Synthesis of Nanosized SSZ-13 Zeolite and Performance of Its Mixed Matrix Membrane for CO2/CH4 Separation
2019-07-15LiuJianqiangLuoYibinLiMinggangShuXingtian
Liu Jianqiang; Luo Yibin; Li Minggang; Shu Xingtian
(SINOPEC Research Institute of Petroleum Processing, Beijing 100083)
Abstract: Nanosized SSZ-13 zeolite was synthesized by traditional hydrothermal method using N,N,N-trimethyl-1-adamantanaminium hydroxide as the structure-directing agent (SDA). The in fluence of different preparative conditions of nanosized SSZ-13 was investigated systematically. The synthetic zeolites were characterized by X-ray powder diffraction(XRD), nitrogen physisorption, and scanning electron microscopy (SEM). By means of the self-assembled method, the thin SSZ-13/polyvinyl alcohol nanocomposite membranes were obtained by incorporating the nanosized SSZ-13 zeolite into the polymeric precursor (polyvinyl alcohol (PVA)). The permeation properties of pure CO2 and CH4 through the mixed matrix membranes (MMMs) were measured. The results showed that the highly crystalline SSZ-13 zeolite in a dispersed nanocrystal form with a controllable particle size of 100 nm could be hydrothermally synthesized by optimizing the synthetic parameters and the selectivity of CO2/CH4 of the MMMs could reach a value of 40 by changing the amount of nanosized SSZ-13 zeolite.
Key words: nanosized SSZ-13; polyvinyl alcohol; permeation
1 Introduction
Natural gas is well-known as the most clean fossil energy and is generally garnering the attention of global countries[1-3]. As there are some impurities in natural gas such as CO2, which can lead to reduced combustion heat and can corrode the pipeline, the natural gas must be purified before utilization[4-7]. Membrane separation[8-14]is a new separation process to deal with natural gas.Compared to the traditional separation process, the membrane separation process has many advantages in terms of simpler equipment, less investment, and low production cost, and therefore it is arousing a widespread interest in the latest years.
Membrane material is the foundation stone of membrane separation process and can significantly influence the permeation and selectivity of membrane. In recent years, some new membrane materials[15-17]with excellent performance for separation of CO2/CH4are continuously emerging, such as the polyimide membrane, the polysulfone membrane, etc. However, as we all know,the polymeric membranes cannot achieve both high permeability and selectivity because of the Robeson upper boundary, which hinders the research and application of current gas separation processes. Research of organicinorganic hybrid membranes becomes the most interesting focus because they possess both advantages of organic and inorganic membranes along with the potential in breaking through the Robeson upper boundary. Zeolites are often selected as inorganic additives to prepared MMMs. As the size of zeolites gets smaller, the separation performance of MMMs turns better[18]. For preparation of membrane with excellent separation performance, zeolites in the MMMs should be subject to nanocrystallization process.
The SSZ-13 zeolite was first synthesized by Zones in 1985[19]. SSZ-13 has a tetrahedral framework composed of double six-membered rings (D-6R) in an AABBCC sequence, the units of which connect to form a cavity with eight-membered ring pore windows. The size of SSZ-13 pore windows is only 0.34 nm, which is between the kinetic gas diameters of CO2(0.33 nm) and CH4(0.38 nm)[20-21]. The SSZ-13 zeolite has a reasonable potential for MMMs synthesis in order to implement selective gas separation.
In this research, by using silica gel, sodium aluminate,deionized water, and NaOH as raw materials andN,N,N-trimethyl-1-adamantanaminium hydroxide as template, the nanosized SSZ-13 zeolite was synthesized by traditional hydrothermal method. Factors affecting the synthesis of SSZ-13 zeolite were systematically investigated. By means of the self-assembled method, the thin SSZ-13/polyvinyl alcohol nanocomposite membranes were obtained by incorporating the nanosized SSZ-13 zeolite into the polymeric precursor (polyvinyl alcohol(PVA)). The permeation properties of pure CO2and CH4through the MMMs were measured.
2 Experimental
2.1 Materials
All chemicals were used directly in our experiments without further purification. Silica gel was acquired from the Evonik Degussa (China) Co., Ltd. Sodium aluminate was acquired from the Sinopec Changling Catalyst Division.N,N,N-Trimethyl-1-adamantanaminium hydroxide solution (25%) was acquired from the Sichuan Zhongbang Technical Development Co., Ltd. NaOH and PVA were purchased from the Sinopharm Chemical Reagent Co., Ltd. These reagents were of the analytical grade purity.
2.2 Synthesis of nanosized SSZ-13 zeolites
The SSZ-13 zeolites were synthesized by the hydrothermal method. At room temperature, sodium aluminate was dissolved in a mixture of deionized water andN,N,N-trimethyl-1-adamantanaminium hydroxide solution under stirring. Then, silica gel and NaOH were added into the mixture under vigorous stirring. Subsequently, the precursor was subjected to hydrothermal treatment conducted at 140—160 °C for 1—4 days according to the dynamic composition method.The resulting solids were filtered and thoroughly washed with deionized water until the effluent water showed a neutral reaction, and then the filter cake was dried in an oven at 120 °C for 12 h. Finally the SDA was removed after being subject to calcination at 550 °C for 2 h.
2.3 Preparation of MMMs
The surfaces of reverse osmosis membrane were coated with PVA and nanosized SSZ-13 zeolite for 1 minute. Then, after having poured out the excess solution, the remaining mixture was dried in an oven at 90 °C for 10 minutes.
2.4 Characterizations
The powder X-ray diffraction (XRD) patterns were recorded on a Rigaku D/MAX-ⅢA X-ray diffractometer using CuKα radiation in the 2θrange of 5°—35°. The adsorption isotherms were performed at -196 °C on a Micromeritics AS-3 instrument using nitrogen (N2) as the adsorbate. The BET surface area was determined by the Brunauer-Emmett-Teller (BET) method. The scanning electron microscopy(SEM) images of zeolite and MMMs were collected on a FEI Quanta 200F scanning electron microscope.
2.5 Performance tests for separation of CO2/CH4
The MMMs were cut into a circle with a diameter of 10 cm, placed above the porous support plate, and loaded into the membrane separator. N2gas was used to adjust the reaction pressure. After the pressure reached the required pressure value and became stabilized, the device was switched to the permeable gas analysis state. The computer could record the date of gas separation automatically.
The permeation rate is calculated by the following equation from the flux of pure gas on the permeating side:
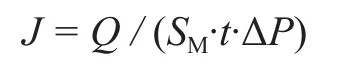
where ΔP, SM,andtare the partial pressure difference of the gases transmembrane, the effective permeating area,and the permeating time, respectively.
The ideal separation factor is calculated based on the permeation rate of pure gases:
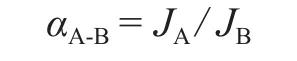
whereJAandJBare the permeation rate for gases A and B,respectively.
3 Results and Discussion
3.1 Effects of the amount of template on the synthesis of nanosized SSZ-13
The quaternary ammonium base, which can significantly influence the grain morphology and size,is commonly used in molecular sieves synthesis as the template. The R2O/ SiO2ratio is known to be one of the most important factors for obtaining the nanosized zeolite. The effects of template on the synthesis of nanosized SSZ-13 zeolite were researched by altering the amount ofN,N,N-trimethyl-1-adamantanaminium hydroxide. The hydrothermal synthesis temperature was 140 °C, which should be kept constant for 3 days. The XRD patterns of SSZ-13 molecular sieve prepared with different amounts ofN,N,N-trimethyl-1-adamantanaminium hydroxide (with the ratio of mR2O to Al2O3equating tox, 1.4x, 1.8x, and 2.2x,respectively)are illustrated in Figure 1. All of the samples showed the standard CHA zeolite diffraction patterns with peaks observed at the 2θof 9.7°, 14.1°, 16.7°, 17.8°,and 20.5°, respectively[22]. No impure crystal phase was observed in the four samples.
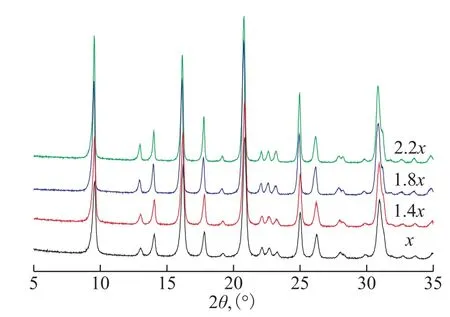
Figure 1 XRD patterns of SSZ-13 samples prepared with different amount of template
The relative crystallinity of SSZ-13 samples is illustrated in Figure 2. The results showed that the relative crystallinity of samples increased with an increasing dosage of the template at the beginning, and reached a maximum when the ratio of mR2O to Al2O3was 1.4x.Then, the relative crystallinity of samples decreased rapidly, when the amount of the template continued to increase.

Figure 2 The relative crystallinity of SSZ-13 samples prepared with different amount of template
The N2adsorption-desorption isotherms of SSZ-13 zeolite samples are shown in Figure 3. All samples exhibited the characteristic features of type-Ⅳ isotherm, in which the adsorption volume increased rapidly under low relative pressure and an obvious hysteresis loop associated with the micropore adsorption was observed. Perhaps the hysteresis loops were produced by the secondary particle piled pores.

Figure 3 N2 adsorption-desorption isotherms of SSZ-13 samples prepared with different amount of template
The textural properties of SSZ-13 zeolite samples are shown in Table 1. The trend of changes in pore properties was similar to that of the relative crystallinity. The micropore area reached a maximum (592 m2/g) when the ratio of mR2O to Al2O3was 1.4x, and subsequently the micropore area began to decrease with an increasing amount of template.
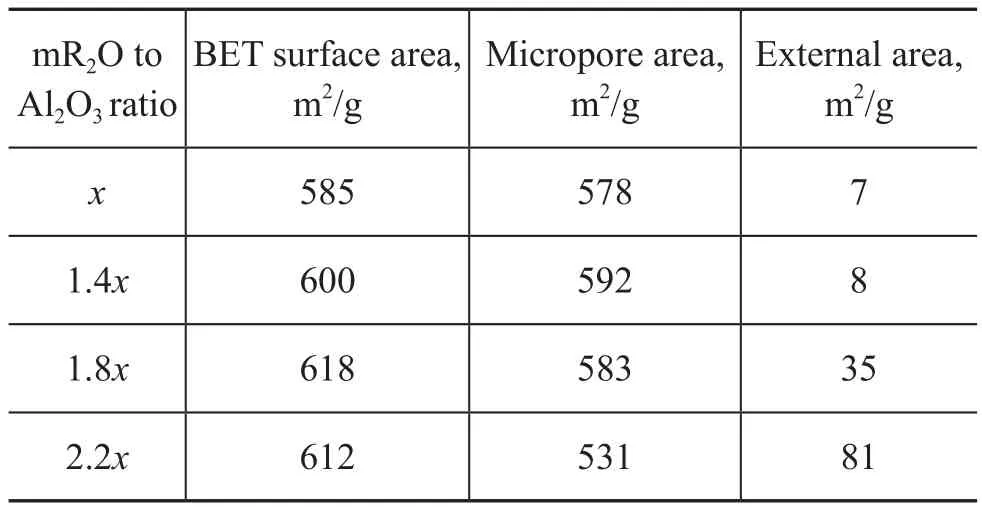
Table 1 The textural properties of SSZ-13 zeolite samples
The SEM images of SSZ-13 zeolite samples are shown in Figure 4. The results illustrated that the size of SSZ-13 zeolite decreased gradually with an increasing amount ofN,N,N-trimethyl-1-adamantanaminium hydroxide.When the mR2O/Al2O3ratio wasx, the big SSZ-13 zeolite particles were made up of some small fuzzy secondary particles. By increasing the mR2O to Al2O3ratio to 1.4x, the particles presented small flaky granule conglomeration. By further increasing the amount of the template, the zeolite particles with a size of below 100 nm became very distinct and independent. Finally, the SEM images of the sample prepared at a mR2O/Al2O3ratio of 2.2xshowed that the sample was not matured.
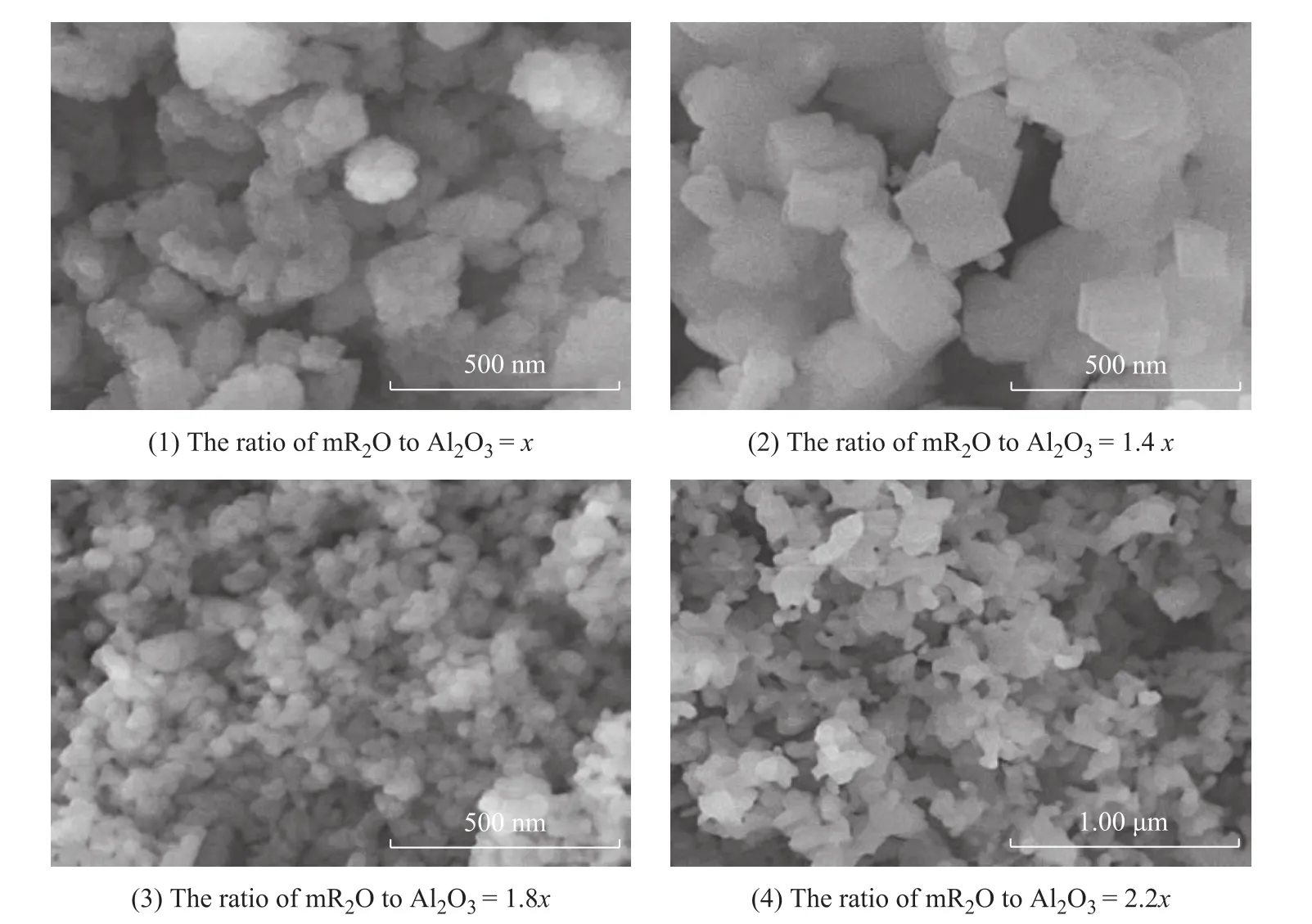
Figure 4 The SEM images of SSZ-13 samples prepared with different amount of template
As we all know, the crystallization of zeolite proceeded in two stages, which corresponded to the nucleation process and the growth process, respectively. The organic template played an important role in both two processes. The quaternary ammonium base acted as a pH regulator and structure-directing agent in the preparation of molecular sieve. With other conditions unchanged, increasing the amount of the template was beneficial to improving the solubility of silicon and aluminum, and also strengthening the inducing effect,which would consequently accelerate the generation of more nucleation. On the other hand, an overhigh amount of quaternary ammonium base could provide excessive OH-ions which would lead to the overhigh solubility of silicon and aluminum and the reduced zeolite crystallinity. Therefore, a proper dosage of template was one of the key factors for synthesis of the nanosized SSZ-13 zeolite with high crystallinity.
3.2 Effects of crystallization time on the synthesis of nanosized SSZ-13
The effects of the crystallization time from 1 day to 4 days on the synthesis of nanosized SSZ-13 zeolites were investigated. The XRD patterns of SSZ-13 zeolites are illustrated in Figure 5. All of the samples showed the standard CHA zeolite diffraction patterns without impure crystal phase.
The relative crystallinity of the SSZ-13 samples is illustrated in Figure 6. The results showed that the relative crystallinity of samples increased with a prolonged crystallization time, and reached a maximum when the crystallization time was 3.5 days. Then, the relative crystallinity of samples decreased rapidly when the crystallization time continued to increase.
The N2adsorption-desorption isotherms of SSZ-13 zeolite samples are shown in Figure 7. All samples also exhibited the characteristic features of type-Ⅳ isotherm.
The textural properties of SSZ-13 zeolite samples are shown in Table 2. The micropore area reached a maximum (602 m2/g) when the crystallization time was 3.5 days, and subsequently the micropore area began to decrease with a further increase of the crystallization time.
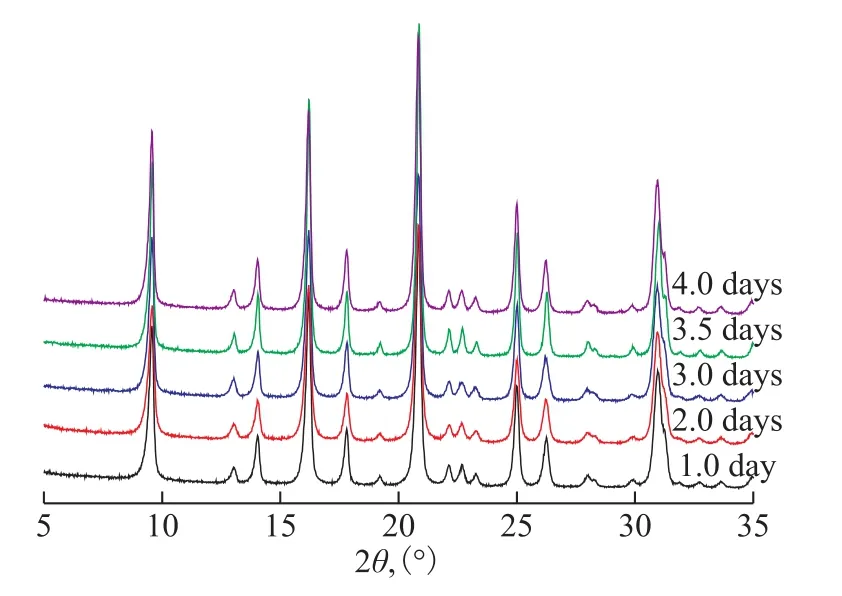
Figure 5 XRD patterns of samples prepared with different crystallization time
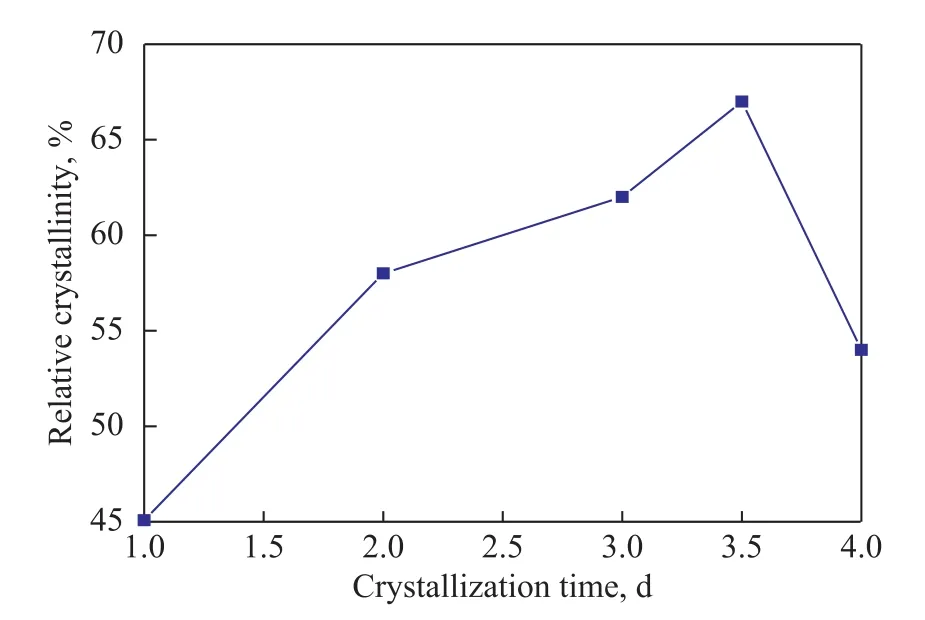
Figure 6 The relative crystallinity of SSZ-13 samples prepared with different crystallization time
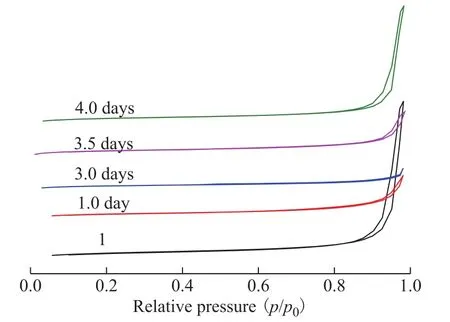
Figure 7 N2 adsorption-desorption isotherms of SSZ-13 samples prepared with different crystallization time

Table 2 The textural properties of SSZ-13 zeolite samples
The SEM images of SSZ-13 zeolite samples are shown in Figure 8. The results illustrated that the size of SSZ-13 zeolites decreased gradually with an increasing crystallization time. When the crystallization time was 1 day, the particles showed a block-like form. When the crystallization time was increased from 2 days to 4 days,the particle size of zeolites was similarly below 100 nm.
3.3 Effects of crystallization temperature on the synthesis of nanosized SSZ-13
The effects of crystallization temperatures from 140 °C to 160 °C on the synthesis of nanosized SSZ-13 were observed. The XRD patterns of SSZ-13 zeolites prepared thereby are illustrated in Figure 9. All of the samples also showed the standard CHA zeolite diffraction patterns without impure crystal phase.
The relative crystallinity of SSZ-13 samples is illustrated in Figure 10. The results showed that the relative crystallinity of samples increased with an increasing crystallization temperature.
The N2adsorption-desorption isotherms of SSZ-13 zeolite samples are shown in Figure 11. All samples exhibited the characteristic features of type-Ⅳ isotherm. But the hysteresis loop of the sample synthesized at 160 °C was obviously smaller than the other two samples.
The textural properties of SSZ-13 zeolite samples are shown in Table 3. The micropore area reached a maximum (625 m2/g), when the crystallization temperature was 160 °C.
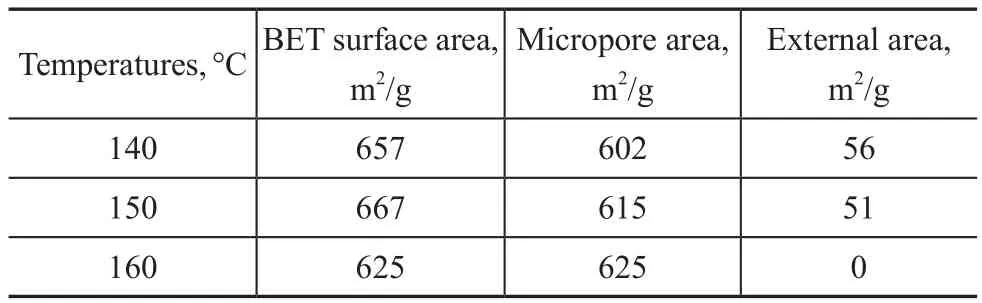
Table 3 The textural properties of SSZ-13 zeolite samples
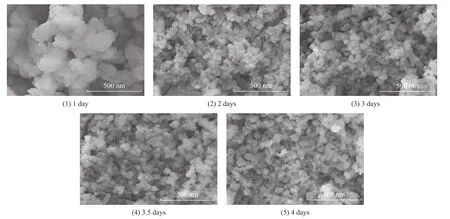
Figure 8 The SEM images of SSZ-13 samples prepared with different crystallization time
Figure 9 XRD patterns of SSZ-13 samples prepared at various crystallization temperatures
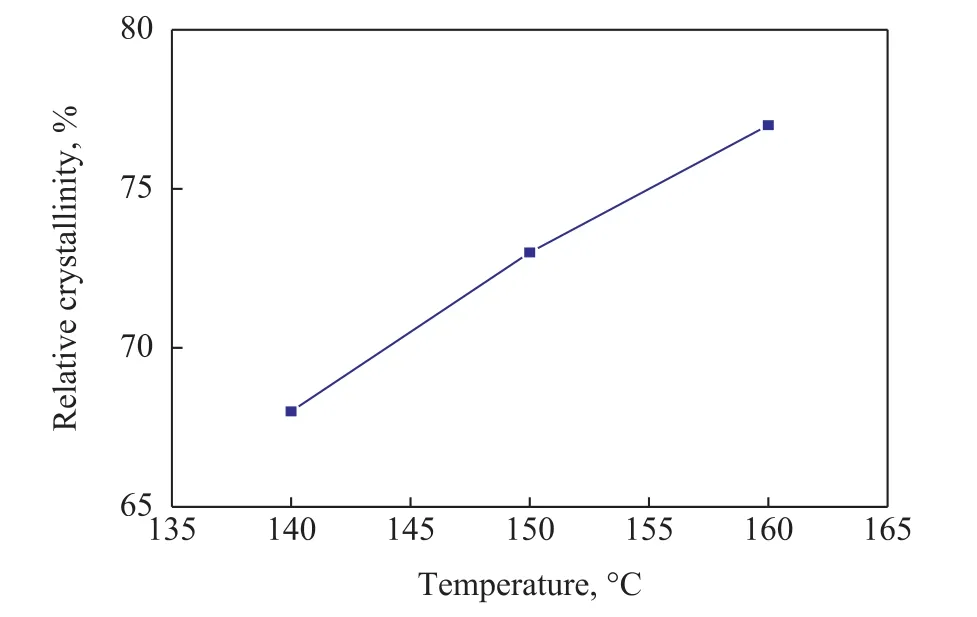
Figure 10 The relative crystallinity of SSZ-13 samples prepared at various crystallization temperatures
The SEM images of SSZ-13 zeolite samples are shown in Figure 12. The results illustrated that the size of zeolites increased gradually with a rising crystallization temperature. Only when the crystallization temperature was 140 °C, the particle size of the zeolites was less than 100 nm.
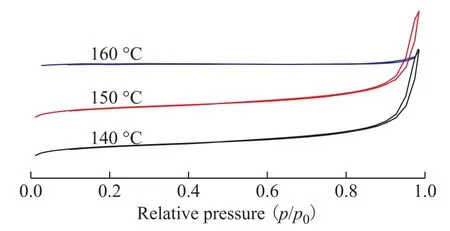
Figure 11 N2 adsorption-desorption isotherms of SSZ-13 samples prepared at various crystallization temperatures
3.4 Gas separation performance of nanosized SSZ-13 composite membranes
The surfaces of reverse osmosis membrane were coated with PVA and the nanosized SSZ-13 zeolite. The effects of nano SSZ-13 in the MMMs were researched by altering the mass fraction of zeolite (from 0 to 2.5%).The SEM images of MMMs are shown in Figure 13. The results showed that the surface of membrane without SSZ-13 zeolite was smooth and defectless. The zeolite could be found clearly after being added into the organic membrane. But no fissure could be discovered.
The gas separation performance of MMMs is shown in Table 4. The results showed that both the permeation rate of CO2and the ideal separation factor of CO2/CH4increased significantly with an increasing mass of nanosized SSZ-13 zeolite. When the mass of molecular sieve exceeded 1.5%, the increase of the permeation rate and the ideal separation factor was not obvious. The gas permeability of the selected gases in the nanosized SSZ-13 composite membranes decreased in the following order: CO2(0.33 nm) > CH4(0.38 nm), which was in agreement with the order of their kinetic gas diameters.As we all know, the transport mechanism of zeolite material was based on this mechanism. According to this mechanism, the separation was caused by the passage of smaller molecules through the pores while the larger molecules were obstructed. It was indicated that the gas permeation in the nanosized SSZ-13 composite membranes obeyed the molecular diffusion mechanism[23].
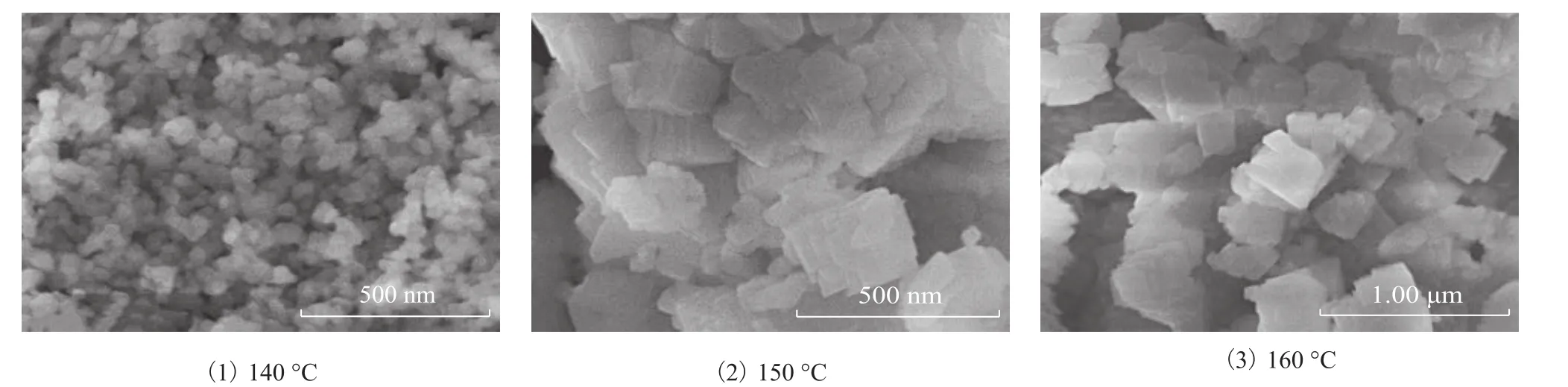
Figure 12 The SEM images of samples prepared at various crystallization temperatures
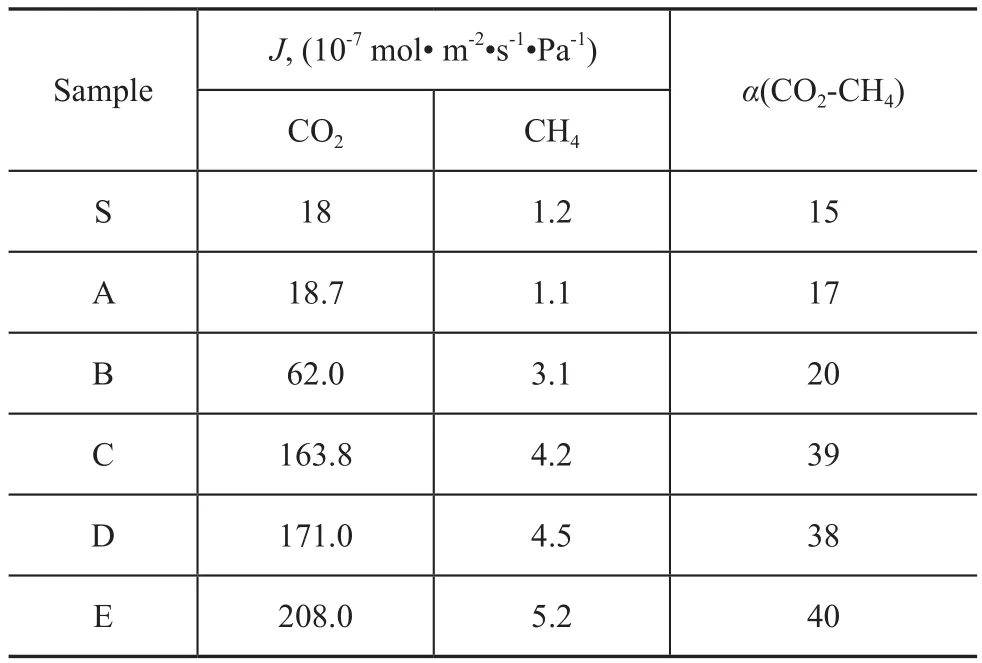
Table 4 Gas separation performance of the nanosized SSZ-13 zeolite composite membranes
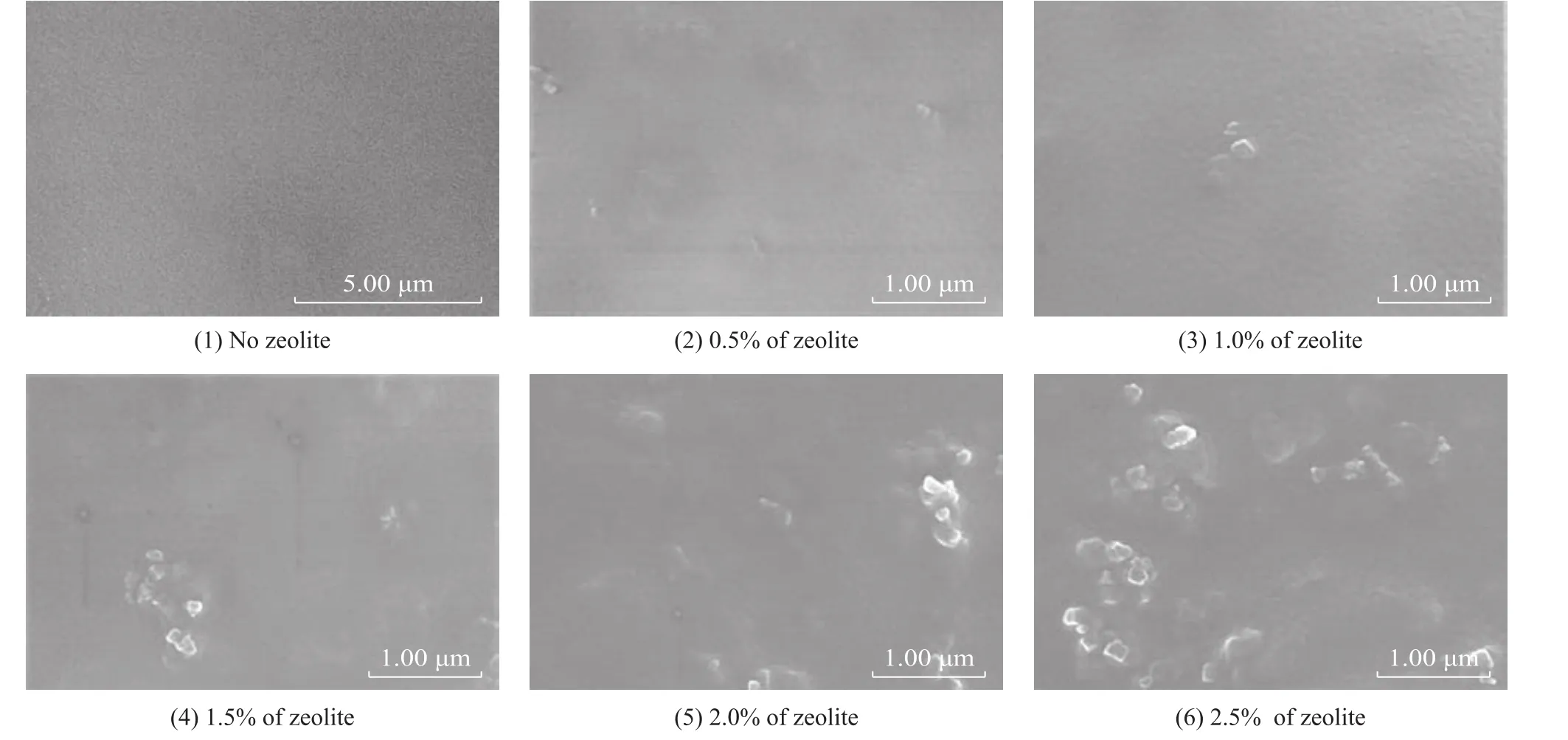
Figure 13 The SEM images of nanosized SSZ-13 MMMs formed with different mass fraction of zeolite
4 Conclusions
Nanosized SSZ-13 zeolite was prepared by traditional hydrothermal synthesis method usingN,N,N-trimethyl-1-adamantanaminium hydroxide as the structure-directing agent (SDA). The particle size of SSZ-13 zeolite was less than 100 nm and the micropore area could reach 602 m2/g.MMMs were prepared with the nanosized SSZ-13 zeolite and PVA. The ideal selectivity of CO2/CH4was above 39 when the mass of SSZ-13 zeolite reached 1.5 %.
杂志排行
中国炼油与石油化工的其它文章
- Methane Storage and Synthesis of HKUST-1 Prepared with Different Solvent
- Optimization of Dividing Wall Column with Heat Transfer Process Across the Wall for Feed Properties Variation
- Novel BiVO4@C3N4@GO Composites with Higher Photocatalytic Performance
- Effect of the Morphology of ZnO Support on the Desulfurization and Regeneration Performance of Ni/ZnO Catalyst
- Research on Hydrogenolysis of Glycerol to 1, 2-Propylene Glycol by Using Supported Raney-Cu/Al2O3
- Intelligent Transformation and Upgrading of Oil Refining& Petrochemical Industries in China: Investigation &Application
