Study on Flue Gas Desulfurization Process with Selective SO2 Removal by N-formylmorpholine
2019-07-15LongJianWangJinFanChenYangMingleiZhongWeimin
Long Jian; Wang Jin; Fan Chen; Yang Minglei; Zhong Weimin
(1. Key Laboratory of Advanced Control and Optimization for Chemical Processes, Ministry of Education,East China University of Science and Technology, Shanghai 200237;2. SINOPEC Zhenhai Refining and Chemical Co., Ltd., Ningbo 315207)
Abstract: Absorptive separation for resource utilization by selective SO2 removal from flue gas is a potential method applicable in practice. A flue gas desulfurization process for SO2 utilization by selective absorption in a lab-scale absorption tower at atmospheric pressure using N-formylmorpholine (NFM) as the absorbent is developed to capture and concentrate the SO2 from flue gas, in which the CO2 content is several orders higher than that of SO2. The investigation of the effects of different operating conditions on the SO2 removal efficiency shows that the SO2 removal efficiency can be obviously enhanced by increasing NFM concentration, or decreasing the absorption temperature, the superficial gas velocity, the gas-liquid ratio, or the SO2 concentration in absorption solution. Under the optimum operating conditions (covering a temperature of 40 °C, a superficial gas velocity of <0.0165 m/s, a gas-liquid ratio of 200—250, a SO2 concentration in lean NFM solution of 0—10 mg/L,and a NFM concentration of 3 mol/L), the SO2 removal rate reaches over 99.5% while the absorption of CO2 is negligible.Similarly, the SO2 removal rate is as high as 99.5% obtained in consecutive absorption-desorption cycles. Desorption experiment results indicate that the absorption of sulfur dioxide is completely reversible and the release of SO2 from NFM is very easy and rapid at 104 °C. The absorption simulation result for desulfurization of flue gas vented from the industrial catalytic cracking regenerator shows that 98.0% of SO2 can be absorbed in the absorber and most of them are released in the desorber. The experimental and simulated results show that the desulfurization ability and regenerability of NFM solution is encouraging for the development of FGD process to capture the SO2 from flue gas.
Key words: flue gas desulfurization; selective SO2 removal; N-formylmorpholine; absorption; simulation
1 Introduction
The substantial increase in anthropogenic emissions during the last decades has caused dramatic changes in air quality and regional climate[1]. The severe air pollution not only can harm the public health, but also can alter the radiation budget and the hydrological cycle[2]. SO2, a primary source of socalled ‘acid rain', which is mainly vented from fossil fuel combustion at the thermal generating plants, the industrial boilers, the metallurgical roasting operations, the Claus sulfur plants and refineries, is one of the main sources of air pollution[3-4]. Removal of SO2from flue gas emitted during fossil fuel combustion has been a worldwide concern[5].
Various flue gas desulfurization (FGD) technologies such as the dry, semi-dry and wet flue gas desulfurization have been investigated and developed[6-8]. The wet FGD process is effective for a variety of fuels and has been widely commercialized to achieve the desired SO2removal rates[9-10].However, the current technology generates a large amount of wet solid waste, which requires extensive wastewater treatment and involves complicated and costly operations[11].Although SO2is one of the key precursors to acid rain and a major air pollutant, it is also an important and useful resource for manufacture of sulfur fertilizer if it is reasonably captured and transformed[12]. Based on this idea, the absorption process employing organic solvents is one of the most promising routes for flue gas purification. Under mild conditions without producing unwanted side products, it has many advantages on the selectivity in the absorption and the possible regeneration of both the absorption medium and the absorbed species[13]. It is mentioned to use organic solvents,which is highly selective to sulfur dioxide while other gaseous components are not absorbed[14]. However, the currently available technologies are not the best candidates. One reason is the intensive energy consumption during the processing of gas streams. Another reason is the production of byproducts or volatile organic solvents. Therefore, exploring suitable solvents for the capture of acidic gases from the perspective of green chemistry is an extensively concerned topic[15].
N-formylmorpholine (NFM) is used as a physical solvent for years for recovery of high-purity aromatics and more recently for the treatment of sub-quality natural gas[16-17].Recently, there has been some research on the physical properties of NFM[18], and its ability to absorb SO2is measured by a stirred vessel with a flat interface[19]and the property of SO2-NFM complex[20]is studied, which indicates that NFM is a promising medium for flue gas desulfurization. However, the validation of NFM desulfurization performance via continuous flue gas absorption-desorption experiments, optimization of its process operating conditions, and simulation for industrial scale process are almost rarely referred to. Process simulation is an essential tool for the study of chemical processes because of its ability to predict the process performance with adjustments in key parameters.
For the development of a regenerative process aiming at flue gas scrubbing, the laboratory-scale experiments with NFM serving as the absorbent by using artificial flue gas were performed to investigate the in fluence of different operating conditions on the SO2removal efficiency in an absorption tower. A simulation model for a SO2capture process,established by the simulation software Aspen Plus Version 8.4, was developed to predict and further demonstrate the desulfurization efficiency of NFM for treating the flue gas vented from the industrial catalytic cracking regenerator.
2 Experimental
2.1 Solvent properties and chemical reactions
NFM, analytical grade reagent of low toxicity, is miscible with water at any concentration. Carbon dioxide, the Henry constant of which is 6.89—25.9 MPa at 298 K—403 K,has tiny solubility in NFM at low pressure[21]. The existence of chemical interaction between SO2and NFM,with the formation of a 1:1 complex, was demonstrated by absorption tests and melting curve measurements.For concentrated solutions of sulfur dioxide, chemical interactions may exist between sulfur dioxide and a 1:1 complex, which would allow for the formation of a 2:1 SO2-NFM complex in the liquid phase[20]. The liquid-phase reaction is supposed to take place as follows:

Absorption of SO2in NFM results in the fast reversible formation of a SO2-NFM complex. The kinetics of the complex (SO2-NFM) formation and decomposition occurring in the liquid phase after SO2absorption indicate that the liquid-phase reaction is very fast, and possibly instantaneous. It is quite encouraging for the development of flue gas absorption processes.
2.2 Materials and Analysis
The artificial flue gas was generated by mixing 4000 μL/L of SO2and 11.5% of CO2with pure N2to simulate the flue gas after the treatment involving heat recovery, dust removal, gas cooling and mist elimination. NFM was selected and diluted by deionized water into various concentrations of the solvent for the regenerative absorption of sulfur dioxide.
The SO2and CO2content in the gas was analyzed by a flue gas analyzer (Model ecom-J2KN, electrochemical sensor,RBR, Germany). The SO2and CO2content in the aqueous solution samples from the bottom of absorber was analyzed by iodometric titration using an auto-titrator and the volumetric method of acidolysis, respectively. The IR spectra were determined with a Nicolet 6700 infrared spectrometer by the coating method. The surface tension of the liquid was determined with a BZY-1 surface tensiometer.
The SO2removal rate,η(%), can be calculated as shown below:

whereQinandQoutare the flow rate of the flue gas at the inlet and outlet (L/h), respectively;CinandCoutare the SO2volume fraction in the flue gas at the inlet and outlet, respectively. In this study, theQinandQoutcan be considered as the same.
2.3 Experimental procedure
The FGD apparatus is outlined in Figure 1. Simulated flue gas, supplied by a high-pressure cylinder, was fed into the absorber after being sent into a buffer tank at the desired low pressure and steady flow. The simulated flue gas measured by a gas flowmeter reacted in the absorption tower on the NFM solution in a countercurrent arrangement. The flow of the lean NFM to the absorption column was controlled by a flowmeter. A carbon steel packed column (with a length of 600 mm, and an inner diameter of 30 mm), filled with cascade mini rings packing with a height of 480 mm, was used as the absorption tower to absorb SO2. The temperature of the towers was controlled by electrical heating. The temperature at the bottom and the top of regeneration column was maintained at 104 °C, and 40 °C, respectively. The rich NFM, operated at a constant flow rate around 5 mL/min, flowed downward through the structured packing bed against the upwardly flowing steam and desorbed SO2. The NFM solution that was depleted of SO2was pumped from the regeneration tower to the lean NFM storage tank via the lean/rich heat exchanger and water condenser. The SO2released from the rich NFM solution after leaving the top of the regeneration tower along with a certain amount of water vapor flowed into the condenser where the water vapor of the gas was condensed and returned to the tower. The non-condensable,water saturated SO2gas stream was then collected.
2.4 Simulation method
Data for operating conditions used in the simulation were gleaned from previous experimental studies. The NRTL-RK method was used for dealing with the SO2absorption system since it was the most versatile and suitable property method for studying the aqueous system. The required data for SO2-NFM and 2SO2-NFM treated as the pseudo-components were created from experimental data and mass balances. The missing binary interaction parameters were predicted from UNIFAC,a group contribution activity coefficient model. The equilibrium distillation model, RadFrac, was used for the absorber and desorber in the Aspen Plus, because the liquid-phase reaction in the absorption-desorption process was very fast, and possibly instantaneous. The chemical equilibrium reactions and column configurations are given in Table 1. The equilibrium constants for Reactions 1—2 are based on the study result of Kermadec, et al., and the equilibrium constants for Reactions 3—7 are calculated by Aspen Plus from the Gibbs energies, while the kinetic parameters for Reactions 8 and 9 are taken from the ENRTL-RK_Rate_Based_MDEA model in Aspen Plus.

Figure 1 Flow diagram of selectivity FGD pilot plant used in the experiments
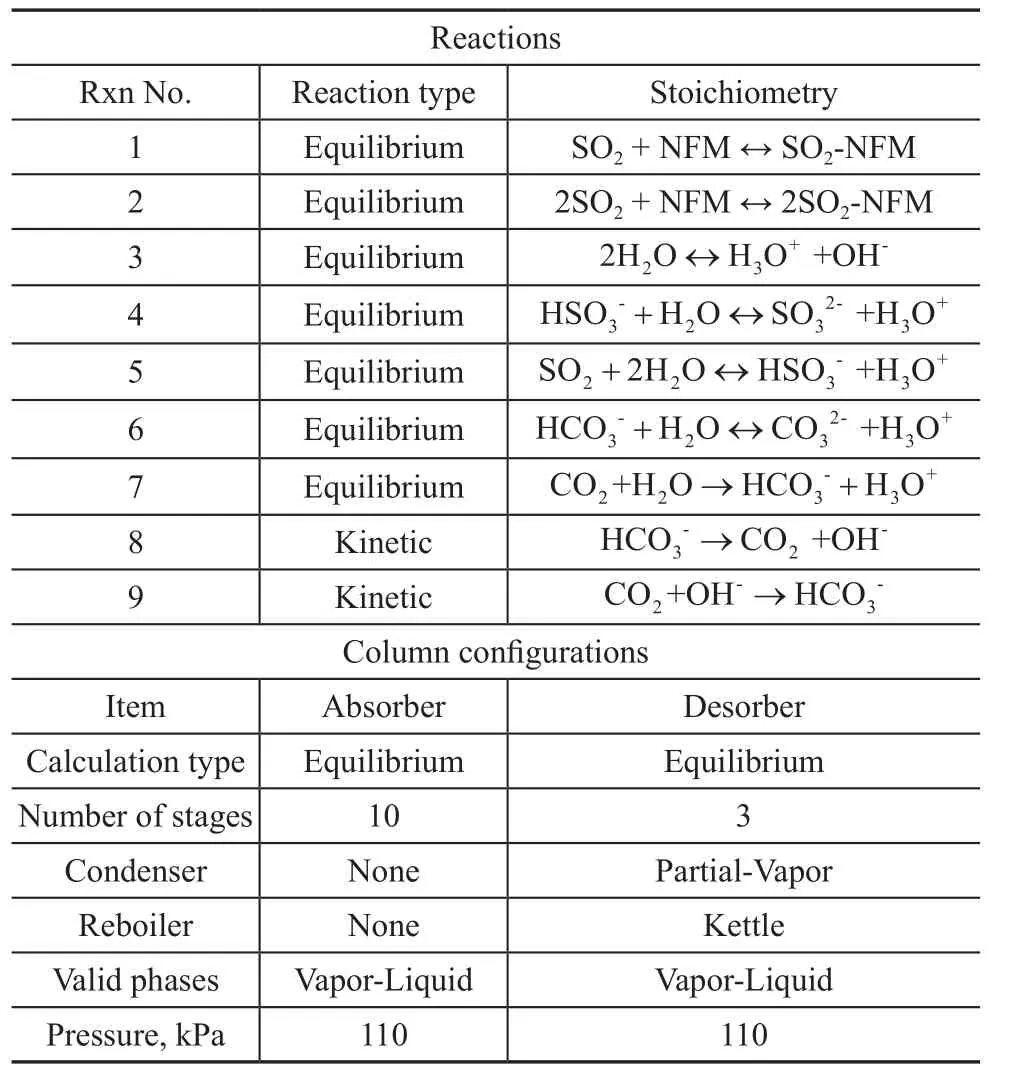
Table 1 List of reactions and column configuration in the Model
The flue gas emitted from industrial catalytic cracking regenerator contains 0.40% of SO2, 0.01% of SO3,0.36% of NO, 0.18% of NO2, 71.70% of N2, 4.07%of O2, 16.64% of CO2, and 6.64% of H2O (in molar fractions). During the desulfurization process,a flue gas stream (FEEDGAS) at a flowrate of 500 m3/h is fed into the bottom of absorber at 40◦C and 120 kPa. The flue gas in the absorption tower reacts on the NFM solution (NFMIN, with a H2O/NFM ratio of 0.67% /0.33%, in molar fractions) which is introduced at a rate of 5 m3/h under conditions covering a temperature of 40◦C and a pressure of 120 kPa in a countercurrent arrangement. The treated gas (SWEETGAS) with a small amount of SO2is released from the top of the column.The rich amine solvent stream (RICHNFM), which leaves the bottom of absorber, is then sent to the heater. The heated rich amine solvent stream (RICHNFM1, 105◦C) is then sent to the desorber, where the ACIDGAS is released at the top of the column.
3 Results and Discussion
3.1 Effect of operating parameters on SO2 removal
3.1.1 Absorption temperature
The SO2removal experiments were carried out to observe the effect of absorption temperature on the desulfurization performance at 30—70 °C, with a simulated flue gas feeding rate of about 0.0183 m/s (superficial velocity)under conditions covering an atmospheric pressure, a NFM concentration of 1.2 mol/L, a SO2concentration in NFM solution of 0 mg/L, and a gas-liquid ratio of 200 (the ratio between the volume flow of flue gas and the volumetric flow of NFM liquid,VG/VL). An obvious in fluence of temperature on the SO2removal efficiency can be clearly found in Figure 2. It is found that the SO2removal efficiency increases obviously as the absorption temperature decreases.In the present result, NFM can clean up SO2quite efficiently.And the SO2removal rate increases from 64.97% to 99.10%,when the temperature reduces from 70°C to 30 °C.
The mass transfer and absorption reaction are carried out between the gas phase and the liquid phase through the gas-liquid interface. There is a stagnant film on both sides of the gas-liquid interface, in which exists the mass transfer and transfer resistance[21]. For absorption system governed by the gas film, the lower temperature, through increasing the gas film side mass transfer coefficient and then enlarging the overall mass transfer coefficient, can improve the mass transfer because sulfur dioxide must spread from the gas phase to the liquid- film, and then the bulk of liquid. SO2solubility in NFM solution increases with a decreasing temperature, which signifies the improvement of gas-liquid mass transfer driving force.Moreover, the dissociation equilibrium of the SO2-NFM complex with an increasing temperature will result in a decreasing SO2removal efficiency.

Figure 2 Effects of absorption temperature on the SO2 removal rate
3.1.2 Superficial gas velocity
The superficial gas velocity, defined as the gas volumetric flow rate divided by the column cross-sectional area, is widely used in the chemical reactor engineering[22]. It is one of the most important parameters in design and operation of various packed bed reactors. In the absorption process, changes in the superficial flue gas velocity can lead to variations in temperature. Consequently,some experiments were also performed to investigate the effect of superficial flue gas velocity ranging from 0.0147 m/s to 0.021 m/s on the SO2removal rate. It is clear from Figure 3 that the desulfurization efficiency declines monotonically with an increasing superficial gas velocity.Selection of superficial gas velocity depends on the situation.In the desulfurization experiments with NFM, it is found that the SO2removal rate remains almost at above 90% when the superficial flue gas velocity is below 0.0165 m/s.
3.1.3 NFM concentration
The effect of NFM concentration ranging from 1.2 mol/L to 3.5 mol/L on the SO2removal rate was investigated. Figure 4 displays the SO2removal rate at various NFM concentrations. The SO2removal rate increases sharply from 90.00% to 99.30% as the NFM concentration increases from 1.2 mol/L to 2.4 mol/L and a further increase shows only a slight rise of SO2removal rate. The SO2removal rate reaches around 99.83% at a NFM concentration of 3.6 mol/L. The increase in NFM concentration leads to an increasing number of useful molecules in the absorbent solution along with the improvement of mass transfer driving force, which has contributed to the increase in SO2removal rate. An excessive high solvent concentration will lead to lower SO2removal efficiency, mainly because the viscosity of the solution increases and therefore the mass transfer resistance would rise. However, the experimental results show that the SO2removal rate still reaches above 99% when the NFM concentration is 3.6 mol/L. This can occur, because the viscosity of NFM is 6.2 mm2/s at 25oC and it can be miscible with water at any ratio as mentioned before, which means that the viscosity of absorbent solution is affected slightly by the increase in the NFM concentration.
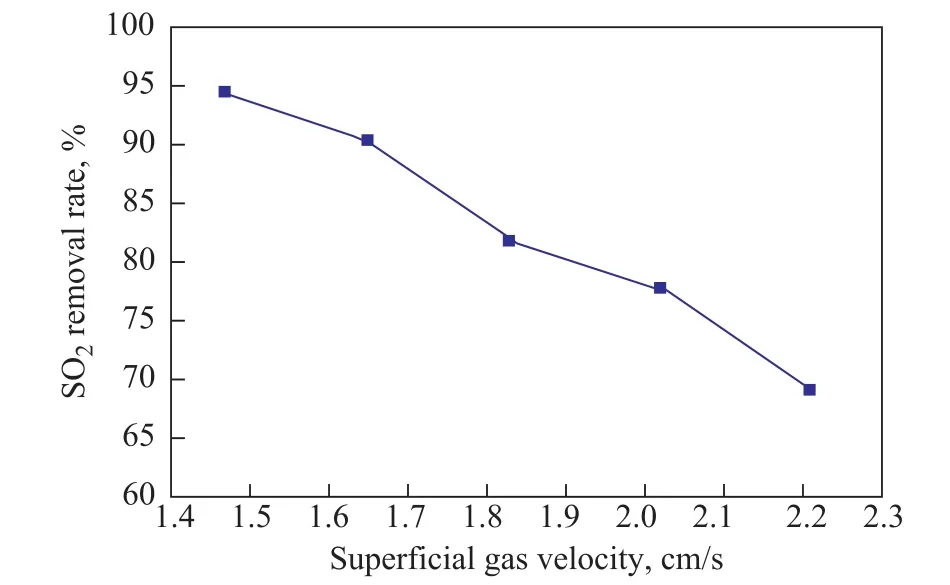
Figure 3 Effects of superficial gas velocity on the SO2 removal rate.
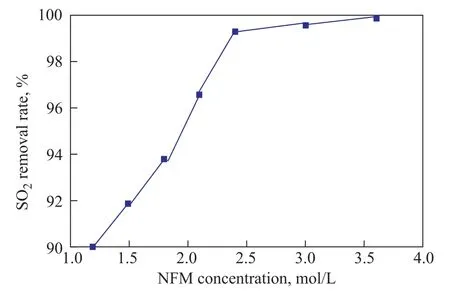
Figure 4 Effects of NFM concentration on the SO2 removal rate
3.1.4 Gas-liquid ratio
The gas-liquid ratio (VG/VL), has been found to be one of the most important criterions for reporting the absorber performance[23]. Different gas-liquid volume ratios, obtained by changing the volumetric flow of NFM liquid, were employed to examine their effects on the desulfurization efficiency under conditions covering a superficial gas velocity of 0.0 165 m/s,an atmospheric pressure, a NFM concentration of 3 mol/L, and a SO2concentration in NFM solution of 0 mg/L. Experimental results are shown in Figure 5. It verifies thatVG/VLis a main parameter for FGD system.The SO2removal rate can be maintained at around 99.5%as theVG/VLratio increases from 150 to 257, and it would decrease to 88.6% at aVG/VLratio of 450.
With an increase in the value ofVG/VLratio measured in the absorber, the gas-liquid interface area for the absorption of SO2decreases when the liquid flow rate is fixed. Consequently, the SO2absorption rate decreases,and the SO2removal efficiency is weakened. However,when theVG/VLratio is too small, the cohesion of droplets will be enhanced, and the effective gas-liquid interface area no longer increases but even decreases, resulting in a smaller mass-transfer coefficient[24]. At this moment, a further decrease in theVG/VLratio becomes meaningless,and the increase in average absorption rate and SO2removal efficiency would slow down. In present study,the SO2concentration in clean gas obtained in aVG/VLratio range of 200—250 is less than 90 mg/m3, which is low enough from the point of meeting the Cleaner production standard for the petroleum refinery industry(HJ/T 125—2003, China). And hence, this range can be considered as optimal.
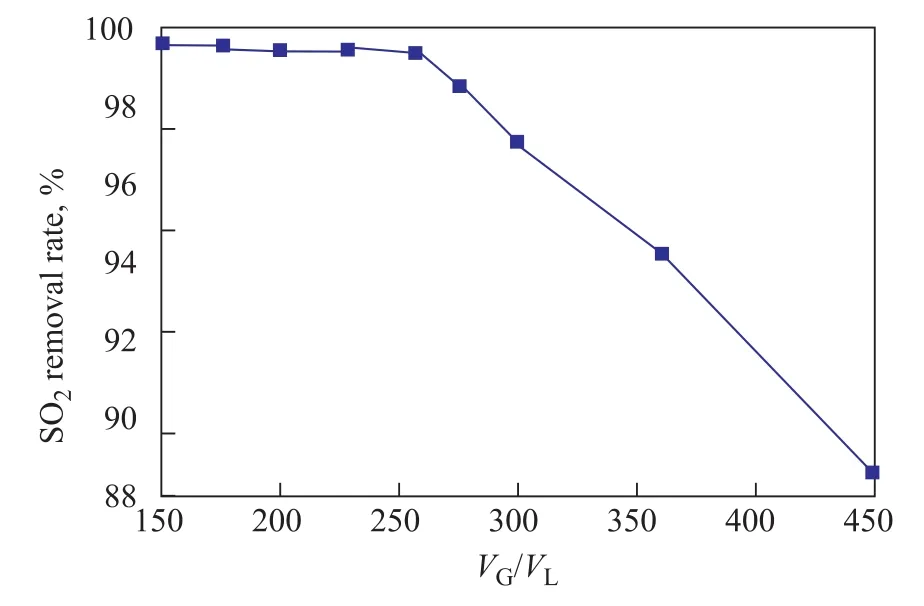
Figure 5 Effects of VG/VL on the SO2 removal rate
3.1.5 SO2 concentration in NFM solution
Generally, the SO2removal efficiency of the regenerative solvent, which is reused in the absorption process,decreases as the SO2content in the regenerative solvent increases. A series of desulfurization experiments were carried out to examine the effect of SO2concentration in the NFM solution diluted to 10—200 mg/L by fresh NFM on the SO2removal efficiency. Figure 6 gives the results of SO2removal rate at various SO2concentrations in the NFM solution.
As the increasing SO2concentration in NFM solution will require larger amount of NFM for absorption, however the liquid flow rate is constant, thus the SO2removal efficiency is predicted to decrease continuously and it is indeed true as shown in Figure 6. On the plus side, a SO2removal rate reaching as high as 94% has been achieved at a SO2concentration of <200 mg/L, which is lower than 98.5% at a SO2concentration of <50 mg/L.
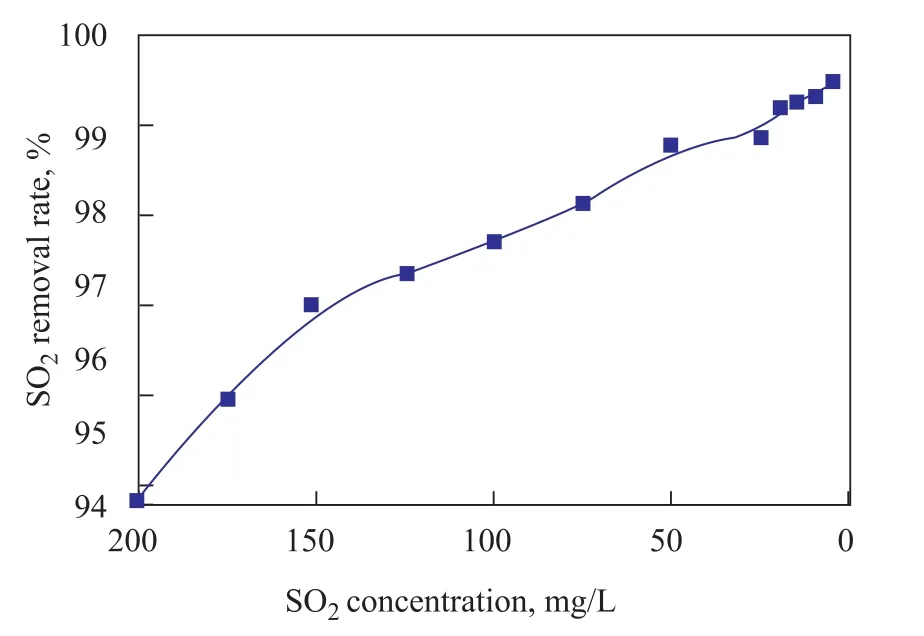
Figure 6 Effects of SO2 concentration in NFM solution on the removal rate for SO2
3.2 Selective SO2 removal by NFM
The solubility of CO2in NFM decreases from 4.22×10-3kmol/kg to 9.43×10-5kmol/kg at 40 °C, when the total pressure of the acid gas reduces from 553.0 kPa to 451.02 kPa[21], which means that a lower solubility can be obtained at a lower atmospheric pressure. Hence, the experiment of CO2absorption by pure NFM was carried out. Some samples of CO2-NFM were obtained at 40 °C using pure CO2with a volumetric flow rate of 100 mL/min which was introduced into the flask filled with 40 mL of pure NFM for 2 h. The IR spectra of the CO2-NFM and the pure NFM are shown in Figure 7. Upon comparing the CO2-NFM spectrum with that of the pure NFM, it is observed that they are basically the same. This indicates that the CO2solubility in NFM is negligible and CO2in flue gas cannot be absorbed by NFM solution during the desulfurization process under atmospheric pressure.The results mentioned above show that NFM has good performance for the selective absorption of SO2from flue gas, from which highly purified SO2stream can be obtained through solvent regeneration.
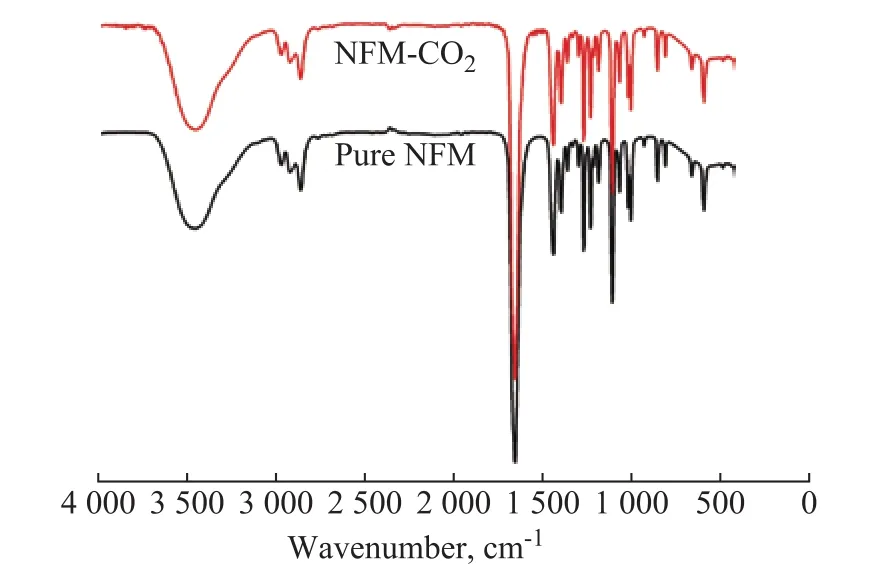
Figure 7 IR spectra of pure NFM and CO2-NFM
3.3 Desorption performance of NFM
The SO2-NFM solution (containing 3 534 mg/L of SO2), formed from 40 mL of pure NFM saturated by the simulated flue gas (containing 4 000 μL/L of SO2) with a volumetric flow rate of 100 mL/min at 40 °C for 2 h,was regenerated at 104 °C for 1.5 h. The SO2content in the lean NFM was too low to be detected by iodometric titration using an auto-titrator. The results for the IR spectra of lean NFM and pure NFM are given in Figure 8.Upon comparing their spectra, it is obvious that there are no distinct changes in chemical shifts and no new peak in the spectrum of the regenerative NFM, which means that the chemical stability of NFM can be maintained.Moreover, in order to use NFM in potential applications,the physical properties such as surface tension (40 °C)and kinematic viscosity (20 °C) of the fresh and lean NFM are measured with the results given in Figure 9.Among the thermodynamic properties, the surface tension is an important and useful indicator related with the heat transfer, mass transfer, and flow property. Surface tension is defined as the difference between the energy in the bulk and the surface per unit area of interface. This parameter is a basic physical property that has a key role in the development, design and simulation of gas absorption process[25]. It is well-known that the tension at the surface of a liquid is one of the most important demonstration of the intermolecular forces composed of both repulsion and attraction terms. The results indicate that the surface tension of lean NFM is equal to 30.7 mN/m, which is lower than that of pure NFM (44.5 mN/m). It is clear that the surface tension of NFM after regeneration is reduced.Moreover, the kinematic viscosity of the regenerated NFM is similar to that of pure NFM. These results indicate strongly that NFM has excellent thermal stability and the potential for recycled use in the absorption process.
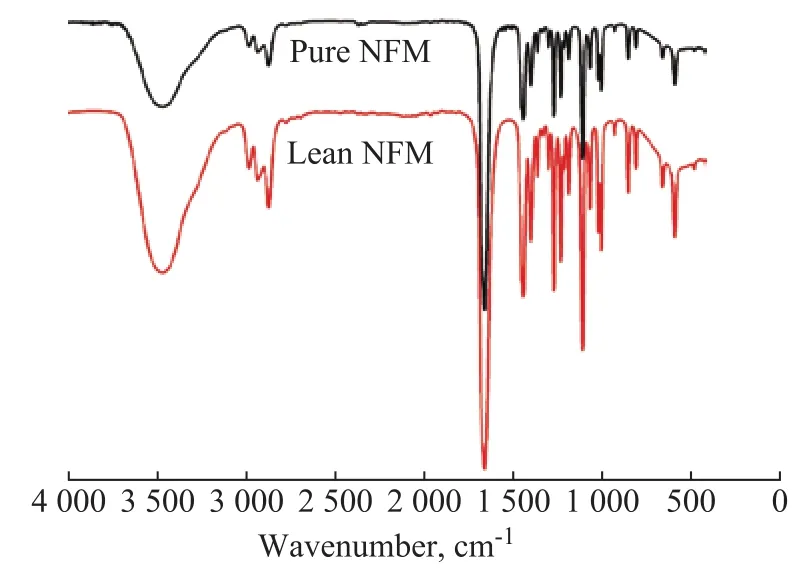
Figure 8 IR spectra of pure NFM and lean NFM
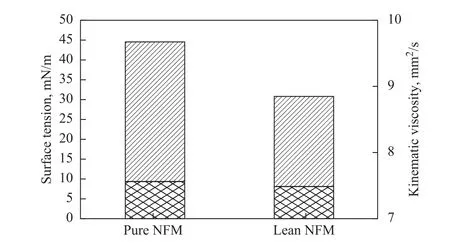
Figure 9 Physical properties of pure and lean NFM
In addition, 80 mL of liquid samples of rich NFM and rich MDEA (with the concentration of both reagents equating to 3 mol/L each), with similar SO2concentration of 577.3 mg/L and 602.18 mg/L, respectively, were collected and analyzed to verify the desorption ability of aqueous NFM when it was being exposed to the operating condition of 104 °C for 1 h in a SO2desorption environment. The comparison result (Figure 10) shows that 99.1% of SO2initially absorbed in NFM is released,while the desorption efficiency of SO2-MEDA system is only 7.2%. SO2can be released from NFM easily and very rapidly. A trace amount of SO2that has remained in the NFM solution indicates that it is possible to regenerate NFM with ease from SO2-NFM in the regeneration unit and recycle in the absorption process.
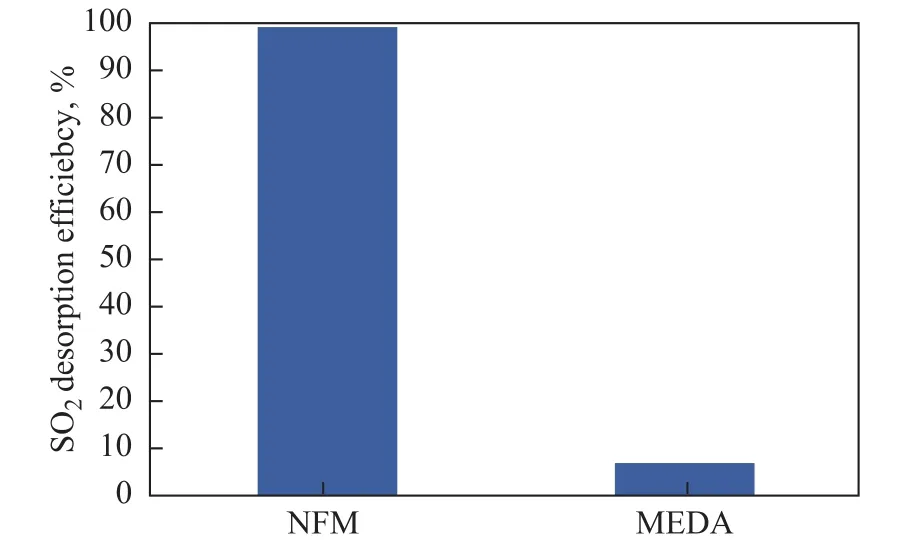
Figure 10 SO2 desorption efficiency of rich NFM and MDEA solution
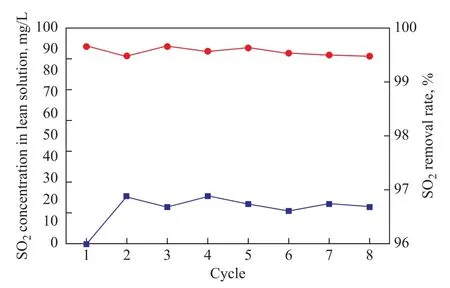
Figure 11 Recycle utilization of NFM solvent and SO2 removal rate
Furthermore, the desulfurization efficiency of NFM solution in consecutive cycles was investigated in the packed absorber. The rich NFM solution was purged in the regenerating column with a flowrate of 100 mL/min at 104 °C, and then was cooled down to ambient temperature before the next absorption experiment was started. The
SO2removal rate obtained in consecutive cycles is shown in Figure 11. The initial SO2concentration in the NFM solution is 0, and then remains at around 4—6 mg/L after regeneration. It is found that the SO2removal rate is as high as 99.5% after 8 cycles and shows no remarkably descending trend. It seems that the NFM is fairly stable for the SO2selective absorption. There are several ways to deal with the removed SO2in industry. Usually it is converted to a useful chemical or fertilizer, for example,by washing the desorbed gas with ammonia solution.It is also possible to convert the desorbed gas as a raw material in sulfuric acid production if this gas is washed by a solution of hydrogen peroxide.
3.4 Simulation
To bypass expensive and time-consuming testing or experiments, an absorption simulation model was developed to predict and further demonstrate the SO2desulfurization efficiency of NFM for treating the flue gas vented from the industrial catalytic cracking regenerator.The flow diagram of the simulation model is given in Figure 12. The simulated results (Table 3) show that the molar flowrate of SO2in sweet gas (0.01 kmol/h)is lower than that of feed gas (0.093 kmol/h) and acid gas (0.075 kmol/h), which means that 89.2% of SO2are absorbed in the absorber and most of them are released in the desorber. The simulation results show that the SO2desulfurization ability and regenerability of NFM solution are in good agreement with the experimental data.

Table 3 Simulation results for flue gas desulfurization
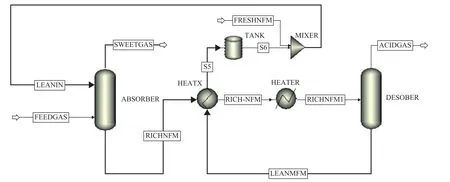
Figure 12 Simulation process flow diagram of flue gas emitted from industrial catalytic cracking regenerator
4 Conclusions
Absorption experiments of the artificial flue gas were carried out to investigate the solvent absorption capacity and selectivity for sulfur dioxide removal under various operating conditions. It is found that the SO2removal efficiency can be obviously enhanced by increasing the NFM concentration, or decreasing the absorption temperature, the superficial gas velocity, the gas-liquid ratio, or the SO2concentration in the absorption solution.The presence of CO2impurities at high concentrations in the simulated flue gas stream does not significantly in fluence the SO2selective absorption capacity of NFM.The amount of SO2in flue gas can be selectively absorbed in NFM solution (3 mol/L) with the SO2removal rate reaching above 99.5% under mild and cost-effective operating conditions covering a superficial gas velocity of below 0.0165 m/s, aVG/VLratio of 200—250, a SO2concentration in NFM solution of 0—10 mg/L at 40 °C,while the massive amount of CO2in flue gas is not absorbed during the desulfurization process at all, as evidenced by the comparison on the IR spectra of CO2-NFM and pure NFM.
In addition, the issues associated with the solvent stability and regenerability of the NFM solution after repeatedly recycled regenerations via the scrubbing process have been investigated. Based on the conclusions on the SO2desorption efficiency and IR spectra of pure NFM and lean NFM, the rich NFM can be easily and rapidly regenerated as compared to the rich MEDA when being heated to 104 °C. Apart from the advantages described above, the SO2absorption capacity (with the SO2removal rate reaching above 99%) was almost retained in consecutive regeneration cycles when NFM solution was exposed to simulated flue gas stream, suggesting that the NFM solution was reasonably stable. The desulfurization simulation results for treating the flue gas emitted from the industrial catalytic cracking regenerator indicate that 89.2% SO2in flue gas can be absorbed in the simulation process. The high absorption fluxes and the easier regeneration of rich NFM achieved in the absorption process under mild operating conditions are quite encouraging for the development of FGD process for capturing the SO2from flue gas.
Acknowledgment:This work was supported by National Natural Science Foundation of China (Major Program:61590923), International (Regional) Cooperation and Exchange Project(No. 61720106008), National Natural Science Foundation of China (No. 61873093), National Science Fund for Distinguished Young Scholars (61725301) and the Fundamental Research Funds for the Central Universities.
杂志排行
中国炼油与石油化工的其它文章
- Optimization of Dividing Wall Column with Heat Transfer Process Across the Wall for Feed Properties Variation
- Numerical Simulation of Optimization of Mixing Tank for Residue Upgrading Reactor
- Biodegradability and Tribological Properties of Mineral Base Oil Enhanced by Caprylic Methyl Diethanolamine Phosphate Ester
- Enhanced Anti-Wear Property of Low Viscosity Engine Oil for Sequence IVB Engine Test Meeting the GF-6 Specification
- Research and Application of Image Recognition Technology in Microscopy Diagnosis of Catalytic Cracking Catalysts
- Evaluation of Inocula and Packing Material for Acceleration of Start-Up in Biofilters
