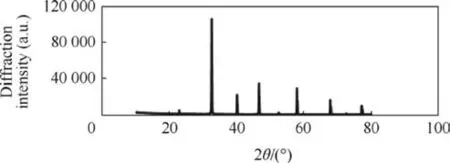
Fig.1. XRD of SrTiO3.

Fig. 2. FESEM of SrTiO3.

Table 2Effect of SrTiO3 on thermal decomposition temperature of AP.
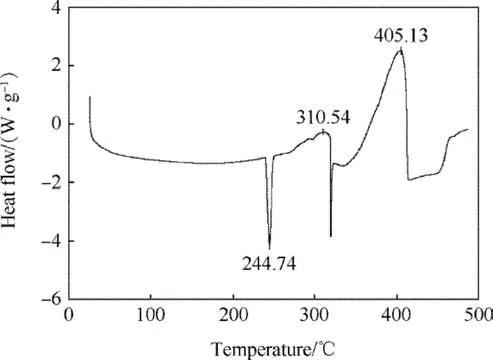
Fig. 3. DSC of ammonium perchlorate.
3.3. Evaluation of SrTiO3 in composite propellants
The propellant compositions (Table 1) were prepared by incorporating SrTiO3at 1.0 and 2.0%level(by replacing an equal amount of coarse AP) in the standard composition. The propellant processing and performance parameters were then studied for all the prepared compositions.
Fig. 4. DSC of mixture comprising 1.427% SrTiO3 and 98.573% AP.
The propellant slurry viscosity, density and mechanical properties data are presented in Table 3. The data indicates that as the content of SrTiO3increases in composition, the EOM viscosity also increases. The increase in EOM viscosity of SrTiO3compositions may be due to lower particle size of SrTiO3in comparison to AP.However, despite the slightly higher viscosity of SrTiO3compositions, the propellant slurry was easily castable.
The cured propellant density data (Table 3) show that the density of the SrTiO3based formulations increases with its content.This behaviour may be due to the higher density of SrTiO3in comparison to AP which was replaced for SrTiO3incorporation in the propellant compositions.The density of SrTiO3and AP was 5180 and 1950 kg/m3, respectively as measured using gas pycnometer.
The mechanical properties data (Table 3) infers that as SrTiO3content in the compositions increase, all the three performance parameters (tensile strength, E-modulus and % elongation)decrease. This behaviour could be due to the less binding (adhesion) of SrTiO3particles with binder compared to AP particles.However,the mechanical properties of developed compositions are sufficient for any case bonded propellant application.
Table 4 shows data on impact and friction sensitivity of the standard composition and the composition having 2% SrTiO3following standard procedures [26-29]. The impact sensitivity values are presented as height for 50%probability of an explosion.The friction sensitivity is presented as the minimum load which will not cause initiation for five consecutive trials. The data in the table reveals that the SrTiO3based compositions are slightly more sensitive towards impact and friction than the standard composition.This may be due to the catalytic action of SrTiO3on propellant thermal decomposition under impact and frictional loading.
Propellant thermal conductivity, diffusivity and specific heat were measured for standard composition as well as a composition having 2% SrTiO3following standard procedures using flash laser thermal apparatus[30].The data obtained are presented in Table 5 which reveals that thermal conductivity, diffusivity and specific heat values of propellant increase with the addition of SrTiO3in the standard propellant composition.
Thermal decomposition study of standard propellant composition and compositions containing SrTiO3was carried out using DSC and the data on endotherm/exotherm are presented in Table 6 and Figs.5 and 6.The standard propellant exhibits an endothermic peak and two exothermic peaks. The endotherm (at around 247°C) is attributed to orthorhombic to cubic phase transition of AP crystals and exotherms are due to decomposition of AP. The incorporation of SrTiO3does not cause an appreciable change in endothermic and the first exothermic peak, however, the second exothermic peak temperature reduces by 24°C. The decrease in second exothermic peak temperature signifies that SrTiO3is catalysing the composite propellant thermal decomposition.
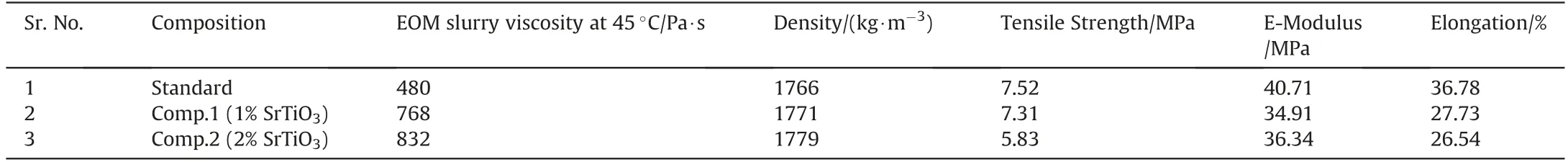
Table 3Effect of SrTiO3 on EOM viscosity, density and mechanical properties of the composite propellants.

Table 4Effect of SrTiO3 on the sensitivity of propellant.
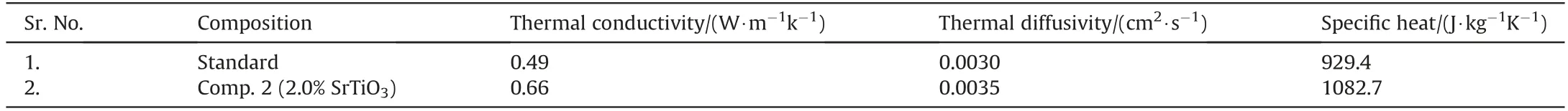
Table 5Effect of SrTiO3 on thermal transport properties of composite propellant formulations.

Table 6Effect of SrTiO3 on composite propellant thermal decomposition.
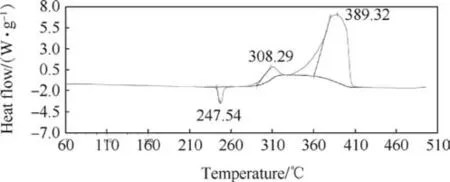
Fig. 5. DSC of standard propellant composition.
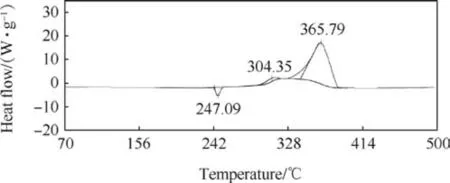
Fig. 6. DSC of 2% SrTiO3 propellant composition (Comp. 2).
Table 7 shows the calorimetric values (cal-val) of the studied propellants.The incorporation of SrTiO3in the standard composite propellant causes a reduction in its cal-val.This behaviour is due to the fact that SrTiO3is not adding any energy to the propellant system and it has been incorporated by part replacement of energetic AP.
The SSBR of studied formulations were measured at different pressures using acoustic emission technique in S.S.bomb under N2environment. The results presented in Table 7 and Fig. 7 show an increase in burning rate with increase in SrTiO3content. SrTiO3at 2.0% level causes more than 12% enhancement in burning rate (at 70 ksc pressure)in comparison to the burning rate of the standard composition.
Strontium titanate,which contains Sr as well as Ti,the catalytic mechanism by which propellant burning rate enhances is very difficult to predict. Electron transfer by redox cycle may enhance because of the multivalent nature of Ti. In addition, there may be the presence of crystal defects in the SrTiO3,which can create holes and electrons (due to the presence of Sr in addition to Ti) in the crystal structure [31,32]. SrTiO3may provide a link for electron transfer from perchlorate ions to the ammonium ions in the AP decomposition step during propellant burning.
SSBR data at different pressures were used to estimate pressure exponent (n-value) of the propellant formulations under study.Table 7 and Fig. 7 present the calculated n-values. The pressure exponent with 2% SrTiO3was 0.46 in comparison to 0.35 of standard composition.This slight increase in pressure exponent shows that premixed behaviour of flame might become more dominant in premixed/diffusion multi-flame of composite propellant combustion by the addition of SrTiO3in propellant composition.
The obtained results can be useful for prediction of combustion of SrTiO3based composite propellants.The pressure exponent(‘n’)is of critical importance in maintaining the stable operation of any rocket motor [33]. It also has significant influence on ignition transient process [33,34]. Transient pressure in the combustion chamber of solid rocket motors is given as
Table 7Ballistic properties of studied compositions using SrTiO3.
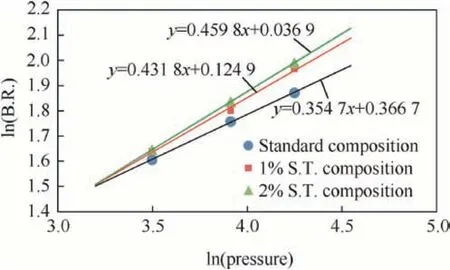
Fig. 7. Effect of pressure on propellant SSBR.
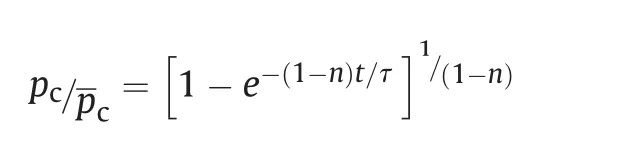
Where

and pcis the steady combustion pressure given as
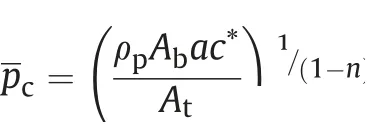
Here,Ab- Propellant burning area
At- Rocket motor nozzle throat area
Vc- Chamber free volume
c*-Characteristic velocity
a- Constant from burning rate relation r =apn
Small changes in Ab can lead to significant changes in chamber pressure(p)and subsequently in burning rate,if‘n’is close to 1.The composite propellant generally has ‘n’ below 0.5.
4. Conclusion
In this study, SrTiO3 was characterized and evaluated in a standard composite propellant having 18%Al.The propellant slurry EOM viscosity increases from 480 to 832 Pa s on the incorporation of 2.0%SrTiO3.The data on friction and impact sensitivity analysis of propellant samples showed that the developed composition with SrTiO3 is slightly more sensitive than the standard composition.The data on the burning rate reveals that in comparison to standard composition,12%enhancement in burning rate was observed with 2% SrTiO3 at 70 ksc.
杂志排行
Defence Technology的其它文章
- Body armour - New materials, new systems Ian G. Crouch*
- Special materials in pyrotechnics VII: Pyrotechnics used in thermal batteries☆
- Real-time calculation of fragment velocity for cylindrical warheads
- Heavy metal free primers: Polymorphism of gadolinium and titanium in the context of GSR glass phase Felice Nunziata
- Mitigation of EDFA transient effects in variable duty cycle pulsed signals
- Ballistic impact properties of woven bamboo- woven E-glassunsaturated polyester hybrid composites
