Evaluation of Inocula and Packing Material for Acceleration of Start-Up in Biofilters
2019-07-15LiChaoZhaoDongfengMuGuiqinSongXiangningZhangTingtingLiuJun
Li Chao; Zhao Dongfeng; Mu Guiqin; Song Xiangning; Zhang Tingting; Liu Jun
(1. SINOPEC Research Institute of Safety Engineering, Qingdao 266000;2. Department of Environmental and Safety Engineering, China University of Petroleum (East China), Qingdao 266580;3. Qingdao Oasis Environmental & Safety Technology Co., Ltd., Qingdao 266555; 4. Center for Safety,Environmental & Energy Conservation Technology, China University of Petroleum (East China), Qingdao 266555; 5. Shandong Provincial Key Laboratory of Water Pollution Control and Resource Reuse, School of Environmental Science and Engineering, Shandong University, Qingdao 266237)
Abstract:Several strategies with different combination of inocula and packing material were investigated to obtain the optimal start-up time and elimination capacity (EC) in toluene biofiltration. The inocula contained the activated sludge and toluene degrading bacteria, and the packing material consisted of different mixing ratios of peat and wood chips. A final toluene load of 21.2 g/(m3·h) was attained step by step in four parallel biofilters. A shortest start-up time of 15 days and a highest EC of 17.0 g/(m3·h) were observed in the biofilter B-4, which was inoculated with a special microbial consortium consisting of three strains of toluene degrading bacteria and was packed with the mixture of peat and wood chips at a ratio of 80:20 (w/w).These results indicated that inoculating pre-acclimatized microbes dramatically shortened the start-up time, and such a composition of packing material could maintain an appropriate environment (with the bed porosity and water content equating to 0.45 and 1.96, respectively) for the growth of dominant toluene degrading bacteria in the biofilter.
Key words: acceleration of start-up; biofilter; inocula; packing material
1 Introduction
Volatile organic compounds (VOCs) emitted from petroleum and chemical industries pose some problems to human health and air pollution. Currently, biofiltration technology has been widely used for low concentration and high flow of VOCs compared to other physicochemical methods due to its low energy consumption and less waste production[1-3]. A considerable amount of research has been conducted to interpret its biodegradation mechanism and to strengthen its pollutant removal efficiency for speeding up the industrial application[4-5].Most researches were focused on the optimization of operation parameters, such as pollutant load, retention time, water content, temperature, pH value, pressure drop,nutrients, etc.[6-8]Furthermore, numerous mathematical models have been proposed to assess the performance of biofilter[9-10].
Moreover, successful start-up is the basis of biofilter and nowadays needs more attention[11]. The completion time is an important indicator to evaluate the start-up of biofilter,which is affected by many factors, such as pollutants,bioreactors configuration and inocula, etc. Gallastegui, et al. reported that it took 8—10 days to complete the startup of a biofilter, which was packed with a commercial soil amendment (ABONLIR) for biodegradation of ethylbenzene and toluene, after inoculating the activated sludge in a biofilter[12]. Similar observations were also reported by Volckaert, et al. during the ethylbenzene biofiltration[13]. On the studies on BTEX (benzene,toluene, ethylbenzene, ando-xylene) degradation,Mohammad, et al. reported microbial acclimation was finished in one month in a biofilter, while Lu, et al.spent over 2 weeks to attain a steady-state condition in a biotrickling filter[14-15]. The fungal biofilters usually take longer to finish the start-up as compared to the bacteria biofilters. For instance, Spigno and De Faveri obtained continuous elimination ofn-hexane after 50-day operation withAspergillus niger[16].Moreover, Sempere, et al.reported a start-up period of around 3 months for VOCs removal in an industrial biofilter, which was far longer than those achieved in laboratory-scale bioreactor[17].
Actually, an inefficient elimination is often observed in the start-up of biofilter. However, there were few studies concerning the acceleration of biofilter start-up so far.Vergara-Fernández, et al. successfully reduced the startup time of fungal biofilters by using appropriate carbon sources[18]. Elías, et al. recommended that the preliminary acclimation strategies should consist of easily degradable carbon source addition and discontinuous acclimation mode[19]. In addition, another effective approach is to inoculate the biofilter with the biomass adapted to the pollutants, which could be purchased from the specialist corporations, or extracted from other active biofilters, or isolated from activated sludge of wastewater treatment plants and other similar sources[20-22]. Through the method mentioned above, Morales, et al. observed a shorter acclimation period of about 10 days in a peat biofilter than those requiring about 2—3 weeks in previous studies for the toluene elimination[23].
In this study, the start-up of four biofilters was conducted by gradually increasing the toluene load in period A, B,and C. The aim of this study was to evaluate the start-up of biofilters with different packing materials and inocula in terms of toluene elimination capacity and start-up time. The microbial population distribution along the bed length was also monitored.
2 Materials and Methods
2.1 Inocula and packing material
Activated sludge collected from a wastewater treatment plant located at Qingdao Oil Refinery Co. (China) was used as one of the inoculum sources after pretreatment with washing and aeration. Other inoculum sources included several strains of bacteria isolated from activated sludge utilizing toluene as carbon source,includingBacillus megateriumJB-1,Micrococcus lutensJB-3, andPseudomonas putidaJB-4. During the start-up, 4 L of the suspension of inoculum sources were placed in a series of biofilters and diluted with 6 L of nutrient solution. The mineral medium contained(g/L): KH2PO4(0.61), NaH2PO4(0.6), FeCl3(0.01),MgSO4(0.05), CaCl2(0.002), and NH4NO3(2.0). The pH value of the medium was adjusted to 7.0±0.2 with a dilute HCl solution (0.1 N). A small quantity of mineral medium (1 L) was added at the end of period A, B,and C.
A mixture of wood chips and peat was used as the biofilter media. The media characteristics are shown in Table 1. Wood chips, as the support of biofilter, were used to maintain more homogeneous gas distribution across the packed bed and to minimize the pressure drop[24]. Thus,the performance of wood chips mixed with different peat addition was compared. The packing material used in this experiment needed disinfection in order to eliminate the in fluence of indigenous microorganisms.

Table 1 Main physico-chemical properties of peat and wood chips employed in the experiments
2.2 Biofilters set-up and operating conditions
Figure 1 presents the experimental biofilter set-up, which is comprised of a gas regulation system (for controlling the inlet toluene concentration, gas flow rate, humidity,and temperature) and a tubular reactor made of plexiglass(in which the packed bed is divided into three equal sections with a total volume of 9.4 L). As for the cylinder,each section is 10 cm in height with an internal diameter of 20 cm, and a 25 cm height of head space is designed for obtaining a uniform inlet gas flow and installing spray nozzles to distribute the nutrient solution or water, while a 21 cm height of bottom space is designed for the out flow of purified air and leachates.
Four biofilters can be operated synchronously, and detailed configuration scheme of each bioreactor is given in Table 2. These biofilters were incubated at (28±3) °C.The temperature of inlet airstream was maintained at(30±2) °C and the relative humidity (RH) was higher than 99.9%. The dilute toluene gas was introduced at the top of the biofilter at a constant flow rate of 1.8 L/min (empty bed retention time(EBRT) of 2.9 min) corresponding to a superficial velocity of 1.7 m/h. The inlet concentration of toluene experienced three acclimation period A(0.2 g/m3), period B (0.5 g/m3), and period C (1.0 g/m3),corresponding to an inlet load of 4.2 g/(m3·h),10.6 g/(m3·h), and 21.2 g/(m3·h), respectively.
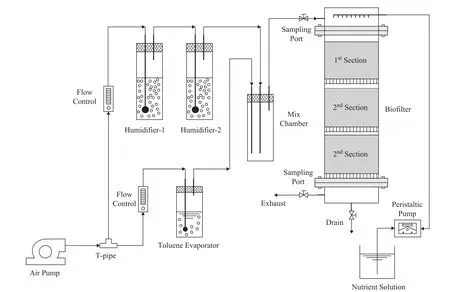
Figure 1 Schematic diagram of experimental biofilter
2.3 Analytical methods
Toluene concentrations were measured using a gas chromatograph SP-342A (BeijingBeifen-RuiliAnalyticalInstrument(Group) Co. Ltd.) equipped with a FID detector.The capillary column was 60 m×0.25 m×0.5 μm and N2(≥99.999%) was used as carrier gas. The temperature of the oven, injector, and detector was maintained at 120 °C, 150 °C, and 200 °C, respectively. Effluent air samples with a volume of 0.1 L were collected using an activated carbon adsorption tube. After extraction with carbon disulfide, these samples were aspirated by a 10 μL gas-tight syringe and 1 μL was injected into the GC. The toluene inlet load (IL, g/(m3·h)), elimination capacity (EC,g/(m3·h)), and removal efficiency (RE, %) of biofilters can be calculated by the following equations:

in which,CinandCoutare inlet and outlet toluene concentration, respectively (g/m3),Qis air flow (m3/h),andVbis the packed bed volume (m3).
The pressure drops across the packed bed was monitored daily with an inclined tube manometer (YYT-2000B, Yiou, precision±2 Pa). The water content (W,dimensionless) was determined with an electronic balance (ACS-6, Helisite, precision±0.2 g), which can be calculated by the following relation:

where,WiwandWrware initial and regular weight of each sectional packed bed, respectively (g),Wdis the weight of dry packing material in each sectional packed bed (g).
2 to 3 grams of the packing material were taken from each section of the biofilters and were mixed with 100 ml of sterile saline solution (0.9% NaCl). The plating method was used to enumerate the total and toluene degrading microbial population according to the standard methods[25]. For toluene degrader enumerations, plates were incubated in a toluene atmosphere. The counts were reported as the colony forming unit (CFU)/g of packing material (wet basis). Besides, the morphology of microbial population was also monitored by a scanning electron microscope (SEM, KYKY-2800B).
3 Results and Discussion
3.1 Toluene removal and start-up time
Biofilter start-up and adaptation to toluene were conducted with different inocula and packing material.
Figure 2 shows theIL,EC, andREin four biofilters tested in this study. In the control biofilter B-1, the raw activated sludge was inoculated in wood chips (Figure 2a). During the acclimation period A, a negligibleECbetween days 0 and 7 showed an initial growth of toluene degraders and the others were eliminated. Subsequently, a gradual increase ofECbetween day 8 and day 15 reflected the rapid reproduction of toluene degraders. Finally, the steady state was attained between day 16 and day 20, with an averageECof 2.2 g/(m3·h) (48.1%RE), which implied that the toluene degraders had dominated the microbial community of packed bed. During the adaptation period B, an increase ofILled to the further growth of toluene degraders and the increase ofEC, and it took 9 days to attain the steady state with an averageECof 6.6 g/(m3·h) (61.2%RE). The same phenomenon was observed in period C, and the final stableEC(12.4 g/(m3·h)) andRE(55.0%) reached between day 45 and day 49. After a total operating time of 50 days(Table 3), 585.8 g/m3, 1 691.0 g/m3, and 3 721.4 g/m3of toluene were consumed; corresponding to 23.76%,47.07%, and 45.81% of degradation in periods A, B, and C, respectively.
In biofilter B-2, the raw activated sludge was inoculated in mixture of peat and wood chips at a ratio of 50:50 (w/w), with the results shown in Figure 2b. During the period A, it was found that the steady toluene uptake, with a higher averageEC(3.0 g/(m3·h)) andRE(67.9%), was attained in a shorter time about 12 days. During the period B, it took more time around 11 days to reach the steady state, similar to that state in the biofilter B-1 with same values of averageECandRE. During the period C, the toluene degradation decreased to a lower level of averageEC(10.4 g/(m3·h)) andRE(48.1%) as compared to the values in biofilter B-1 even though the steady state was reached quickly in 5 days. After 49 days of operation, the toluene degradation of each period was 42.26%, 47.15%,and 42.85%, respectively (Table 3).
Compared with biofilter B-1, biofilter B-2 showed a shorter start-up time with a mixture of peat and wood chip (at a ratio of 50:50 w/w). However, such a mixing ratio led to low water content of packed bed which caused microbial death andECreduction. Thus the inocula and mixing ratio of packing material were further optimized in the next operation of biofilters.
In biofilter B-3, three strains of toluene degrading bacteria were inoculated in the wood chips. As shown in Figure 2c,a small amount of toluene was fed in initial 14 days(period A) and nearly 44.15% of toluene was removed.AfterILincreased successively on the 15thday and the 28thday, the toluene consumption in period B was 1 722.2 g/m3corresponding to 52.04% of degradation, and in period C, 56.21% of toluene (around 3 309.4 g/m3) was consumed. Compared to B-1 and B-2, a more complete elimination of toluene was obtained in biofilter B-3 in the total process and each period. The steady toluene uptake was reached after 9 days in period A, 7 days in period B,and 5 days in period C, respectively (Table. 3).
In biofilter B-4, three strains of toluene degrading bacteria as B-3 were inoculated in a mixture of peat and wood chips at a ratio of 80:20 (w/w). As shown in Figure 2d, the biofilter B-4 presented a shortest startup time in all experiments, which was around 5 days in each period and 15 days in total. Moreover, the biofilter B-4 also showed a highestECandREin steady state,with the details presented below: period A: 4.0 g/(m3·h)and 81.0%; period B: 9.2 g/(m3·h) and 79.6%; period C:17.0 g/(m3·h) and 75.6%. After 30 days of operation,the total toluene consumption was approximately 6 259.6 g/m3, corresponding to 68.8% of degradation.
Compared to biofilter B-1 and B-2, biofilters B-3 and B-4, which were inoculated with toluene degrading bacteria, showed more efficient toluene degradation(>50%) and saved about half of the adaptation time in the start-up period. In addition, the comparison of results between biofilters B-3 and B-4 indicated that a high mixing ratio of peat to wood chips (80:20,w/w) reduced the start-up time from 22 days to 15 days and increased the toluene degradation by 15.6%. This improvement could be attributed to the larger surface area of granular peat, which was beneficial to adhesion and growth of microorganisms on the packing material.
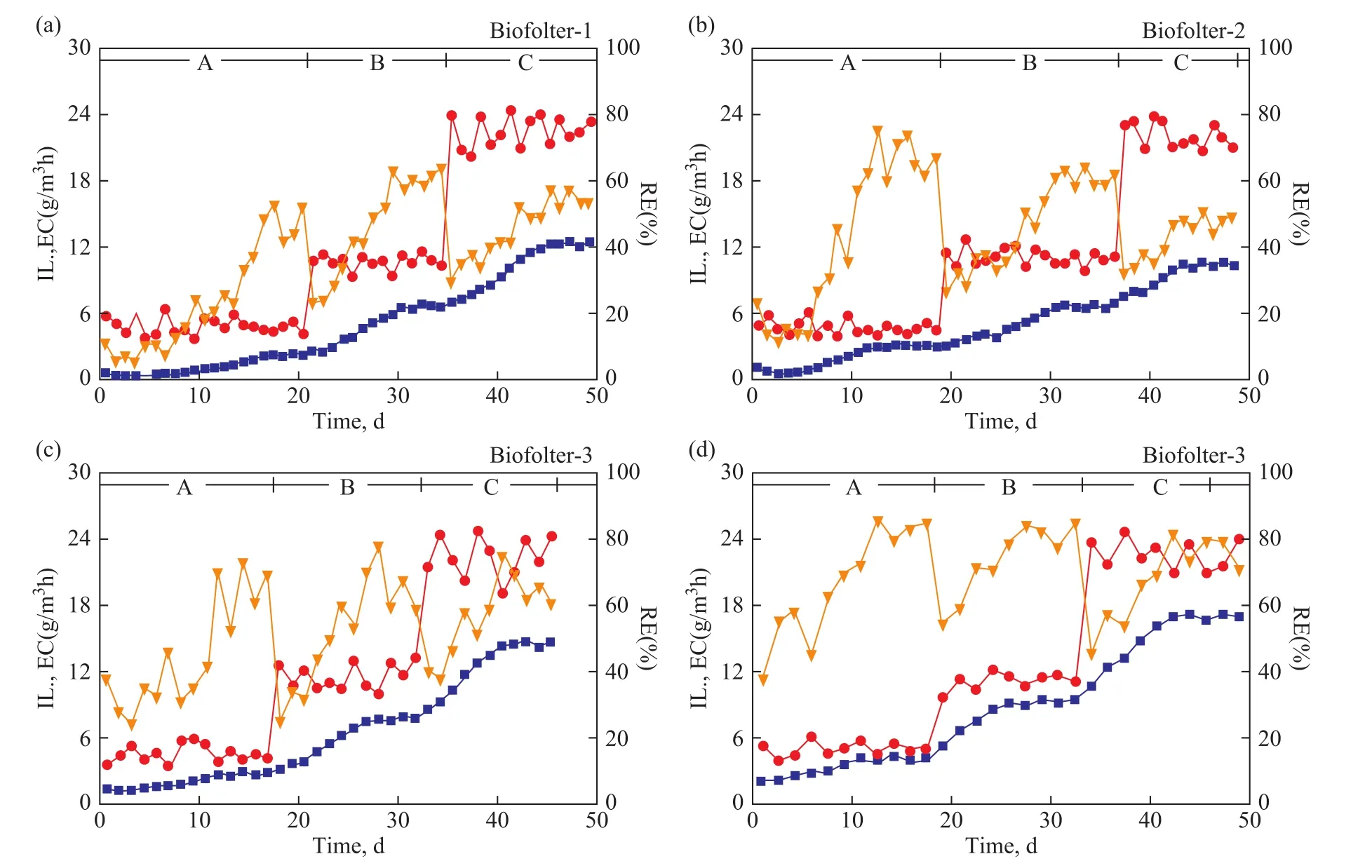
Figure 2 Evolution of the IL, EC and RE of toluene over time in four biofilters B-1, B-2, B-3 and B-4
3.2 Bed pressure drops
The variations in pressure drop for each biofilter as a function of time are shown in Figure 3. The biofilters B-1, B-2, and B-3 exhibited steady pressure drop up to 26.2 Pa/m, whereas an increase of pressure dropfinally reached 209.2 Pa/m in biofilter B-4. The increase of pressure drop could be attributed to the growth of microbes on the fillers, which might lower the bed voidage in biofilter B-4 (0.45). While the biofilters with high bed voidage (B-1: 0.67; B-2: 0.64;B-3: 0.69) would maintain the low pressure drop. Thus, a particular attention must be paid to the bed voidage and its proper value should be in the range of 0.4—0.8[26].
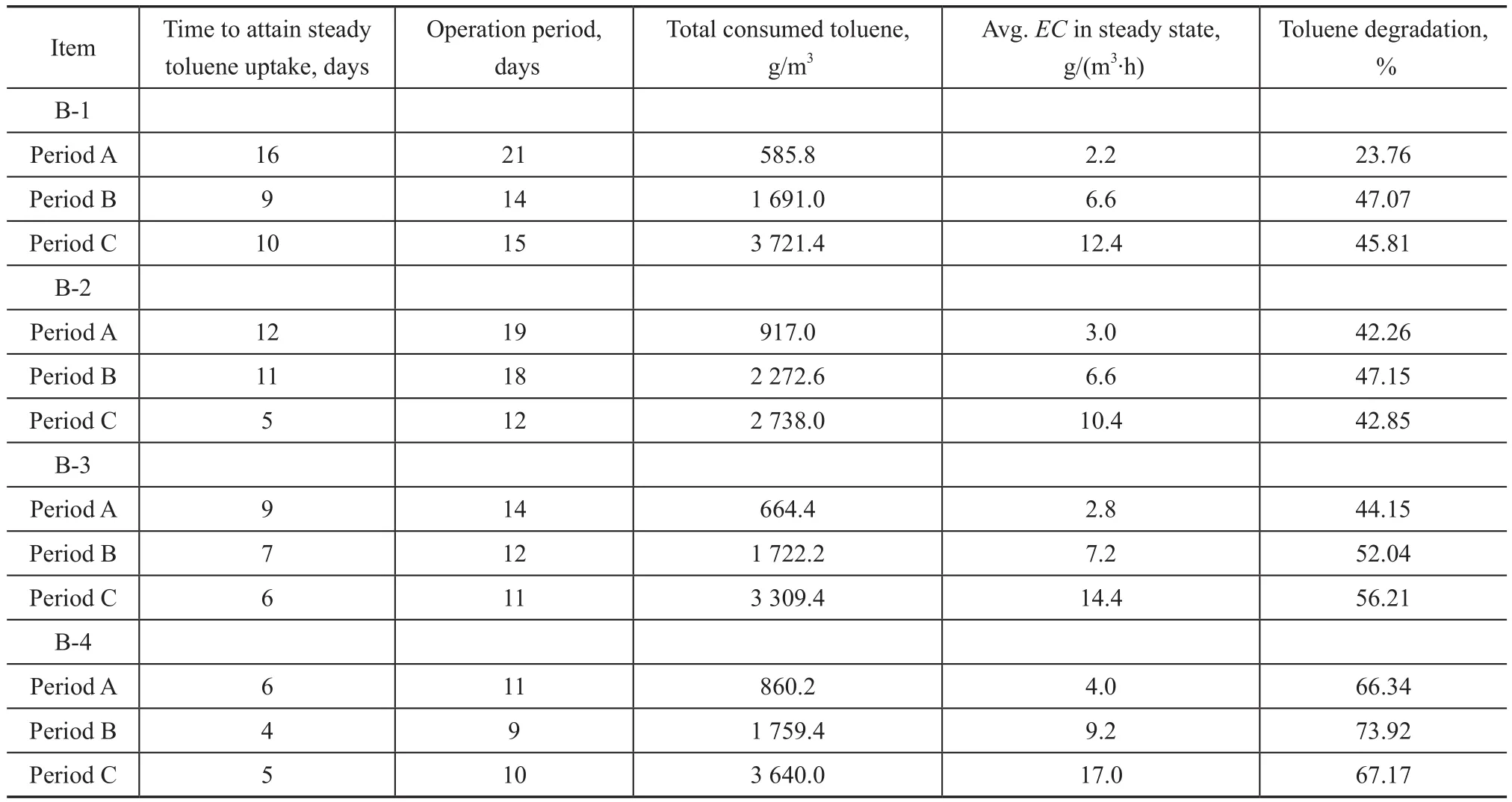
Table 3 Biofilters operated with three concentration gradients, time to attain steady toluene uptake, operation time, total toluene consumption, EC average in the steady state and total toluene removal.

Figure 3 Pressure drops across each biofilter during the start-up period
3.3 Water content
Water content is one of the key parameters for biofilter operation, and the optimal values ranges from 0.66 to 1.5[27]. Excess water reduces biofilter voidage, which can lead to the formation of anaerobic zones, increasing gasliquid interfacial resistance and pressure drop. Lower water content promotes the preferential pathways of gas flow and inhibits the metabolic activity. The water content of biofilters was determined at the beginning and the end of start-up period, as shown in Table 4. Throughout the experiments, the inlet air stream was humidified to prevent the drying of packed bed. At day 0, the water content was homogeneous in each section, the average value in biofilters B-1 and B-3 was 2.51, and the average value in biofilters B-2 and B-4 was 1.57. At the end of start-up period, the water content in biofilters B-1 and B-3 was stable due to the good water holding capacity of wood chips.
In biofilters B-2 and B-4, the wood chips mixed with different amount of peat could lead to different effects on the final bed moisture content. Specifically, the water content of packed bed had a slight increase in biofilter B-4 having a higher peat mixing ratio (80:20w/w), while it dramatically dropped from 1.58 to 0.29 when the mixing ratio of peat and wood chips was 50:50 (w/w) in biofilter B-2. This phenomenon could be ascribed to the systematical effects of exothermic microbial reaction and mixed fillers with high surface area.
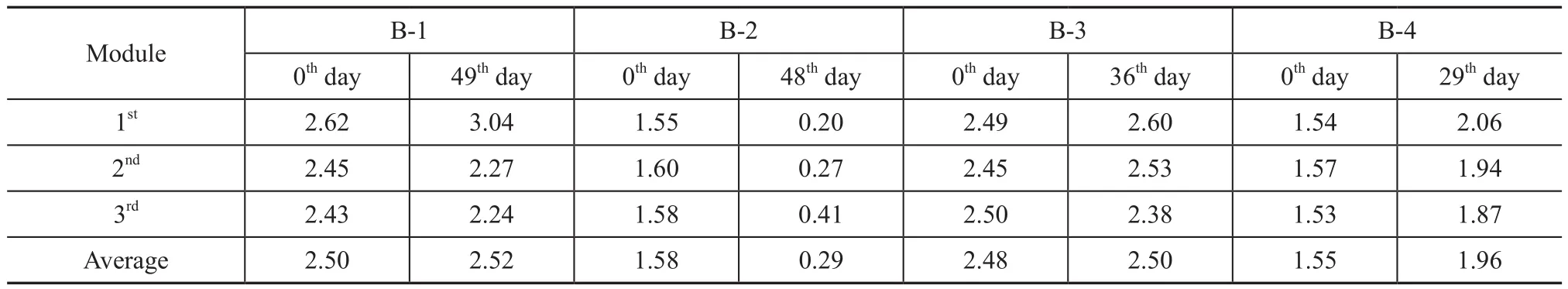
Table 4 Water content of each section in four biofilters at beginning and end of start-up
3.4 Microbial status
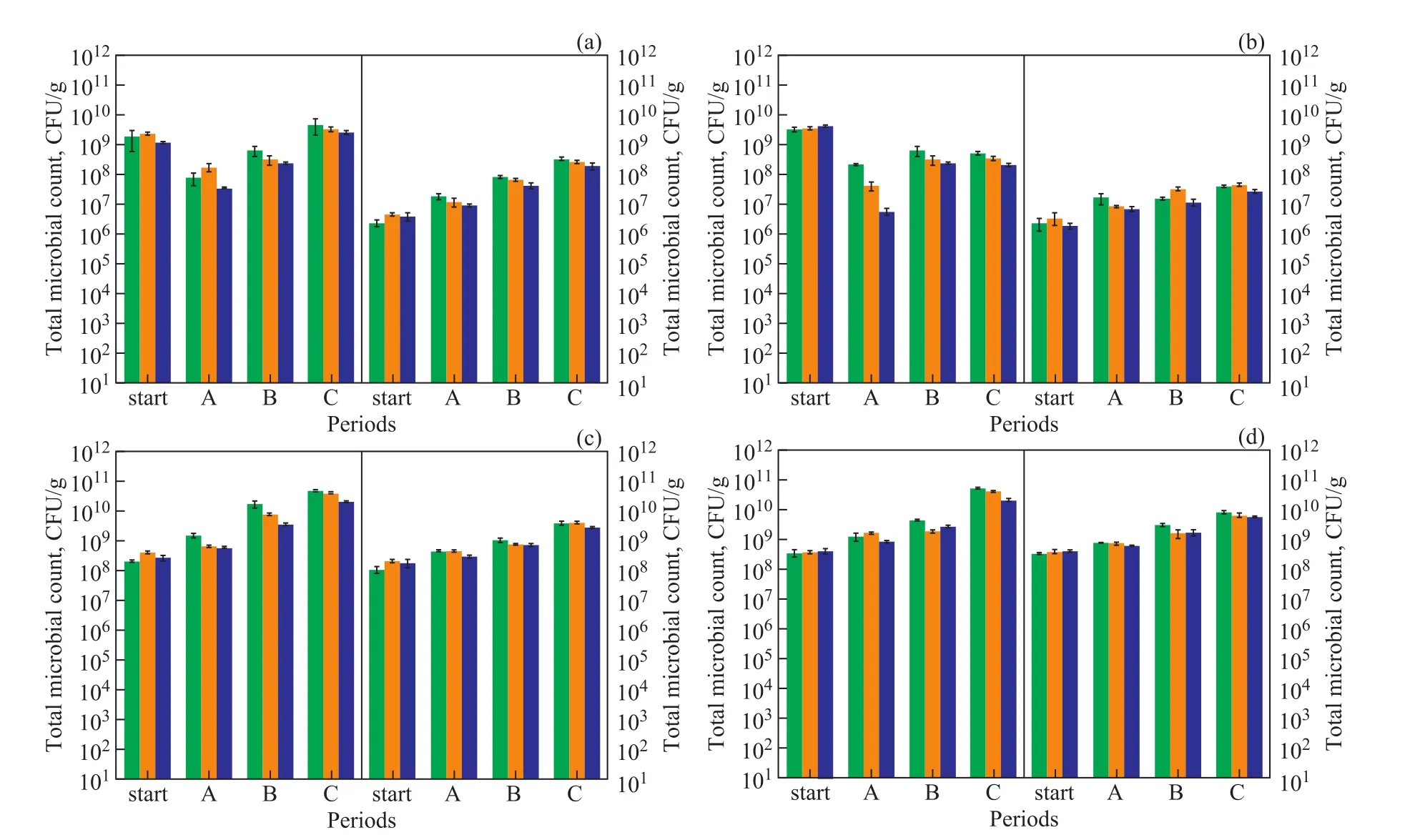
Figure 4 The total microbial counts and toluene degrader counts in biofilters B-1(a), B-2(b), B-3(c) and B-4(d) at different period of start-up
The biomass growth on the packing material was regularly analyzed by microbial counts. The total microbial counts and toluene degrader counts in each section of the packed bed at the end of each period are presented in Figure 4. For the biofilters inoculated with activated sludge (B-1 and B-2), an obvious acclimation process was identified in period A, in which total microbial counts decreased by 10-fold to 3.1×108CFU/g and the toluene degraders increased by 10-fold to an average count of 2.8×107CFU/g. In the next period, total microbial counts and toluene degrader counts increased in stages withIL. Lower microbial growth rate of the biofilter B-2 was ascribed to lost control of water content in packed bed. In the biofilters B-3 and B-4, acclimation process was avoided by inoculating toluene degraders instead, so a higher microbial growth rate was obtained.At the end of start-up period, the toluene degrader counts of B-3 and B-4 were 3.8×109CFU/g and 7.2×109CFU/g,respectively. These values were similar to those biofilters inoculated with acclimated activated microbes for xylene elimination. In addition, total biomass statistics showed that the toluene degraders accounted for 10%—20% of the total microbial counts. At the beginning of start-up period, the toluene degrader counts decreased along with the gas flow, which indicated that most toluene removal occurred in the first two sections. At the end of start-up period, the difference of toluene degrader counts between three sections had gradually disappeared, which was related to similar performance of each section at a high tolueneIL.
Figure 5 presents the morphology of the biofilm on the packing material at the end of start-up period. A mature biofilm composed of a mass of coccoid and rod-shaped bacteria and a few of filamentous fungi were observed in biofilters B-1, B-3, and B-4. In particular, the fungi presented in the biofilters B-3 and B-4 inoculated with only three strains of toluene degrading bacteria suggested the formation of diverse microbial communities. In Figure 5b, a sparse distribution of bacteria and fungi validated the biomass measurement results of biofilter B-2.
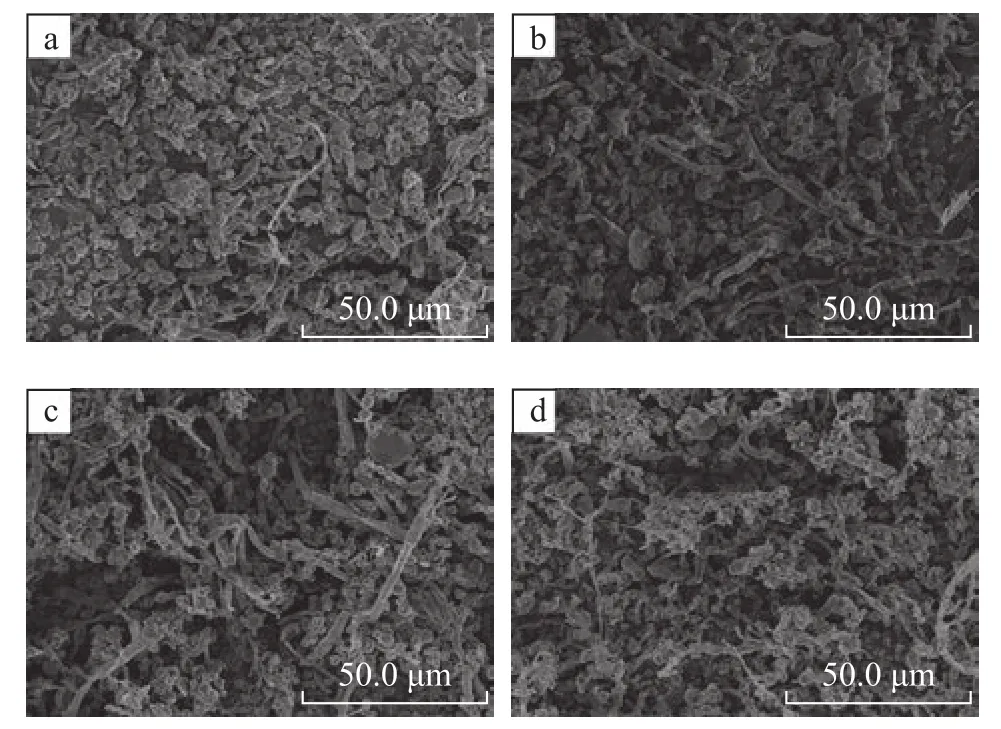
Figure 5 SEM of microbes taken from 1st section of the biofilters B-1(a), B-2(b), B-3(c) and B-4(d) at the end of start-up operation
4 Conclusions
This research evaluated the influences of inocula and packing material on start-up of biofilter by two indicators including the start-up time andEC. The main conclusions were as follows: (I) When being inoculated with adapted microorganisms instead of raw activated sludge, the startup time was reduced from 35 days to 22 days and the total toluene degradation increased from 40% to 50%. (II)It is beneficial for shortening start-up time by packing the mixture of peat and wood chips instead of the wood chips alone. However, the mixing ratio needed special attention because it had a significant in fluence on the biofilterECby changing the water content and microbial community of packed bed. The biofilterECwas increased with a higher peat mixing ratio (80:20 w/w), but was decreased with a lower peat mixing ratio (50:50w/w).
Acknowledgements:The authors gratefully acknowledge the financial supports by the National Natural Science Foundation of China (Grant No. 21505156), the Fundamental Research Funds for the Central Universities (24720122043), and the Scientific Research and Technological Development Projects of PetroChina Co., Ltd. (2013F-2101).
杂志排行
中国炼油与石油化工的其它文章
- Optimization of Dividing Wall Column with Heat Transfer Process Across the Wall for Feed Properties Variation
- Numerical Simulation of Optimization of Mixing Tank for Residue Upgrading Reactor
- Biodegradability and Tribological Properties of Mineral Base Oil Enhanced by Caprylic Methyl Diethanolamine Phosphate Ester
- Enhanced Anti-Wear Property of Low Viscosity Engine Oil for Sequence IVB Engine Test Meeting the GF-6 Specification
- Study on Flue Gas Desulfurization Process with Selective SO2 Removal by N-formylmorpholine
- Research and Application of Image Recognition Technology in Microscopy Diagnosis of Catalytic Cracking Catalysts
