Synthesis of MOR/SAPO-11 Composite Molecular Sieve via Seed Crystallization for n-Alkane Hydroisomerization
2019-07-15SunNaWangHaiyanMaYuxiangYangZhanxuKangLei
Sun Na; Wang Haiyan; Ma Yuxiang; Yang Zhanxu; Kang Lei
(1. College of Chemical Engineering, China University of Petroleum, Qingdao 266580;2. School of Chemistry and Materials Science, Liaoning Shihua University, Fushun 113001)
Abstract: n-Alkane isomerization is a critical reaction that can affect parameters in oil refining, such as the gasoline octane number and diesel oil solidifying point. In this study, a catalyst support, mordenite (MOR)/silicoaluminophosphate(SAPO)-11 composite zeolite with core/shell structure, was synthesized by hydrothermal method with MOR acting as the seed for crystallization. The crystal structure, elemental composition, surface area, pore volume, and acidity of the catalyst was thoroughly characterized. In addition, the catalytic performance of the as-obtained Pt/MOR/SAPO-11 in the hydroisomerization of n-dodecane was tested. The results indicated that the properties and catalytic performance of the composite molecular sieve were quite different from those of the pure zeolites and physical mixture of MOR and SAPO-11(MOR+SAPO-11). Compared with the physical mixture, MOR and SAPO-11 were more tightly bound in MOR/SAPO-11 because of chemical bonding. Moreover, the acidity and pore structure were favorable to the catalytic hydroisomerization of n-dodecane. Pt/MOR/SAPO-11 exhibited higher isomerization activity than the Pt-loaded pristine MOR and MOR+SAPO-11. Thus, the core-shell composite molecular sieve has promising industrial applications as the catalyst support.
Key words: composite zeolite; core-shell structure; MOR; SAPO-11; hydroisomerization
1 Introduction
n-Alkane isomerization is an important reaction in oil refining, because it has a wide range of favorable influence on the process, including the improvement of the gasoline octane number, reduction of the diesel oil solidifying point, and improvement in the performance of low-temperature processing of lube base oil[1]. Mordenite(MOR)[2], silicoaluminophosphate (SAPO)-11[3], Beta[4],ZSM-22[5], and SAPO-41[6]zeolites showed high activity and selectivity in the isomerization ofn-alkanes. SAPO-11 was mainly used in isomerization of long-chain alkanes to improve the low-temperature flow properties of diesel fuel and lubricating oil. It has suitable acidity and channel structure, but the pore diameter is generally less than 1 nm. This is unfavorable to the mass transfer of large organic molecules, which consequently can limit the catalytic performance of the zeolite[7]. On the other hand,MOR shows excellent catalytic performance in amination,alkane isomerization, and xylene isomerization because of its excellent heat and acid resistance[8]. However, its hydrothermal stability is poor, which would increase the generation of coke and lead to deactivation of the catalyst[9].The formation of a composite made of two kinds of molecular sieves as a means to overcome the shortcomings of each component has become a popular research topic[10-18]. In recent years, a new type of composite, the porous nanocomposite with a core-shell structure, has been gaining an increasing attention[19]. Zhu, et al.[20]prepared a SAPO-11/MOR composite molecular sieve by kneading and mixing, and investigated the effect of this preparation method on catalyst performance in hydroisomerization reaction. The prepared Pt-SAPO-11/MOR molecular sieve had evenly dispersed particles, small spacing, and higher yield of heterogeneous branched alkanes. Generally, a physical mixture is expected to have poorly connected zeolite frameworks because of the large space. In contrast,the composite molecular sieves synthesized by chemical methods not only exhibit the structural characteristics of the zeolite components[21], but also possess a framework of zeolites connected by chemical bonds. These composite molecular sieves have a reasonable distribution of acidity,good shape-selective performance, and hydrothermal stability; thus, they have potential applications in isomerization reactions[22]. Zhang, et al.[23]synthesized a Beta/MCM-22 composite molecular sieve with a core-shell structure through a two-step hydrothermal crystallization method. Ren, et al.[24]prepared a Y/Beta composite molecular sieve using a stepwise derivative method and expounded on the mechanism of synthesis of core-shell composite molecular sieves.
This study adopted the seed crystallization method,which would cover the MOR surface with a layer of SAPO-11 molecular sieve with high Si/Al ratio, to prepare a core-shell composite molecular sieve with MOR serving as the core and SAPO-11 serving as the shell (MOR/SAPO-11). This configuration is expected to improve the surface acidity of MOR, make it more modest in catalytic reaction. The physical characteristics of the MOR/SAPO-11 composite molecular sieve,along with those of the pure zeolites and corresponding physical mixture (MOR+SAPO-11) were determined. In addition, the catalytic performance of the zeolites in the hydroisomerization ofn-dodecane was investigated.
2 Experimental
2.1 H-MOR alkali treatment
H-MOR powder was dipped in an equal volume of NaOH solution (0.2 mol/L). The impregnated samples underwent hydrothermal treatment at 333 K for 30 min. Alkali vapor etching of the samples by 0.2 mol/L NaOH solution was then conducted at 358 K for 2 h. In this process, the ratio of the alkali and solid sample was 30 mL/g. The products were ion-exchanged three times in ammonium chloride solution at 353 K. After ion exchange, the products were washed with distilled water, dried at 383 K for 12 h, and then calcined at 813 K for 4 h.
2.2 Synthesis of core-shell MOR/SAPO-11 composite zeolite
The MOR/SAPO-11 composite zeolite was synthesized by hydrothermal method according to the following procedure: Twenty grams of MOR zeolite [n(SiO2)/n(Al2O3)=25, Catalyst Plant of Nankai University,Tianjin, China]were added to 100 mL of distilled water, and the mixture (pH = 8.0) was stirred for 30 min. H3PO4(85%, Sinopharm Chemical Regent Co.,Shanghai, China), pseudoboehmite (70% Al2O3, Chinalco,Beijing, China), and distilled water were mixed and stirred for 2 h. The slurries were mixed with the hybrid template, which was a mixture of di-n-propylamine and diisopropylamine (mDPA/mDIPA mass ratio of 1,Sinopharm Chemical Reagent Co., Shanghai, China).The molar ratio of the reactants in this synthesis was set to 0.1SiO2: 1.0Al2O3: 1.0P2O5: 0.6DPA: 0.6DIPA:50H2O. The mixture was vigorously stirred for 2 h to form a homogeneous slurry with pH = 6.0. The gel was transferred to an autoclave and heated at 190 °C for 48 h. After crystallization, the MOR/SAPO-11 composite zeolite product was washed, dried at 100 °C overnight,and then calcined at 600 °C for 12 h to completely remove the template.
2.3 Preparation of catalysts
In a 20-mesh to 40-mesh sieve, MOR, SAPO-11,MOR/SAPO-11, and MOR+SAPO-11 zeolite carriers,respectively, were loaded with 0.5% of Pt by wet impregnation method using H2PtCl6solution as the metal precursor. The resulting catalysts were dried at 373 K and calcined at 732 K for 4 h. These samples were labeled as Pt/MOR, Pt/SAPO-11, Pt/MOR/SAPO-11, and Pt/MOR+SAPO-11, respectively.
2.4 Characterization
To identify the phase structure, powder XRD patterns were collected by a D8 Advance X-ray powder diffractometer (Bruker) with Cu Kα radiation (λ= 0.15418 nm) operated at 40 kV and 40 mA.
N2isothermal adsorption-desorption was performed at the temperature of liquid nitrogen using an Autosorb IQ2-MP gas sorption analyzer (Quantachrome Instruments). Each sample (ca. 200 mg) was degassed for 8 h at 573 K and 10-6mmHg before data measurement. The specific surface area (SBET) was calculated according to the Brunauer-Emmett-Teller (BET) method, while the pore volume(Vpore) was obtained byt-plot analysis of the adsorption isotherm.
IR spectra were recorded using a Nicolet iS50 Fouriertransform IR spectrometer (Thermo Fischer Scientific).The IR spectrometer was equipped with an in-situ cell with CaF2windows. About 15.0 mg of each sample was pressed into a self-supported disc, which was introduced into the cell. The disc was first heated to 673 K for 2 h with air flow and was subsequently cooled down to room temperature under vacuum. The IR spectra were recorded following contact of the samples with pyridine vapor.The temperature was then increased incrementally for the measurement.
SEM was performed using a Quanta 400F scanning electron microscope (Hitachi). The morphology and chemical composition of the catalysts were determined by TEM-EDS using a JEM-2100F field emission electron microscope (JEOL Ltd.).
2.5 Evaluation of catalytic performance
n-Dodecane hydroisomerization was conducted in a continuous flow, tubular fixed-bed micro-reactor loaded with 3.0-g of catalyst operating under ambient pressure.The catalyst was activated in-situ under H2flow at 673 K for 4 h prior to the commencement of reaction. The H2/n-dodecane molar ratio was 200, and the reaction pressure was 2.0 MPa at a volumetric hourly space velocity(LHSV) of 2.0 h-1. Products were analyzed on-line by a 7820A gas chromatograph (Agilent) equipped with a HPPONA capillary column (50 m × 0.2 mm × 0.5 μm) and a flame ionization detector.
3 Results and Discussion
3.1 Characterization
3.1.1 XRD analyses
The XRD patterns of MOR, SAPO-11, MOR/SAPO-11,and MOR+SAPO-11 are shown in Figure 1. The XRD pattern of MOR/SAPO-11 only shows the diffraction peaks of MOR and SAPO-11, indicating the presence of both components in the intergrowth zeolites. Moreover,the intensity of the diffraction peaks at 8.1°, 9.8°, 21.2°,22.4°, and 25.8° are stronger for MOR/SAPO-11 than for MOR+SAPO-11, indicating the higher crystallinity of the former.

Figure 1 XRD patterns of various zeolites
3.1.2 N2 adsorption-desorption analyses
Figure 2 shows the N2adsorption-desorption isotherms of SAPO-11, MOR, MOR/SAPO-11, and MOR+SAPO-11 zeolites. Monolayer adsorption occurs on all samples at a relative pressure (p/p0) of below 0.1, indicating the characteristic micropore framework[25].
The isotherms of MOR+SAPO-11 and MOR/SAPO-11 are between those of SAPO-11 and MOR, indicating that they are a mixture of the latter two zeolites. Furthermore, the difference between the MOR/SAPO-11 and MOR+SAPO-11 isotherms reflects the difference between the connection of SAPO-11 and MOR in the two composites.
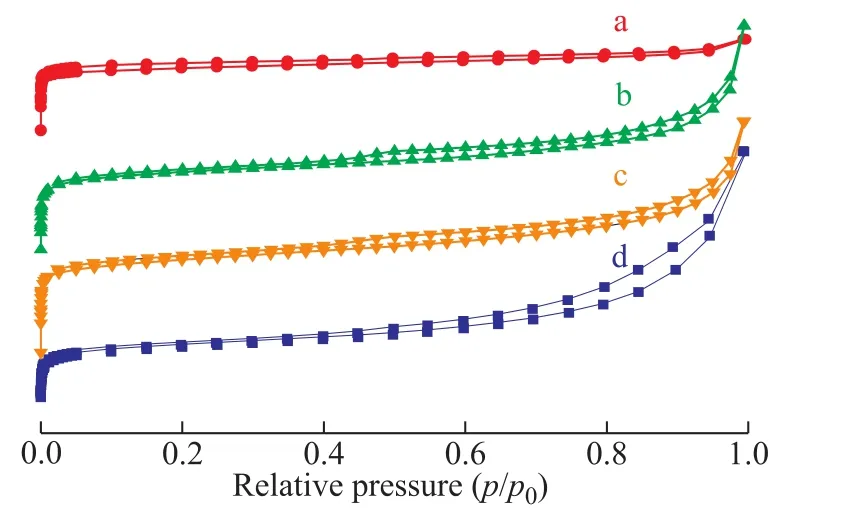
Figure 2 N2 adsorption-desorption isotherms of samples

Table 1 Physical properties of catalysts
Table 1 shows that theSBETvalues of MOR/SAPO-11 and MOR+SAPO-11 are between those of MOR and SAPO-11; moreover, theSBETvalue of MOR/SAPO-11 is lower than that of MOR+SAPO-11. The mass ratio of MOR and SAPO-11 is 1:1 in the MOR/SAPO-11 composite molecular sieve. The average of the microporous BET surface areas (Smicro) of SAPO-11(132.3 m2/g) and MOR (373.4 m2/g) is 252.8 m2/g,which is close to theSmicroof MOR/SAPO-11(256.2 m2/g). Therefore, the MOR zeolite channel was not blocked after addition of SAPO-11 during the synthesis of MOR/SAPO-11.
3.1.3 SEM and TEM-EDS analyses
The SEM images of MOR, SAPO-11, MOR/SAPO-11,and MOR+SAPO-11 in Figure 3 show significant differences in the morphologies of the samples. MOR has porous structural defects on the crystal surface after alkali etching (Figure 3(a)). SAPO-11 presents a uniform spherical morphology and particle size distribution(about 4 μm-5 μm) (Figure 3(b)). SAPO-11 and MOR are independent in MOR+SAPO-11, which was prepared by physical mixing; thus, the SEM image of the composite shows a mixture of columnar MOR crystals and spherical SAPO-11 particles (Figure 3(d)). Moreover, the SAPO-11 molecular sieves are surrounded by an uneven distribution of MOR. On the other hand, in MOR/SAPO-11, SAPO-11 molecular sieves grew in the outer surface, resulting in uniform coverage of MOR (Figure 3(c)). This image shows that after alkali treatment of modified MOR, the SAPO-11 molecular sieves can uniformly and firmly adhere to MOR. However, the SEM image of MOR/SAPO-11 shows that pseudo-spherical aggregates accumulated with columnar crystals without individual SAPO-11 or MOR particles. MOR zeolite particles may be completely covered by SAPO-11 molecular sieves,forming a core/shell structure with MOR and SAPO-11 as the core and shell, respectively. These results indicate that MOR and SAPO-11 were combined more tightly in MOR/SAPO-11 than in MOR+SAPO-11. Furthermore, more external surface area was formed owing to the increased interspaces between the SAPO-11 and MOR particles in MOR/SAPO-11, as confirmed by the adsorption results.

Figure 3 SEM images of samples.
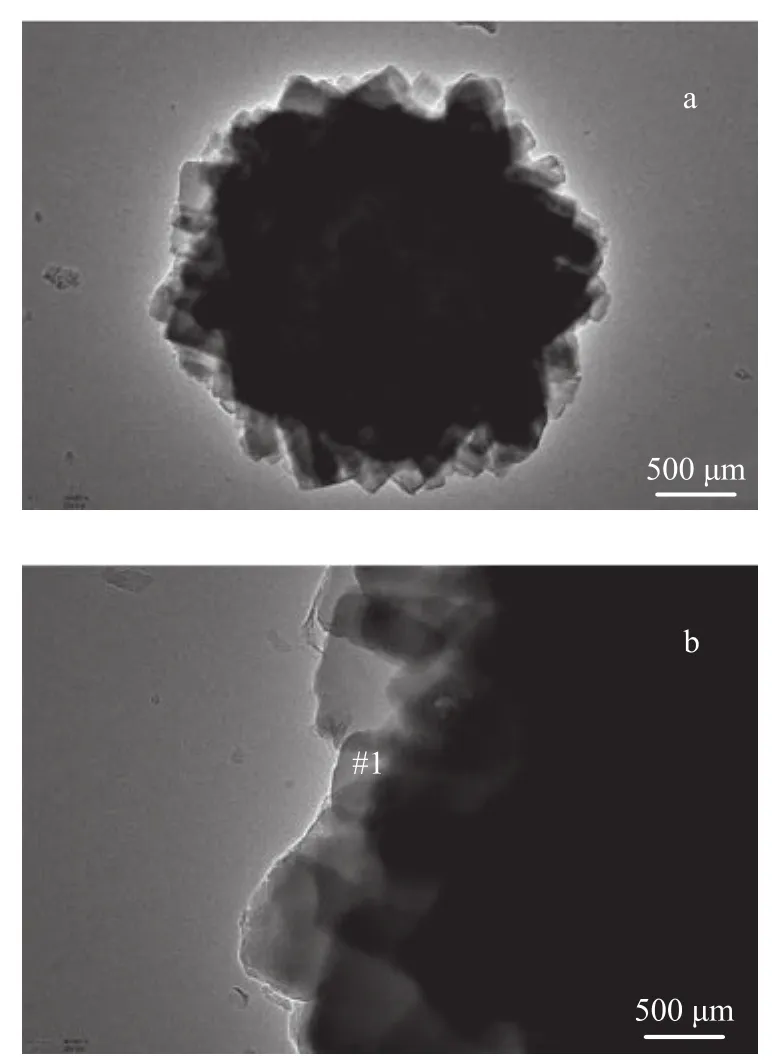
Figure 4 TEM images of the MOR/SAPO-11 composite zeolite
The results of the TEM-EDS analysis of MOR/SAPO-11 are shown in Figure 4 and Table 2. The TEM image of MOR/SAPO-11 in Figure 4(a) shows rectangularly packed, pseudo-spherical aggregates, which can be more clearly seen in Figure 4(b). Table 2 shows the chemical composition of MOR/SAPO-11 identified by the EDS analysis. On the exterior (shell) of MOR/SAPO-11 (#1 in Figure 4(b)), the Si, Al, and P contents are approximately the same as those for SAPO-11. On the other hand, then(SiO2)/n(Al2O3) ratio of MOR/SAPO-11 is more than two times compared to that of SAPO-11. These results confirm that MOR/SAPO-11 is made up of a SAPO-11 shell and a MOR core.
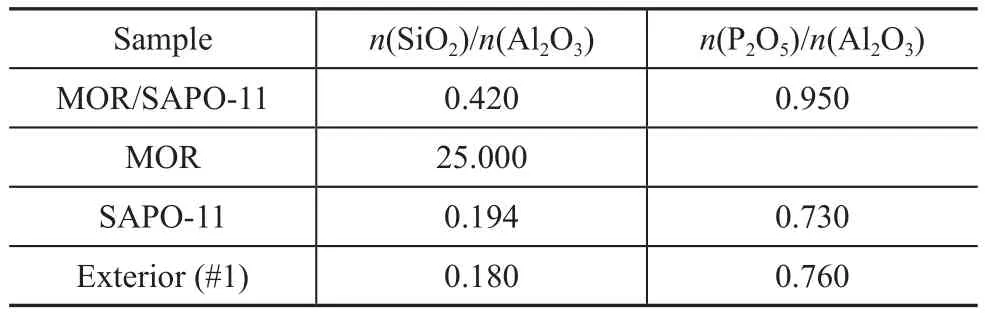
Table 2 Chemical composition of the samples identified by TEM-EDS analysis
3.1.4 Acidity characterization
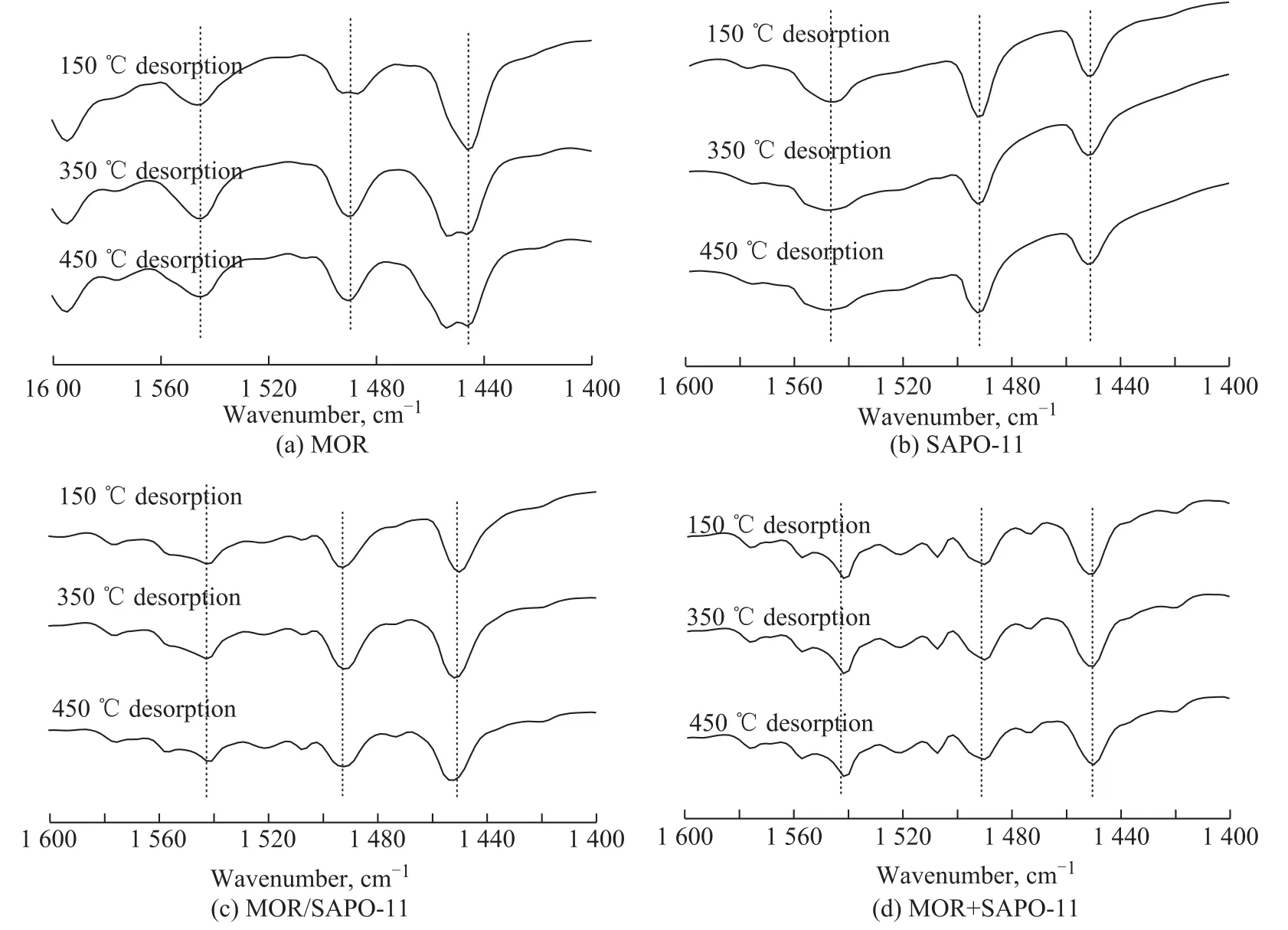
Figure 5 IR spectra of pyridine adsorbed on the samples at different temperatures
Figure 5 shows the IR spectra of pyridine adsorbed on MOR, SAPO-11, MOR/SAPO-11, and MOR+SAPO-11,respectively, at different temperatures. There are three pyridine absorption bands corresponding to C-C bending vibration within the range of 1 600 -1 400 cm-1. The peaks at 1 540 cm-1and 1 450 cm-1correspond to pyridine adsorption on the Brønsted (B)-and Lewis (L)-acidic sites, respectively, while the strongest peak at 1 490 cm-1corresponds to pyridine adsorption on both the B- and L-acidic sites. These data prove that all four samples include the B- and L-acidic sites[26].

Table 3 Acidic properties of samples at different temperatures determined by Py-IR
The acidity of MOR, SAPO-11, MOR/SAPO-11,and MOR+SAPO-11 was also determined by Py-IR spectroscopy. The density and distribution of acidic sites were estimated from the areas of the corresponding IR bands. The results are summarized in Table 3. The weak,strong, and total acidic sites, and the density of B-acidic sites decrease in the following order: MOR > MOR+SAPO-11> MOR/SAPO-11 > SAPO-11. The ratio (B+L)150/(B+L)350characterizes the distribution of weak and strong acidic sites.Based on this parameter (Table 3), the four catalysts have more weak acidic sites than strong ones. This ratio decreases in the following order: MOR/SAPO-11 > MOR+SAPO-11> SAPO-11 > MOR. MOR/SAPO-11 had the highest B150/L150, B350/L350, and Btotal/Ltotalamong the samples investigated,indicating that this composite molecular sieve catalyst has the highest distribution of B-acidic sites.
The presence of defect structures in the framework and formation of extra-framework aluminophosphate demonstrated that MOR and SAPO-11 are chemically bonded in MOR/SAPO-11. Thus, the density and distribution of acidic sites in this composite is different to those in MOR+SAPO-11. The B/L ratio of MOR/SAPO-11 is higher than other catalysts, which also confirms the chemical bonding in MOR/SAPO-11.
3.2 Evaluation of catalyst performance in hydroisomerization reaction

Figure 6 Conversion of n-dodecane during hydroisomerization reaction over various catalysts as a function of temperature
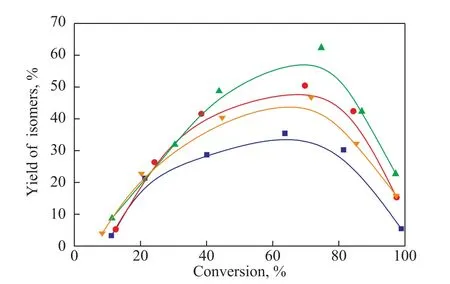
Figure 7 Isomerization yield plotted against n-dodecane conversion
Figure 6 shows the conversion ofn-dodecane over various catalysts, which decreases in the following order:Pt/MOR > Pt/MOR+SAPO-11 > Pt/MOR/SAPO-11 >Pt/SAPO-11. The isomer yields of the four catalysts are shown in Figure 7. The Pt/MOR/SAPO-11 catalyst has the highest isomer yield, while the Pt/MOR+SAPO-11 catalyst has an isomer yield which is intermediate to that of the pure zeolite catalysts.Dodecane isomerization reaction follows the “orifice catalytic mechanism”[27]. Isomerization of long-chain alkanes mainly occurs in the H-SAPO-11 molecular sieve surface and orifice[28]. Hydroisomerization and hydrocracking ofn-alkanes constitute a pair of competing parallel reactions. The first reaction is carbon ion formation, followed by the recurrence of key C-C rearrangement and isomerization, and finally, the cracking of the carbon ions. The higher the isomerization of the branched chain is, the stronger the cracking activity would be. An effective way to enhance isomerization on the surface is to expose more micropores, which expands themass transfer channel and reduces the diffusion resistance between molecular mass transfer and isomerization activity.

Table 4 Product distribution of n-C12 hydroisomerization over Pt-supported catalysts
The Pt/MOR/SAPO-11 catalyst has high catalytic activity, and isomerization reaction mainly occurs on the B-acidic active sites. Being consistent with the Py-IR characterization results, the heterogeneous hydrocarbon yield increases with an increasing ratio of B- and L-acidic sites. Thus, the distribution of the B-acidic site has a significant in fluence onn-dodecane isomerization.
In a physical mixture of SAPO-11 and a molecular sieve catalyst with a large aperture, the spacing is larger; thus,the distance between molecular sieves for the diffusion of reactants, intermediates, and products is longer. This will cause side reactions (cracking reaction), increased probability of shape-selective catalysis, and reduction of synergy between the two kinds of molecular sieves.Without solving these problems, the isomer selectivity cannot be improved.Claude, et al.[29]investigated long-chain alkane hydroisomerization over the Pt/H-ZSM-22 catalyst. Their study indicated that short alkanes such as decane and undecane are mainly converted by the orifice mechanism,while for dodecane and larger alkanes, the contribution of the key-lock mechanism increases with an increasing carbon number. In addition, for hydroisomerization taking place over medium-pore zeolite catalysts, the orifice mechanism favors branching at the end position. As shown in Table 5, the hydroisomerization ofn-dodecane over Pt/SAPO-11 mainly occurs via the orifice mechanism. The Pt/MOR+SAPO-11 catalyst and the Pt/MOR/SAPO-11 catalyst bring forth quite different distributions of mono-branched isomers compared with the pure zeolite catalysts. Isomers with branching at the C2position are rarely detected for the composite zeolite catalysts, which favor C5and C6mono-branched isomers. Thus, the hydroisomerization ofn-dodecane over the composite zeolite catalysts mainly occurs via the key-lock mechanism.
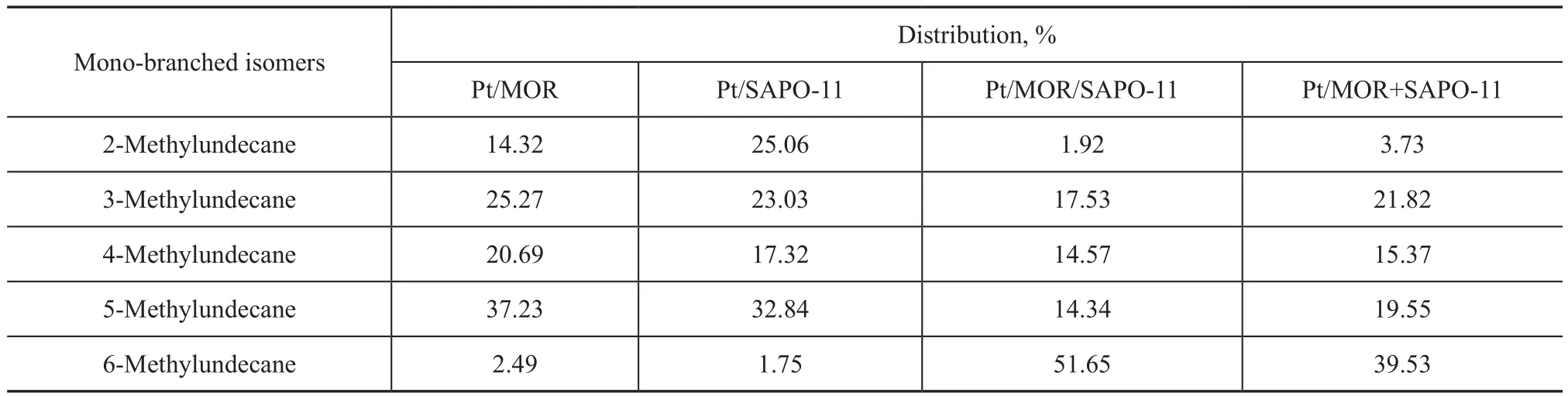
Table 5 Distribution of mono-branched isomers from the isomerization of dodecane
4 Conclusions
The MOR/SAPO-11 composite molecular sieve was synthesized by hydrothermal method using the MOR zeolite as the seed. MOR zeolite particles are uniformly and tightly covered by SAPO-11 molecular sieves,resulting in a core/shell structure. Chemical bonding of MOR and SAPO-11 led to a composite molecular sieve with more weak and B-acidic sites. Compared with the single and physically mixed molecular sieve catalysts,n-dodecane hydroisomerization over the Pt/MOR/SAPO-11 catalyst had the highest yield of multi-branched isomers, and the total isomer yield reached 53.09% at a conversion equating to 96.92%. The relatively high isomerization selectivity of the core-shell MOR/SAPO-11 composite zeolite indicates that it can be successfully utilized forn-alkane isomerization in oil refining.
Acknowledgement:We thank the National Natural Science Fund of China (2016-Z0030), the Natural Science Foundation of Liaoning Province (L2017LQN008, L2016020), and the Fushun Science & Technology Program (2011ZX05039-003).
杂志排行
中国炼油与石油化工的其它文章
- Effect of the Morphology of ZnO Support on the Desulfurization and Regeneration Performance of Ni/ZnO Catalyst
- Optimization of Dividing Wall Column with Heat Transfer Process Across the Wall for Feed Properties Variation
- Methane Storage and Synthesis of HKUST-1 Prepared with Different Solvent
- Research on Hydrogenolysis of Glycerol to 1, 2-Propylene Glycol by Using Supported Raney-Cu/Al2O3
- Synthesis of Nanosized SSZ-13 Zeolite and Performance of Its Mixed Matrix Membrane for CO2/CH4 Separation
- Intelligent Transformation and Upgrading of Oil Refining& Petrochemical Industries in China: Investigation &Application
