Research on Hydrogenolysis of Glycerol to 1, 2-Propylene Glycol by Using Supported Raney-Cu/Al2O3
2019-07-15ZhangLanxiCaoYongzhengWangHuiguoLiNingLuoGuohuaXuXin
Zhang Lanxi; Cao Yongzheng; Wang Huiguo; Li Ning; Luo Guohua, ; Xu Xin,
(1. College of Chemical Engineering, Beijing Institute of Petrochemical Technology, Beijing 102617;2. Beijing Key Laboratory of Fuels Cleaning and Advanced Catalytic Emission Reduction Technology,Beijing 102617)
Abstract: New supported Raney-Cu catalyst was prepared from pseudo-boehmite powder and copper aluminum alloy powder. It could be used in a continuous- flow fixed-bed reactor and it was also a good catalyst for hydrogenolysis of glycerol to propylene glycol after the two raw materials were processed by the following procedures: molding, drying, calcination, and leaching. XRD, H2-TPR, as well as SEM technique were employed to study the physicochemical properties of the catalysts, and the hydrogenolysis of glycerol to 1,2-propylene glycol (1,2-PDO) was used as a probe to evaluate the performance of supported skeletal copper catalysts. The test result indicated that CuAl2 was the main crystal phase of precursor after the dried strips were calcined at 850 °C in air. A part of CuAl2 was oxidized to α-Al2O3, which was the main contributor to the strength of Raney-Cu/Al2O3, while the remaining CuAl2 was converted to active skeletal copper after the leaching process. The effects of reaction temperature, hydrogen pressure, LHSV, hydrogen-oil ratio, glycerol concentration, and alkaline additives on the catalytic performance were studied in water or ethanol, respectively. Conversion of glycerol and selectivity of 1,2-PDO were 30.9% and 91.4% in water, or 99.0% and 73.6% in the ethanol system, respectively, when the reaction was performed at a temperature of 215 °C, a H2 pressure of 3 MPa, a LHSV of 1.0 h-1, a hydrogen-oil ratio of 500, and a glycerol concentration of 20% (ethyl alcohol solution).
Key words: glycerol; hydrogenolysis; 1,2-PDO; Raney-Cu/Al2O3; solvent effect
1 Introduction
Biodiesel industry experiences rapid development since biodiesel, as a kind of potential substitution for fossil fuels, can effectively alleviate the current energy shortage.However, the crude glycerol, a by-product of biodiesel industry restricts the increase of biodiesel production and the overcapacity of glycerol has a negative effect on the benign development of biodiesel industry because about 1 gallon of crude glycerol can be formed along with the production of 9 gallons of biodiesel[1-3]. This fact means it is necessary to work out a new method to convert glycerol to high-value products as a way to support the development of biodiesel industry.
Conversion of glycerol to 1,2-PDO is a valuable route since 1,2-PDO is primarily used in polymer synthesis.1,2-PDO can be used as raw material for the production of cosmetics, antifreeze, and preservative[4-6]. Traditionally,noble metal catalysts (Ru, Rh, Pt, Pd)[7-10]and nonnoble metal (Cu, Ni, Co)[10-12]catalysts are used in the glycerol hydrogenolysis reaction. Although the noble metal catalysts exhibit high activity at low temperature[11],the selectivity for 1, 2-PDO is not high, which restricts their further applications. Instead, the Cu-based catalysts show excellent selectivity to 1, 2-PDO due to the good ability to break the C-O bonds and poor ability to break the C-C bonds[12-13]. Generally, the Cu-based catalysts are prepared by the co-precipitation method with the nitrate solution of Cu, Al, Zn, or Mg. Nevertheless, the catalysts prepared by this method exhibit poor stability since the active Cu on catalyst surface is prone to aggregation when the reaction is performed at high temperature and pressure. Recently, the researches mainly focus on how to improve the stability of Cu-based catalysts. For example,a catalyst with similar crystal lattice can be obtained by thermal decomposition or a catalyst with high dispersion and stability can be obtained by adding carriers.
The Raney-Cu may be a good candidate catalyst for glycerol hydrogenolysis as its porous structure and large specific surface area can improve the stability of Cu catalyst. Traditionally, the Raney-Cu catalysts are prepared by leaching copper aluminum alloy powder in sodium hydroxide solution at specific temperature. Although it can bring about high catalytic activity to catalysts, and this method can be easily under control, the poor compressive strength of conventional Raney Cu restricts its application.In this paper, novel supported Raney-Cu/Al2O3catalyst was prepared and its application in conversion of glycerol to 1,2-PDO was studied. It could be used in the fixedbed reactor because its high compressive strength and the effects of reaction temperature, hydrogen pressure, LHSV,hydrogen/oil ratio, glycerol concentration, and alkaline additives on the catalytic performance were also studied.
2 Experimental
2.1 Catalyst preparation
The supported Raney-Cu/Al2O3was prepared by the following processes: Copper-aluminum alloy powder (with a mass ratio of Cu/Al=48/52, and a particle size of <150 μm)and pseudo-boehmite powder (type: P-DF-03, with a pore volume of ≥0.5 mL/g, provided by the China Aluminum Corporation Shandong Branch) were mixed adequately prior to addition of 15% HNO3solution. Subsequently,the mixture was extruded into strip by a banded extruder and was then dried at 120 °C. The dried strips were calcined at 850 °C for 4 h and the obtained precursor was leached at 70 °C in 20% NaOH solution for 4 h to get the supported Raney-Cu/Al2O3. Finally, the catalyst was washed by deionized water several times to remove residual alkali and was then preserved in water.
2.2 Characterization
The powder X-ray diffraction (XRD) was conducted on a SHIMADZU XRD-7000 Advance X-ray diffractometer using CuKα radiation (λ=0.15418 nm) to characterize the phase composition and crystallite size. At a tube voltage of 40 kV and a tube current of 30 mA, the scanning angle(2θ) range was 10°—90° with a scanning speed of 4 (°)/min. The temperature-programmed reduction (TPR) was carried out on a Chemisorb 2720 instrument produced by the Micromeritics Instrument Corp. to determine the sample reduction behavior. The sample (0.10 g) was heated to 1 000 °C at a temperature increase rate of 10 °C/min in an atmosphere composed of 10% H2/Ar at a flow rate of 20 mL/min, with the amount of H2consumed in the test being monitored by a TCD. The Brunauer-Emmett-Teller(BET) surface area of the Raney-Cu/Al2O3was measured by N2adsorption on a Micromeritics ASAP 2020 N apparatus.The morphology was observed with a SUPPA 55 scanning electron microscope (SEM) produced by the Carl Zeiss AG.
2.3 Catalytic test
The continuous hydrogenation of glycerin over Raney-Cu/Al2O3catalysts was conducted on a fixed-bed reactor. The inner diameter of tube reactor was 12 mm and 10 mL of Raney-Cu/Al2O3(particle size 380 μm ~830 μm) were loaded in the isothermal section of the reactor. The catalyst was subject to in-situ pre-reduction using H2stream at 280 °C for 3 h at a space velocity of 400 h-1(GHSV) before each experimental run.Hydrogenation experiments were performed at 200 °C under a reaction pressure of 3.0 MPa at a space velocity of 1.0 h-1(LHSV) and a hydrogen-oil ratio of 500:1.
2.4 GC Analysis
The GC analysis was conducted by a SHIMADZU GC-14C gas chromatograph equipped with a FID detector and a HP-FFAP capillary column (50 m × 0.25 mm ×0.25 μm). The reaction mixture was collected and charged into a cylindrical bottle with 1,4-butanediol as an internal standard. The analysis condition covered an initial column temperature of 160 °C, which was kept for 1 min prior to raising the column temperature at a temperature increase rate of 10 °C/min to 200 °C for 20 min, with the FID temperature maintained at 250 °C.
3 Results and Discussion
3.1 Crystal phase of Raney-Cu/Al2O3
The supported Raney-Cu/Al2O3, its precursor, and carrier were investigated by XRD to analyze the change of main crystal structure in the course of preparation of the catalyst. Figure 1 shows the XRD spectra of Cu-Al alloy powder (curve B), the pure Al2O3carrier (curve A) which was calcined at 850 °C, and its precursor (curve C). Curve B exhibits that the main crystal phase of Cu-Al alloy powder is CuAl2whereas the diffraction peaks of AlN,Cu9Al4and α-Al2O3appeared with the diminished CuAl2diffraction peak after calcination at 850 °C. These results indicate that the reaction of CuAl2abides by the following calcination process:
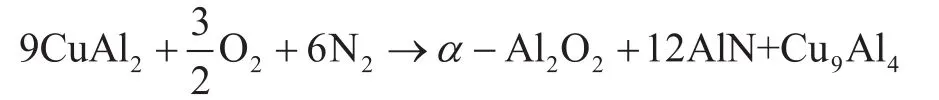
The XRD pattern of pure Al2O3carrier calcined at 850 °C(curve A) shows only the characteristic diffraction peaks of δ-Al2O3, indicating that α-Al2O3in the precursor(curve C) does not emanate from the carrier. Instead, it comes from the oxidation of CuAl2in the atmosphere:a part of metallic Al is oxidized to α-Al2O3and AlN.Therefore, α-Al2O3, the main contributor to the strength of the catalyst, is generated by the oxidation of metal Al in CuAl2alloy phase. The XRD pattern of activated precursor (curve D) exhibits that the diffraction peaks of Raney-Cu appear at the positions of 2θ= 43.19°,50.29° and 73.88° coupled with the disappearance of CuAl2, which means that the hydrogenation activity is originated from the de-aluminum activation of CuAl2.The Cu9Al4phase is insoluble in lye and it remains in the catalyst as there is no obvious change of the characteristic diffraction peaks of Cu9Al4during the activation process.This can retain the porous structure of skeleton Cu and prevent the agglomeration of copper particles during the hydrogenation process. Since AlN has higher thermal conductivity, the existence of the two phases is conducive to improving the structural stability and thermal stability of Raney-Cu/Al2O3catalyst.
Figure 2 shows the H2-TPR spectra of the catalyst measured from room temperature to 800 °C. Since the skeletal Cu has high adsorption properties at low temperature[14], the sharp hydrogen consumption peak located at about 60—230 °C can be assigned to hydrogen adsorption of skeletal Cu and a part of CuO species, which have smaller crystal size in the catalyst.The peak located at 230—340 °C can be assigned to larger crystal-size CuO in the catalyst. The absorption peak located at 590 °C can be attributed to Cu9Al4crystal phase: α (Cu) + γ2(Cu9Al4) → β (AlCu3)[15], a part of H2is separated from the catalyst because the new-born AlCu3shows poor ability of H2consumption.The last peak in the profiles can be assigned to the reduction of CuAl2O4[16].

Figure 1 XRD patterns of pure carrier calcined at 850 °C(A); alloy powder (B); precursor calcined at 850 °C (C); and corresponding catalyst (D)
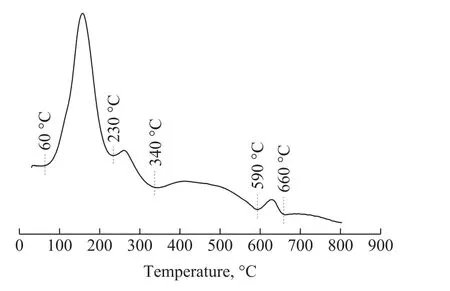
Figure 2 H2-TPR pro files of the Raney-Cu/Al2O3 catalyst
The SEM image of the Raney-Cu/Al2O3(Figure 3) shows Cu particles in the catalyst. The spherical particles are quite small and are dispersed uniformly on the catalyst surface.
3.2 Performance of the Raney-Cu/Al2O3 catalysts on glycerol hydrogenolysis
3.2.1 Effect of reaction temperature on glycerol hydrogenolysis
Reaction temperature has a significant influence on the reaction rate, equilibrium conversion, product selectivity, and catalyst stability for glycerol hydrogenolysis. The mechanism demonstrates that dehydrogenation of glycerol on copper, when it is performed under acidic conditions, can lead to formation of glycerin aldehyde in equilibrium with its enolic tautomer, since it is an endothermic reaction.Then, hydrogenation of the intermediate unsaturated aldehyde occurs because it is an exothermic reaction. This fact means that the lower reaction temperature may result in an incomplete reaction and higher temperature will impair the second step of hydrogenation process. Therefore, an appropriate reaction temperature is crucial to the activity and stability of the Cu-based catalysts. The effect of reaction temperature on the reaction products has been studied under conditions covering a H2pressure of 3 MPa, a LHSV of 1 h-1, and a H2/oil ratio of 500 using a 20% glycerol solution (water and ethanol).
Figure 4 exhibits that the glycerol conversion improves obviously with an increasing temperature in both systems.The conversion of glycerol increases from 4.4% to 51.2% in water and from 43.7% to 99.0% in ethanol,respectively, with the reaction temperature increasing from 170 °C to 230 °C. This is achieved mainly because of the chemical equilibrium[17]. The conversion rate of glycerol in alcohol is higher than that in water system,denoting that the water system is bad for hydrogenation of acetol.
With an increasing reaction temperature (from 170 °C to 230 °C), the selectivity for 1,2-PDO decreases from 89.2% to 84.3%. Meanwhile, the selectivity of acetol increases from 2.3% to 10.3% in the water system. Acetol is the precursor of 1,2-PDO and the hydrogenation process of acetol is an exothermic reaction[18-19]. Although higher temperature is beneficial to glycerol dehydration leading to the formation of acetol, it is unfavorable to the succeeding hydrogenation process of acetol. This means high temperature may prevent the production of acetol, the intermediate product of the reaction, from being converted to 1,2-PDO in time. However, when the selectivity of 1,2-PDO increases from 51.2% to 88.3%, the selectivity of acetol remains stable in the ethanol system. According to chemical equilibrium of glycerol dehydration reaction,the reaction can proceed toward the positive direction in anhydrous system but it has no obvious effect on the second step of hydrogenation. Although higher temperature is unfavorable to hydrogenation reaction, it is beneficial to ethanol reforming to produce hydrogen. The acetol can be converted into 1,2-PDO in time and the selectivity of acetol basically remains unchanged.
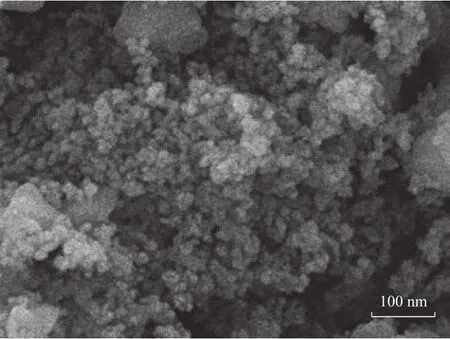
Figure 3 SEM images of the Raney-Cu/Al2O3 catalyst
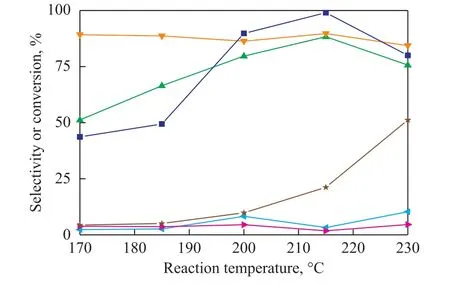
Figure 4 Effect of reaction temperature on glycerol hydrogenolysis in different solutions (ethanol or water)
3.2.2 Effect of reaction pressure on glycerol hydrogenolysis
Leaving my sister, I sought out the tarnished7 hero. Not in such high spirits himself, he greeted me with a somber8 expression. Casually9, I mentioned that I knew the importance of the day and that I just happened to have a lovely arrangement of flowers sitting unclaimed in my vehicle, and that if he could think of a good use for them, he was more than welcome to nonchalantly remove them from their spot of waiting.
Figure 5 shows that the reaction pressure plays a signi ficant role in 1,2-PDO selectivity. In the water system, the selectivity of 1,2-PDO improves from 45.7% to 89.7%when the reaction pressure increases from 1.5 MPa to 3.5 MPa. However, the conversion of glycerol and the selectivity of intermediate product acetol decrease from 25.6% and 45.8% to 15.0% and 2.3%, respectively.According to the literature reports, there is competitive adsorption relationship between glycerol molecules and H2molecules on active sites of the catalyst[20]. The adsorption of H2molecules on the Raney-Cu/Al2O3catalyst is obviously stronger than glycerol molecules as the pressure improves, leading to the decrease of glycerol conversion. Furthermore, higher H2partial pressure increases the number of active hydrogen species on the catalyst surface, which is beneficial to the hydrogenation of acetol to produce 1,2-PDO[21]. In the ethanol system, the selectivity of 1,2-PDO increases from 56.2%to 87.3%. The highest conversion of glycerol is 99.0%at 30 bar, while the reaction pressure has little effect on the selectivity of the intermediate products acetol and alcohol.
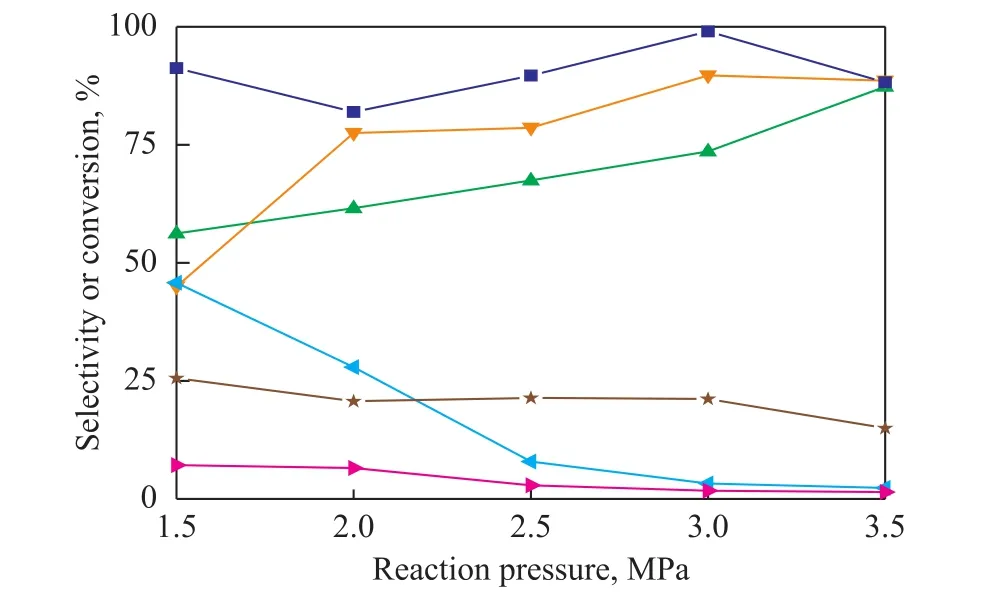
Figure 5 Effect of H2 pressure on glycerol hydrogenolysis in different solutions (ethanol or water)
3.2.3 Effect of hydrogen-oil ratio on glycerol hydrogenolysis
Figure 6 shows the effect of hydrogen/oil ratio (from 100∶1 to 500∶1) on the catalytic performance of Raney-Cu/Al2O3catalyst for glycerol hydrogenation. The effect is not obvious in the water system, the glycerol conversion and 1,2-PDO selectivity slightly decreases from 30.4% and 90.4% to 21.2% and 89.7%, respectively. On the contrary, the glycerol conversion increases significantly from 66.3% to 99.0% and 1,2-PDO selectivity decreases from 76.9% to 73.6% in the ethanol system. There is no obvious effect on the selectivity of acetol.
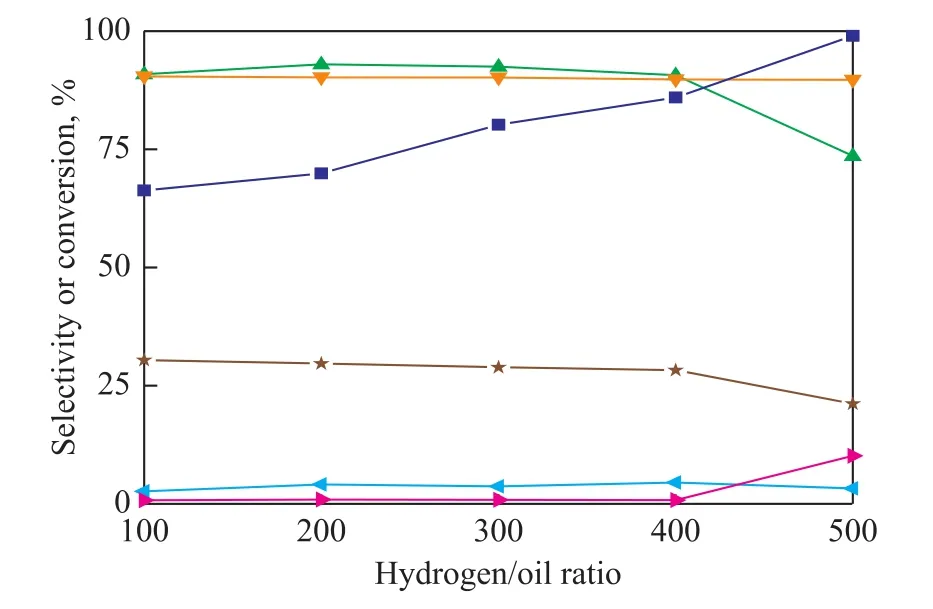
Figure 6 Effect of hydrogen/oil ratio on glycerol hydrogenolysis in different solutions (ethanol or water)
3.2.4 Effect of LHSV on glycerol hydrogenolysis
It is shown in Figure 7 that the conversion of glycerol decreases from 35.9% to 15.1% with the increase of LHSV from 0.6 h-1to 1.4 h-1in the water system while it has no obvious effect on catalytic performance in the ethanol system. Generally speaking, LHSV will affect the conversion and selectivity of catalyst through affecting the residence time of the glycerol on Raney-Cu/Al2O3[22].Lower space velocity will lead to longer residence time on the catalyst, which can improve the glycerol conversion.With the increase of LHSV, the residence time of glycerol molecules on the catalyst decreases so that glycerol conversion decreases. It is noteworthy that the selectivity of 1,2-PDO and acetol is almost unchanged, indicating that the hydrogenation reaction of acetol alcohol on the supported skeleton copper catalyst is relatively stable.This means that the hydrogenation of acetol alcohol is not the control step of glycerol hydrogenolysis to 1,2-PDO[23].
3.2.5 Effect of glycerol concentration on glycerol hydrogenolysis
Figure 8 shows that the glycerol concentration has a significant effect on glycerol conversion over the Raney-Cu/Al2O3catalyst in the water system. It decreases from 27.9% to 19.3% with an increasing concentration of glycerol, while the glycerol conversion increases from 94.4% to 97.1% in the ethanol system. The increase in conversion of glycerol in the ethanol system should be attributed to the lower polarity of ethanol. Besides, the change of glycerol concentration has no effect on the selectivity of 1,2-PDO.

Figure 7 Effect of LHSV on glycerol hydrogenolysis in different solutions (ethanol or water)
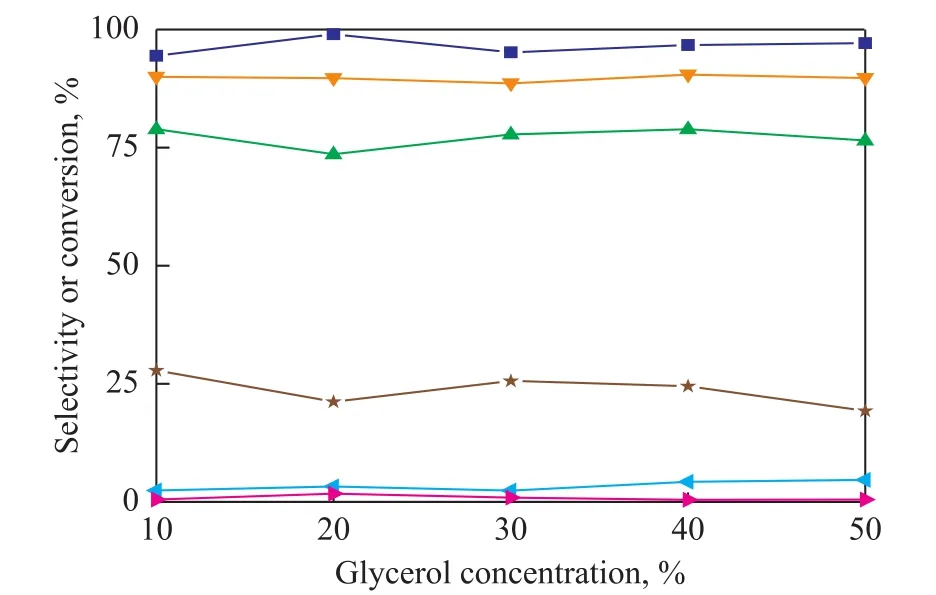
Figure 8 Effect of glycerol concentration in different solutions (ethanol or water)
Table 1 depicts that the six kinds of base additives can improve the conversion of glycerol in the water system.Na2CO3and KOH are the best additives as the glycerol conversion rate is increased from 43.1% to 68.6% and 62.8%, respectively. It is noteworthy that the basic additives can also decrease the selectivity of 1,2-PDO and increase the selectivity of EG. The increase of the pH value of the reaction system makes the C-C bonds on the Raney-Cu/Al2O3catalyst selectively increase to a certain extent, which leads to higher selectivity of ethylene glycol.
In addition, the carbonate of alkali metal is better than the corresponding hydroxide for promoting the ability of breaking the C-C bonds on the Raney-Cu/Al2O3catalyst.The selectivity of the byproduct EG increases to 11.5%and 10.0%, respectively when K2CO3and Na2CO3are
3.2.6 Effect of base additives on glycerol hydrogenolysis
The pH value of raw materials also has a significant effect on the hydrogenolysis of glycerol over the catalyst according to literature reports[9,23], denoting that the catalytic performance of Raney-Cu/Al2O3can be improved by adding suitable acid or alkali auxiliaries.Since the crude glycerol, an alkaline byproduct from biodiesel industry, may result in the inactivation of acid additive, 6 kinds of alkaline additives are selected in this experiment in the water system and the concentration of alkaline additives is 0.2 mol/L (with Li2CO3concentration equating to 0.1 mol/L). The results are shown in Table 1.used as alkali additives. This makes the separation of the subsequent products 1,2-PDO more difficult. Moreover,the larger the radius of the alkali cation, the higher the selectivity of ethylene glycol, which decreases in the following order: K2CO3> Na2CO3> Li2CO3, KOH >NaOH > LiOH. The literature report shows that[24]the difference in glycerol hydrogenolysis properties over the Raney-Cu/Al2O3catalysts caused by alkaline additives can be attributed to the size of alkali metal cation radius and different surface competitive adsorption capacity of the catalyst.
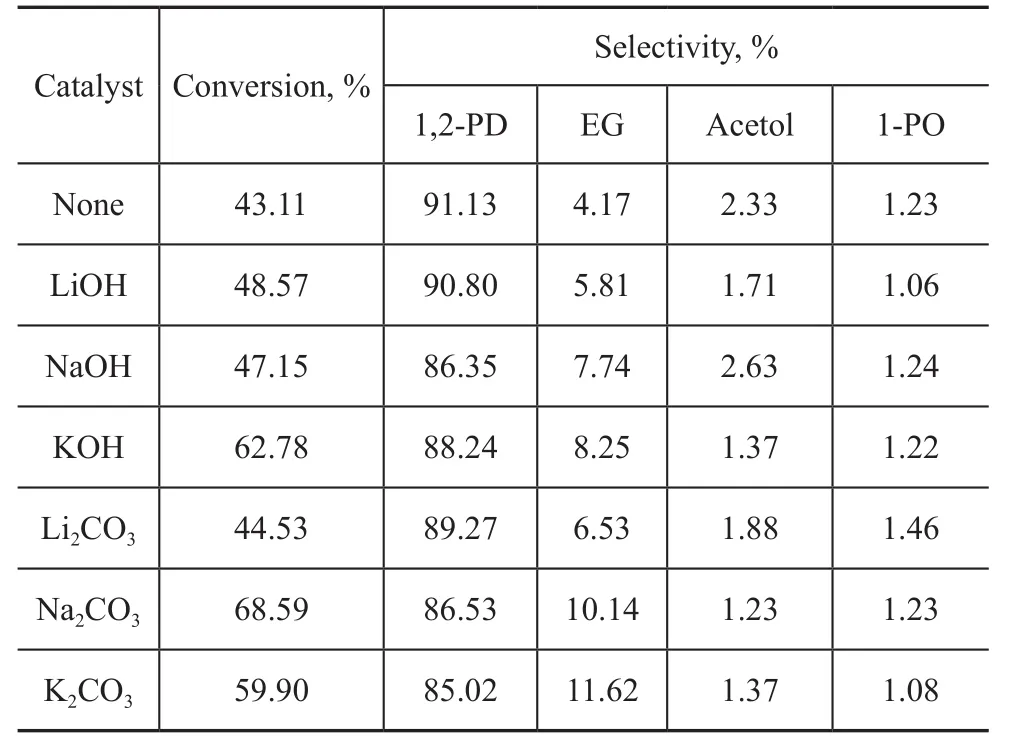
Table 1 Effect of alkali additives on catalytic performance of Raney-Cu/Al2O3 catalyst for converting glycerol to 1,2-PDO
3.2.7 Stability of Raney-Cu/Al2O3 in different solutions for 100 h reaction process
As we can see from Figure 9, the catalyst shows high selectivity of 1,2-PDO (higher than 90.0%) in water but the conversion of glycerol is lower than 60%. The catalyst exhibits bad stability in the water system, while the catalytic activity of the catalyst decreases rapidly from 52.5% to 14.9% with an increasing reaction time.Wang[17]reported that the solvent effect would result in sintering due to the presence of water. The lower surface tension was beneficial to improving the dispersion of Cu particles, which serve as a key factor determining the glycerol conversion. Higher polarity of water can facilitate the removal of 1,2-PDO from the catalyst surface, contributing to higher selectivity to 1,2-PDO.The catalyst has a lower selectivity of 1,2-PDO equating to about 70.0% in the ethanol system and the glycerol conversion rate over the catalyst is almost 100% without any downward trend.
4 Conclusions
The prepared supported Raney-Cu/Al2O3catalyst exhibits good glycerol conversion and 1,2-PDO selectivity. The selectivity of 1,2-PDO and the conversion of glycerol are 89.7% and 21.2% in water, respectively, while the selectivity of 1,2-PDO and conversion of glycerol are 79.7% and 100%in ethanol, respectively, under conditions covering a reaction temperature of 215 °C, a P(H2) of 3 MPa, a LHSV of 1.0 h-1,a hydrogen/oil ratio of 500, and a glycerol concentration of 20%. It can be seen that the reaction solvent has a significant influence on the hydrolysis performance of glycerol on the Raney-Cu/Al2O3catalyst, which is attributed to the different solvent polarity and surface tension. Furthermore, the addition of basic additives in the reactants can significantly promote the glycerol hydrogenolysis activity of Raney-Cu/Al2O3catalyst.Na2CO3is the best additive and the glycerol conversion rate can increase from 43.1% to 68.6% while the EG selectivity can increase from 4.2% to 10.1% in the water system.

Figure 9 100 h reaction time for glycerol conversion over supported Raney-Cu/Al2O3 in different solutions (ethanol or water)
Acknowledgement:This work was financially supported by the Importation and Development of High-Caliber Talents Project of Beijing Municipal Institutions(CIT&TCD20130325) and the Project of Construction of Innovative Teams and Teacher Career Development for Universities and Colleges under Beijing Municipality (IDHT20180508).
杂志排行
中国炼油与石油化工的其它文章
- Optimization of Dividing Wall Column with Heat Transfer Process Across the Wall for Feed Properties Variation
- Numerical Simulation of Optimization of Mixing Tank for Residue Upgrading Reactor
- Biodegradability and Tribological Properties of Mineral Base Oil Enhanced by Caprylic Methyl Diethanolamine Phosphate Ester
- Enhanced Anti-Wear Property of Low Viscosity Engine Oil for Sequence IVB Engine Test Meeting the GF-6 Specification
- Study on Flue Gas Desulfurization Process with Selective SO2 Removal by N-formylmorpholine
- Research and Application of Image Recognition Technology in Microscopy Diagnosis of Catalytic Cracking Catalysts
