Preparation and property of CL-20/BAMO-THF energetic nanocomposites
2019-07-16TengChenYnZhngShungfengGuoLiumingZhoWeiChenziHoLeiXioXingKeWeiJing
Teng Chen , Yn Zhng , Shung-feng Guo , Liu-ming Zho , Wei Chen , G-zi Ho ,Lei Xio , Xing Ke , Wei Jing ,*
a National Special Superfine Powder Engineering Research Center of China, School of Chemical Engineering, Nanjing University of Science and Technology,Nanjing, 210094, PR China
b Xi'an Modern Chemistry Research Institute, Shaanxi, 710065, PR China
Keywords:CL-20/BAMO-THF nanocomposites Kinetic Thermodynamic Impact sensitivity
A B S T R A C T A sol-gel freezing-drying method was utilized to prepare energetic nanocomposites based on 2, 4, 6, 8,10, 12-hexanitro-2, 4, 6, 8, 10, 12-hexaazaisowurtzitane (CL-20) with 3, 3-Bis (azidomethyl) oxetanetetrahydrofuran copolymer (BAMO-THF) as energetic gel matrix. Scanning electron microscopy (SEM),X-ray diffraction (XRD),Raman,Fourier-transform infrared spectroscopy(FT-IR) and differential thermal analyser (DTA) were utilized to characterize the structure and property of the resultant energetic nanocomposites. Compared with raw CL-20, the average particle sizes of CL-20 in CL-20/BAMO-THF energetic nanocomposites were decreased to nano scale and the morphologies of CL-20 were also changed from prismatic to spherical. FT-IR detection revealed that CL-20 particles were recrystallized in BAMO-THF gel matrix during the freezing-drying process. The thermal decomposition behaviors of the energetic nanocomposites were investigated as well. The thermolysis process of CL-20/BAMO-THF nanocomposites was enhanced and the activation energy was lower compared with that of raw CL-20,indicating that CL-20/BAMO-THF nanocomposites showed high thermolysis activity. The impact sensitivity tests indicated that CL-20/BAMO-THF energetic nanocomposites presented low sensitivity performance.
1. Introduction
2,4,6,8,10,12-hexanitro-2,4,6,8,10,12-hexaazaisowurtzitane(CL-20)is currently one of the most powerful nitramines explosive.The superior performances, such as high energy density, high detonation velocity and high detonation pressure, make CL-20 a promising candidate for both military and civil use[1-3].However,the high energy level of CL-20 also brings in high sensitivity,which will hinder the pervasive application. Hence, materials with reduced sensitivity as well as maintain a high energy is still a challenging. In the past decades, high energy level and low sensitivity energetic nanocomposites have got considerable attention[4-9]. The nano-scaled particles of the energetic nanocomposites with shortened the mass transfer and diffusion distance can enhance the reactivity and the amount of released energy.Besides,compared with a single component material, multicomponent structured composites may acquire inimitable property through reinforcement or synergies.
Up till now, various energetic nanocomposites have been fabricated via sol-gel method, including CL-20/Fe2O3[10], RDX/Fe2O3[11],AP/SiO2[12],AP/RDX/SiO2[13],RDX/RF[14],HMX/AP/RF[15]and so on.Although these energetic nanocomposites exhibited high thermolysis activity and low sensitivities,the presence of the inert gel matrix can weaken the energy property.Thus,an effective way to overcome these shortcomings is using an energetic matrix to replace an inert matrix [16,17].
3, 3-Bis (azidomethyl) oxetane (BAMO) and tetra hydro-furan(BAMO-THF) copolymer possess several -N3groups in the polymer chain,the heat of formation are positive,and the sensitivity are low [18], which make it a promising candidate for energetic gel matrix.
In this work, we have demonstrated a facile sol-gel method to prepare CL-20/BAMO-THF nanocomposites using different concentrations of CL-20. The as-synthesized CL-20/BAMO-THF energetic nanocomposites were characterized by SEM, XRD,Raman, FT-IR and DTA. The kinetics and thermodynamics parameters of CL-20/BAMO-THF nanocomposites were calculated. Besides, the impact sensitivities were also tested.
2. Experimental
2.1. Materials
BAMO-THF copolymer (BAMO/THF=5:5, hydroxyl value of 0.36 mmol/g)was purchased from the 806thInstitute of the Eighth Academy of China Aerospace Science and Technology Corporation;2, 4, 6, 8, 10, 12-hexanitro-2, 4, 6, 8, 10, 12-hexaazaisowurtzitane(CL-20, micro grade) was provided by Liaoning Qing yang Chemical Co.,Ltd.;Acetone and ethyl acetate were purchased by Shanghai Lingfeng Chemical Reagent Co., Ltd.; Hexamethylene diisocyanate(HDI) and ditinbutyl dilaurate (DBTDL) were purchased from Aladdin Chemical Co., Ltd. All the materials were used as received without further purification.
2.2. Preparation of CL-20/BAMO-THF nanocomposites
For instance,5 mL of acetone and ethyl acetate mixed solvent(v:v=1:1)were used to dissolve 0.768 g of BAMO-THF,0.2 g of CL-20,0.032 g of HDI and 13 μL of DBTDL. The mixture was vigorously magnetic stirred at 50°C for 30min. After dissolving completely,the mixture was kept under 50°C for gelation. During this period,cross-linked gel matrix was formed, due to the reaction between the -OH groups in BAMO-THF and the isocyanate groups of HDI.After that, the wet gels were aged for 3 days and dried by vacuum freeze-drying method to obtain CL-20/BAMO-THF nanocomposites.Different mass ratios of CL-20 in CL-20/BAMO-THF nanocomposites were also synthesized. The mass ratios of the CL-20 in the CL-20/BAMO-THF nanocomposites were 25%, 45% and 60% and the assynthesized samples were labelled as CL-200.25, CL-200.45and CL-200.60, respectively.
2.3. Characterization and tests
The morphology and structure of the as-prepared nanocomposites were analysed by scanning electron microscopy (SEM,JEOL JSM-7500), X-ray diffraction (XRD, Bruker D8), Fouriertransform infrared spectrometer (FT-IR, Bruker Vector 22) and Raman scattering spectra (JY HR 800).
The thermolysis properties of the as-prepared nanocomposites were studied by differential thermal analyser(DTA,TA-Q 600).The samples were tested in Al2O3crucible with about 1.0 mg in mass.The analysis was performed under a pure nitrogen flow(40 mL⋅min-1)at the heating rates of 5,10,15 and 20°C⋅min-1,and the temperature was ranged from 50°C to 400°C. The impact sensitivity tests were conducted according to GJB772A-97.Twentyfive drops for each group and two groups for each sample were tested. The results were shown in terms of special height (H50)standing for the drop height of 50% explosion probability.
3. Results and discussion
3.1. SEM
Fig.1 shows the SEM images of BAMO-THF blank gel(a),raw CL-20 (b) and CL-20/BAMO-THF nanocomposites (c-d), respectively.From Fig. 1, it can be seen that BAMO-THF gel possess porous network structures and plentiful pores are formed.The SEM images of raw CL-20 are shown in Fig.1(b).The particle sizes of raw CL-20 are in micro-grade, presenting prismatic shape. In contrast, the network structures disappear in the CL-20/BAMO-THF nanocomposites and the shape of CL-20 were also changed from prismatic to spherical. Moreover, the average sizes of CL-20 particles are decreased to nano scale, which is mainly caused by the restriction effect of BAMO-THF matrix.
3.2. XRD
Fig. 2 shows the diffraction patterns of raw CL-20 and CL-20/BAMO-THF nanocomposites. The amorphous phase of BAMO-THF blank gel can affect the intensity of the diffraction peaks of the CL-20/BAMO-THF nanocomposites.The diffraction peaks of the CL-20/BAMO-THF nanocomposites become weak and the diffraction angles of CL-20/BAMO-THF nanocomposites are the same compared with those of raw CL-20, which indicate that CL-20 are recrystallized in BAMO-THF blank gel.
3.3. FT-IR
In Fig. 3 (a), the peaks located at 1769 cm-1, 1296 cm-1and 1099 cm-1are ascribed to the shearing vibration of N-H,stretching vibrations of C-N and C-O, indicating the formation of urethane bonds. The peak centred at 2096 cm-1is attributed to the azide bond (-N3) [19]. Fig. 3 (b) shows the FT-IR spectrum of raw CL-20[20]. It can be observed that the peak at 1562 cm-1is assigned to the NO2asymmetric stretching and the peak at 1386 cm-1is related to the NO2symmetric stretching.The peak at 1284 cm-1is ascribed to the NO2symmetric stretching and N-NO2stretching.The peaks 1181 cm-1and 1042 cm-1are associated with in-plane flexural vibration of CL-20 ring. The peaks at 980 cm-1, 934 cm-1and 882 cm-1are related to the bending vibrations of CL-20 ring. The spectra of as-prepared CL-20/BAMO-THF nanocomposites are illustrated in Fig.3(c).The characteristic peaks for both BAMO-THF and CL-20 can be seen in the CL-20/BAMO-THF nanocomposites,such as -N3, N-NO2and ring vibration of CL-20, which indicate that CL-20 particles were merged in the BAMO-THF gel matrix.
3.4. Raman
The Raman spectra are shown in Fig. 4. Different molecular vibrational mode can be observed in CL-20 spectrum. The bands located at 823 cm-1, 834 cm-1and 855 cm-1are assigned to NO2deformation.The bands appeared at 1050 cm-1and 1124 cm-1are ascribed to N-N stretching vibration and asymmetric C-H stretching, respectively. The bands at 1247 cm-1, 1275 cm-1,1308 cm-1,1338 cm-1and 1382 cm-1are related to the symmetric NO2stretching, while, the band centred at 1576 cm-1is attributed to asymmetric NO2stretching. The bands at 1626 cm-1and 1606 cm-1are associated with C-N stretching. In view of CL-20/BAMO-THF nanocomposites, the characteristic peaks of CL-20 are gradually appeared in the composites, which indicated CL-20 was trapped in the BAMO-THF gel matrix.
3.5. DTA
Fig. 5(a-e) show the DTA curves for BAMO-THF blank gel, raw CL-20 and the CL-20/BAMO-THF nanocomposites at the heating rates of 5,10,15,and 20°C⋅min-1,respectively. It can be seen that the low-temperature decomposition (LTD) and high-temperature decomposition (HTD) process are appeared in the CL-20/BAMOTHF nanocomposites. Compared with the decomposition temperature of CL-20, the LTD temperatures for CL-200.20, CL-200.45and CL-200.60are decreased by 58.8°C, 52.7°C and 46.3°C at the heating rate of 20°C⋅min-1, respectively. It is known that the particle size of the energetics can affect thermal decomposition process [21,22]. When the particle size of CL-20 are decreased to nanoscale, the mass transfer and diffusion distance are greatly reduced,which can accelerate the decomposition of CL-20/BAMOTHF nanocomposites.
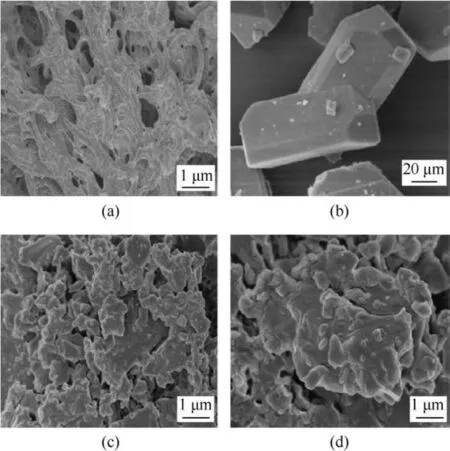
Fig.1. SEM images for(a) BAMO-THF blank gel, (b) raw CL-20, (c) CL-200.20 and (d)CL-200.60.
The thermal decomposition kinetics parameters can be calculated by the Kissinger equation [23].
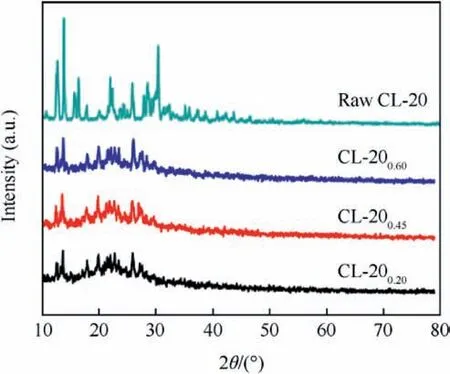
Fig. 2. XRD patterns of raw CL-20 and CL-20/BAMO-THF nanocomposites.

Where β is the heating rate (K⋅min-1); R is the ideal gas constant(8.314 J(mol⋅k)-1); Ea is the activation energy (J⋅mol-1); Tpis the temperature of exothermic peak in DTA curves (K); A is the preexponential factor(s-1);
As illustrated in Fig.6,straight lines are obtained when ln(β/T2p)is plotted against 1/TPand the activation energies can be obtained from the slope of straight line. Both of the LTD and HTD kinetic parameters for CL-20/BAMO-THF nanocomposites are calculated and the calculated results are summarized in Table 1.
The thermodynamic parameters, including the activation enthalpy (△H≠), the activation entropy (△S≠) and the activation Gibbs free energy of (△G≠) at Tp0were calculated on the basis of equs. (2-4) [21,24] and the calculated results are also listed in Table 1.
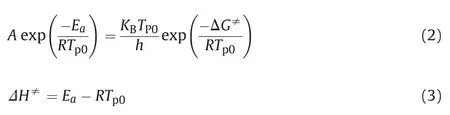
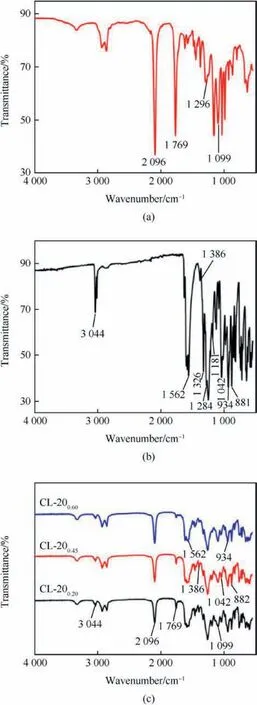
Fig. 3. FT-IR spectra of (a) BAMO-THF, (b) CL-20 and(c) CL-20/BAMO-THF nanocomposites.
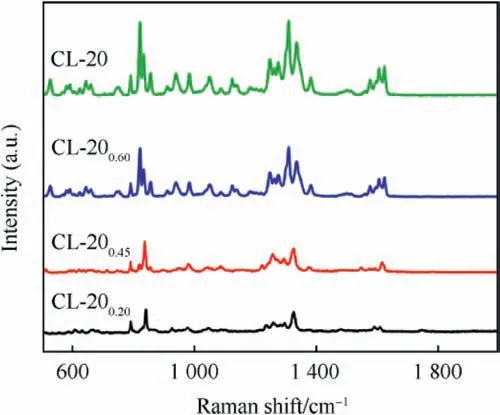
Fig. 4. Raman spectra of CL-20 and CL-20/BAMO-THF nanocomposites.

Where KBand h are the Boltzmann (KB=1.381×10-23J⋅K-1) and Planck constants(h=6.626×10-34J⋅s-1), respectively.
For BAMO-THF blank gel and raw CL-20,the activation energy is estimated to be 192.1 kJ⋅mol-1and 235.3 kJ⋅mol-1, whereas, the LTD activation energy for CL-200.20, CL-200.45and CL-200.60are estimated to be 152.8 kJ⋅mol-1,170.0 kJ⋅mol-1and 189.3 kJ⋅mol-1,respectively.In Comparison to raw CL-20,the activation energy for CL-200.20, CL-200.45and CL-200.60in LTD are decreased by 82.5 kJ⋅mol-1, 65.3 kJ⋅mol-1and 46.0 kJ⋅mol-1. The smaller the activation energy is,the higher the thermal decomposition activity is. Hence, the thermolysis activities of CL-20/BAMO-THF nanocomposites are higher than CL-20.
The △H≠herein are ranked as CL-200.20<CL-200.45<CL-200.60, which means the decomposition process becomes more thermodynamically unfavorable when the contents of CL-20 increase.The positive values of △G≠indicate the thermolysis is not spontaneous process. The positive values of △S≠demonstrate that the transition state presents more activated reaction sites and greater confusion degrees than the reactant molecules. The △S≠values for CL-20/BAMO-THF nanocomposites are positive and arranges in an ascending order of CL-200.20<CL-200.45<CL-200.60,which imply that more decomposition products are formed when increasing the content of CL-20. The calculated results indicate that the energetic nanocomposites are stable and cannot be activated unless sufficient energy (≥△H≠) is absorbed by their molecules [21].
The critical temperature of thermal explosion (Tb) can be calculated based on equ.(5-6)[21,25]and the calculated results are shown in Table 2.

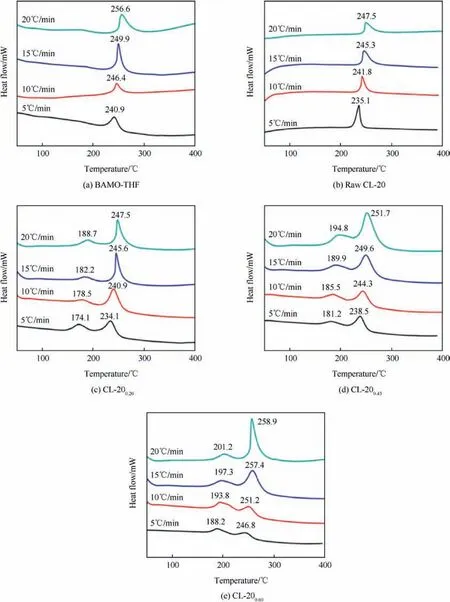
Fig. 5. DTA curves for (a) BAMO-THF, (b) raw CL-20, (c) CL-200.20, (d) CL-200.45 and (e)L-200.60 under various heating rates.
Where a, b and c are coefficients; β is the heating rate; Tp0is the extrapolated peak temperature when β→0;
The thermal stability of CL-20/BAMO-THF nanocomposites varies with the content of CL-20.An increasing trend can be observed when referring to the values of Tb,which indicate the content of CL-20 has a direct influence on the thermal stability of the nanocomposites.
3.6. Impact sensitivity
As shown in Fig. 7, the H50values of the CL-20/BAMO-THF nanocomposites increase when BAMO-THF increase and the values are higher than that of raw CL-20,indicating the addition of BAMO-THF can decrease the impact sensitivity of raw CL-20 effectively. It is commonly considered that the particle sizes of energetic materials have a direct influence on the sensitivity.Based on the hotspot ignition theory,the amount of defects and voids are decreased remarkably in the nano-sized particles, which will reduce the probability of formation of hotspots[21].Moreover,CL-20 particles are trapped in BAMO-THF and the porous gel matrix can consume parts of external stimuli, which will make it too difficult to generate a large hot spot.Hence,the explosive particles are hard to be ignited and the sensitivities are decreased.
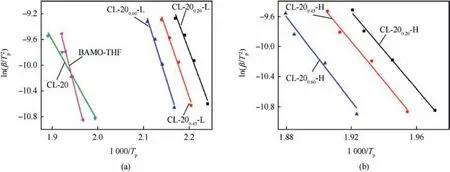
Fig. 6. Dependence of ln(β/T2p) on 1/Tp for BAMO-THF, raw CL-20 and BAMO-THF/CL-20 nanocomposites.

Table 1Summary of kinetic and thermodynamic parameters.
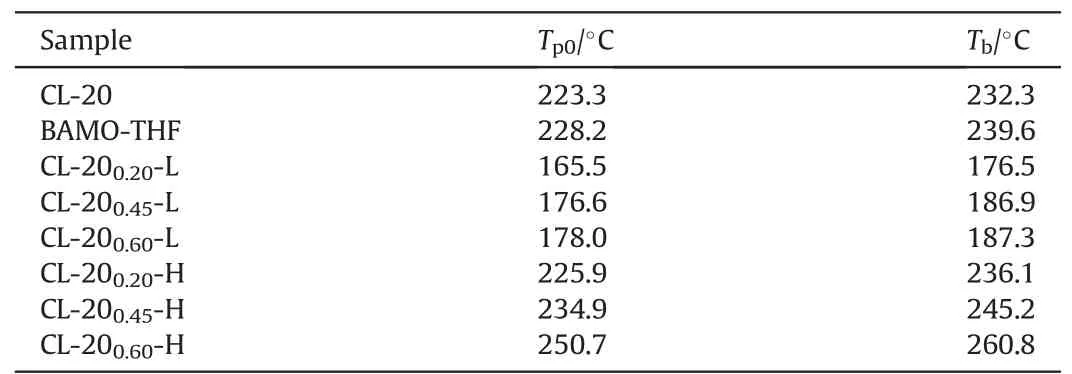
Table 2The calculated Tb for CL-20, BAMO-THF and CL-20/BAMO-THF in LTD and HTD processes.
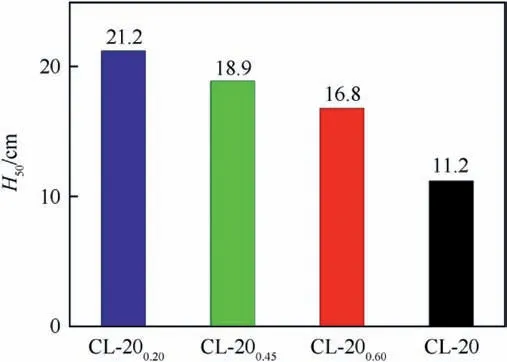
Fig.7. Schematic diagram of characteristic height H50 for CL-20 and CL-20/BAMO-THF nanocomposites.
4. Conclusion
This paper introduces a facile sol-gel method to fabricate CL-20 based energetic nanocomposites with BAMO-THF as gel matrix.The average particle sizes of CL-20 particles in CL-20/BAMO-THF nanocomposites are greatly reduced, implying BAMO-THF gel matrix can restrict the growth the CL-20 crystal in the freezing-drying process. The thermolysis activities of the CL-20/BAMO-THF nanocomposites illustrated in this paper are higher than that of CL-20.Whereas, the impact sensitivities of CL-20/BAMO-THF nanocomposites are notably lower than raw CL-20.These results suggest that the as-prepared CL-20/BAMO-THF nanocomposites possess high thermolysis activity and low sensitivity. The energetic nanocomposites illustrated in this paper can be a very promising ingredient for military use, which may broaden the application of energetic nanocomposite materials.
杂志排行
Defence Technology的其它文章
- Body armour - New materials, new systems Ian G. Crouch*
- Special materials in pyrotechnics VII: Pyrotechnics used in thermal batteries☆
- Real-time calculation of fragment velocity for cylindrical warheads
- Heavy metal free primers: Polymorphism of gadolinium and titanium in the context of GSR glass phase Felice Nunziata
- Mitigation of EDFA transient effects in variable duty cycle pulsed signals
- Ballistic impact properties of woven bamboo- woven E-glassunsaturated polyester hybrid composites
