Research Progress in Refining Process for Production of Caprolactam by Beckman Rearrangement Reaction
2019-07-15WangHaoZhangDejiangFanYingqiXieLiLuoYibinZongBaoning
Wang Hao; Zhang Dejiang; Fan Yingqi; Xie Li; Luo Yibin; Zong Baoning
(SINOPEC Research Institute of Petroleum Processing, Beijing 100083)
Abstract: Caprolactam, which is mainly produced via the Beckmann rearrangement reaction, is an important chemical material. The strict specifications of caprolactam products make the refining process of caprolactam extremely important.According to the different rearrangement process, the refining process of caprolactam is also different. The main refining sequence of liquid phase rearrangement products includes extraction, ion exchange, hydrogenation, and distillation.The refining process of gas phase rearrangement products is conducted mainly through distillation, crystallization, and hydrogenation. In this paper, the research progress of caprolactam refining technology will be summarized according to different rearrangement reaction processes, which will provide a reference for the selection of appropriate caprolactam refining process.
Key words: caprolactam; Beckman rearrangement; refining; oximation
1 Background
As an important chemical material, caprolactam is mainly used for synthesis of polycaprolactam fibre (nylon-6),PA6 engineering resin, PA6 film, etc., which are common components of consumer goods and industrial products.In 2016, the caprolactam production reached 2.15 Mt/a in China[1-2].
The above materials are produced through polymerization of caprolactam monomer. However, the presence of impurities will affect the polymerization rate of caprolactam and reduce the molecular weight of the polymer[3]. Premium grade caprolactam generally has a purity of more than 99.99%, a potassium permanganate(PM) value of more than or equating to 22 000 s, an absorbance of less than 0.04 at 290 nm, and a basicity of less than 0.01. Thus, the refining process is an important step in the production of caprolactam.
The impurities in caprolactam mainly come from the following sources: (1) reactants or intermediates; (2) byproducts formed in the production process; and (3) wetting and deterioration of caprolactam during storage and transport[3]. Different caprolactam production processes lead to different impurities in crude caprolactam, which will affect the refining process. At present, about 95%of caprolactam is prepared from cyclohexanone oxime by the Beckman rearrangement. Generally, aromatic hydrocarbons are used as raw materials. One process of caprolactam preparation from benzene is shown below.
The main Beckmann rearrangement process is carried out in liquid phase. The selectivity of liquid Beckmann rearrangement process can reach 98%. In this process,cyclohexanone oxime reacts with fuming sulfuric acid at 90—120 °C firstly to produce lactam sulfate, which reacts with ammonia into caprolactam. 0.5 ton of ammonium sulfate with low added value is formed when 1 ton of caprolactam is produced.
The technological progress in the caprolactam industry mainly focuses on reduction of the byproduct ammonium sulfate. The Sumitomo Corporation of Japan industrialized the gas-phase Beckman rearrangement process in 2003,with the caprolactam selectivity reaching 96.5%, which was lower than that obtained from the liquid-phase rearrangement process, albeit without formation of the by-product ammonium sulfate with low added value,so its refining process was shorter, greener, and more environmentally friendly. The gas-phase Beckmann rearrangement is the direct conversion of cyclohexanone oxime gas to caprolactam in the presence of catalyst at about 360 °C. A composite oxide solid acid catalyst was used early, but the MFI zeolite is used instead now[4].
In this paper, the refining processes for two different rearrangement processes are summarized to provide a reference for the selection of caprolactam refining schemes.
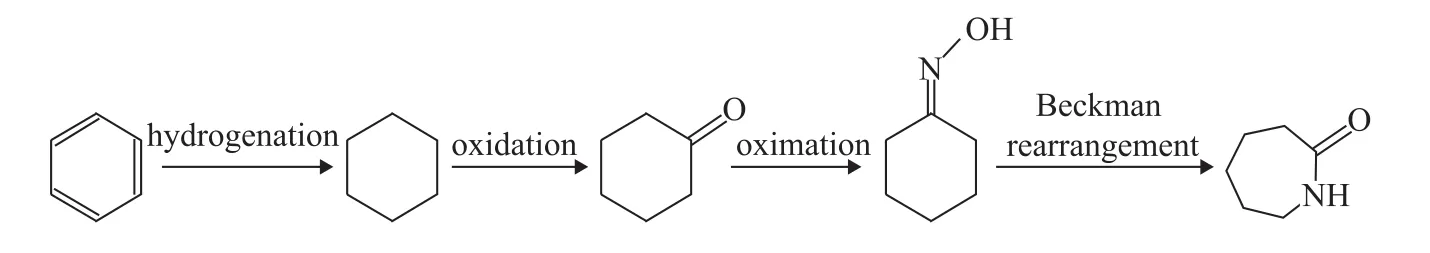
Figure 1 Schematic diagram of reaction process for caprolactam preparation from benzene
2 Refining Process for Liquid Phase Beckman Rearrangement Process
The crude caprolactam prepared by the liquid-phase Beckman rearrangement reaction generally contains 65%—70% of caprolactam, 1%—1.5% of ammonium sulfate, water, and other impurities[5-7]. The refining process of crude caprolactam prepared via the liquid Beckman rearrangement reaction generally consists of the following steps: (1) extraction; (2) ion exchange;(3) hydrogenation; and (4) distillation[8].
2.1 Extraction
Caprolactam refining by extraction is mainly based on the different partition coefficients between caprolactam and its impurities in two different solvents. Refining by extraction can greatly remove the by-product ammonium sulfate and some water-soluble impurities. Extraction is always followed by water stripping, which can remove some lipid-soluble impurities. The extractants commonly used in industry include benzene, toluene and chlorinated hydrocarbons. Water is used as the solvent in the stripping process.
Wang Lin, et al.[9]investigated the optimal operating conditions for extraction by benzene, and found that the extraction process conducted under conditions covering a temperature of 45 °C, a pH value of 6.5, a crude caprolactam content of 76%—76.5%, and a benzene/caprolactam volume ratio of between 2.8:1 and 2.9:1 had the best extraction effect.
As for the extractor, the SINOPEC Baling Petrochemical Company used a new rotating disk extraction column for extraction[10]. Van Delden, et al.[11-12]investigated the hydrodynamic model of caprolactam extraction by toluene in a pulse disc column and experimentally verified the characteristics of the model.
Benzene and chlorinated hydrocarbons are very toxic,so new extractants were investigated in some studies.Wijtkamp, et al.[13]determined the phase equilibrium data ofn-heptanol-caprolactam-water-ammonium sulfate system. Van Bochove, et al.[14]determined the phase equilibrium data of 2-heptanone-caprolactam-waterammonium sulfate system. Based on these data, van Delden, et al.[7]experimentally investigated the extraction effects of low toxic extractants. They comprehensively evaluated many different extractants by determining:(1) the partition coefficients of extractants to caprolactam in 10% caprolactam aqueous solution at 20 °C;(2) the mutual solubility between extractants and water at equilibrium; (3) the composition of the equilibrium phase in the whole industrial operating range; and(4) the physical properties of extractants (density,viscosity, and interfacial tension), and found that higher polarity of the solvent or more polar solvent in the mixed solvent led to bigger partition coefficients of caprolactam in the extractants. They finally chose a 40% mixture of heptanol and heptane serving as the extractant and adopted an extractant-to-feed ratio of 3 in the extraction process and a feed-to-solvent ratio of 0.67 in the stripping process. Subsequently Van Delden, et al.[15]further investigated the equilibrium phase distribution of four impurities (cyclohexanone, aniline,N-methylcaprolactam and cyclohexanoformamide) in the above mixed extractant. The mixed extractant realized a 10% lower total impurity content than toluene used as the extractant.Van Delden, et al.[16]also investigated the hydraulic characteristics and mass transfer model of caprolactam extraction while using the mixed extractant in a pulse disc column. Gong Xingchu, et al.[17]chose a mixture of n-octanol (60%) and cyclohexane as the mixed extractant.Chen, et al.[18]investigated the caprolactam extraction by ionic liquids and found that the anion and cation effects were the main factors that could influence the partition coefficient of caprolactam.
On the whole, the extractant is generally selected in such a manner to ensure a high partition coefficient ratio of caprolactam and its impurities in the extractant and to reduce the mutual solubility between the extractant and the original solvent. In addition, in spite of low energy consumption in the extraction-stripping process,the energy is consumed mainly during the extractant recovery. Solvent usually is recovered by distillation, and the economics of calculation process should be taken into account during selection of an extractant for caprolactam refining.
2.2 Ion exchange
Ion exchange is mainly carried out between ions in solid ion exchanger and ions in dilute solution in order to extract or remove some ions from the solution. Ion exchange usually occurs after extraction-stripping in order to remove residual ammonium sulfate from the aqueous solution of caprolactam[19]. In some studies, ion exchange resins are also used for decolorization of crude caprolactam. A large amount of water and inorganic acids and bases are used during regeneration of ion exchange resins in order to increase the pressure in the subsequent wastewater treatment unit. Moreover, the ion exchange resin regeneration process is often a key step that can limit the efficiency of refining process.
Liu Houzhi and Liu Yaochi[20]studied the removal efficiency of four kinds of home-made ion exchange resins, which were widely used. They found that the ion exchange reaction was endothermic; the adsorption capacity of the resins increased with an increasing temperature, initial concentration, and contact time at 298—323 K; the cation exchange resin D001 had a maximum NH4+exchange capacity of 61.51 mg/g, and the anion exchange resin 717 had a maximum SO42-exchange capacity of 7640.4 mg/g.
In addition to the adsorption efficiency of impurity ions,the regeneration of ionic resins is mainly taken into account in this process. Zhang Yuxin, et al.[21]disclosed that chromatographic resin was introduced to the ion exchange system in order to solve the problem that could lead to easy contamination of anion exchange resins and difficulty in regeneration of these resins as referred to in their patent.
The decolorization by ion exchange resin is mainly based on the fact that some of the color impurities in caprolactam contain carboxyl groups, which are ionized by weakly alkaline aqueous solution of caprolactam leading to the exchange of carboxyl groups-containing impurities with anionic resins. Yuan, et al.[22]investigated the decolorization of caprolactam by ion exchange resin and found that the AMTX202 resin had excellent decolorization effect and the ion exchanged brownish black crude caprolactam solution became clear. In their subsequent study, Zhang, et al.[23]further investigated the decolorization by activated carbon.
2.3 Refining by hydrogenation
It is very difficult to remove some impurities from crude caprolactam by extraction and distillation, but it is very easy to remove them by distillation after hydrogenation of caprolactam[24]. Traditional hydrogenation process is carried out in a slurry bed reactor in the presence of noncrystalline nickel or Raney nickel catalyst, but this process has low hydrogenation efficiency and large loss of catalyst. The magnetically stabilized bed reactor developed by the SINOPEC Research Institute of Petroleum Processing is a reactor for hydrogenation in the presence of magnetic field under the action of magnetic particles-supported catalyst. Such reactor can improve the conversion efficiency and reduce the catalyst consumption. Meng, et al.[24]determined the optimum operating conditions of the magnetically stabilized bed reactor, including a temperature range of 80—100 °C,a pressure of 0.5—1.0 MPa, a liquid space velocity of 30—50 h-1, a hydrogen/caprolactam ratio of 0.7—1.5, and a magnetic field intensity of 15—35 kA/m, which could lead to an increase of PM value of caprolactam from 50 s to 2 000 s and a decrease of catalyst consumption by 60%.
In addition to nickel catalysts, Cheng Shibiao, et al.[25]introduced the use of palladium and oxidized rare earth as main catalyst at a reaction pressure range of 0.1—5.0 MPa and a caprolactam mass space velocity of 0.5—50 h-1mentioned in their patent. The crude caprolactam treated by the catalyst had a PM value of greater than 10 000 s and an extinction value of less than 0.05. Tu Chunyan and Cheng Shibiao[26]proposed the use of cerium modified palladium/activated carbon as the catalyst to refine crude caprolactam by hydrogenation and found that the 2%Pd-4%CeO2/activated carbon catalyst had very high catalytic performance, and could catalyze the hydrogenation of crude caprolactam, leading to an increase of caprolactam purity to 99.9955% and a permanganate value (PMs) of 24 000 s, indicating that this catalyst was more active than the 5% Pd/C catalyst.
2.4 Refining by distillation
Refining by distillation mainly is used to remove impurities, such as water, light and heavy components,etc., from crude caprolactam based on a difference in volatility between the caprolactam and its impurities.Caprolactam has a boiling point of 270 °C (under atmospheric pressure), which is often between the boiling point of light component and the boiling point of heavy component. To avoid deterioration of heated caprolactam during distillation, it is necessary to operate under vacuum, which would lead to an increase of energy consumption.
During distillation, an alkali solution is often added to crude caprolactam to react with some organic acid impurities to form salts, which can be easily separated from the heavy residue at the bottom of the tower[19].
2.5 Other refining technologies
In addition to the above-mentioned refining methods,some new refining methods are also referred to in some studies. These methods are briefly introduced here.
2.5.1 Membrane distillation
Membrane distillation is mainly used to remove water from the caprolactam-water system. A semipermeable membrane is used to remove water from caprolactam at different permeation rates. Although this scheme cannot thoroughly remove water, the membrane distillation can be used for removal of water from the caprolactam system with high water content in order to reduce the energy consumption in the distillation process. Generally speaking, the permeation rate and separation factor are negatively correlated during membrane distillation, so both the distillation efficiency and the separation effect should be taken into account during selection of semipermeable membrane.
Zhang, et al.[27]studied the process of water removal by using a cross-linked polyvinyl alcohol (PVA) membrane as the pervaporation membrane before removal of water from crude caprolactam by distillation. The pervaporation performance of crosslinked PVA membrane was further studied. The experimental data showed that the glutaraldehyde crosslinked PVA had good pervaporation effect, and 0.5% of cross-linked PVA membrane had better caprolactam-water separation effect, while the separation efficiency decreased with the increase of temperature and reached a peak value, about 1 650, at a water content of around 40%.
In their study, Zhu, et al.[28]used a semi-permeable membrane with glutaraldehyde crosslinked sodium alginate-polyvinyl pyrrolidone membrane serving as the top layer and polyacrylonitrile ultrafiltration membrane serving as the substrate to obtain a permeation rate of 1 634.4g/m2·h and a separation factor of 1 610.6 under conditions covering a polyvinyl pyrrolidone membrane content of 20%, a temperature of 323 K, and a caprolactam content of 50%.
Li, et al.[29]studied the effect of chitosan/poly(acrylic acid)composite membrane on pervaporation dehydration of caprolactam solution to obtain a separation factor of 7804 under conditions covering a polyacrylic acid content of 30%, a temperature of 313 K, and a caprolactam content of 70%. Konjac glucomannan crosslinked glutaraldehyde membrane for water removal from caprolactam achieved a maximum separation factor of 3 531[30]. For the 70% caprolactam-water system, the chitosan/konjac glucomannan mixed membrane had a separation factor of 3 000[31]. In the subsequent study, they further investigated the osmotic dehydration effect of composite nano-silica or titanium dioxide osmotic membrane on separation of the caprolactam-water system[32-33].
2.5.2 Crystallization
The crystallization process of liquid phase rearrangement product is often used in separation of the caprolactamwater system. Current studies focus on melt crystallization and evaporative crystallization.
Melt crystallization mainly is based on the difference in melting point between caprolactam and impurities.Current studies aim at removal of water. The melt crystallization process has low energy consumption due to the absence of any solvents, but needs relatively complicated melting crystallization equipment. N. C.Bouropoulos, et al.[34]studied the effect of different conditions on the suspension melt crystallization of caprolactam and found that the caprolactam crystals would have higher water content at a higher cooling rate and tend towards formation of aggregates in the suspension. The solid-liquid separation during suspension melt crystallization restricts its application[35]. Chianese,et al.[36]used a centrifuge for melt crystallization and employed the preheated nitrogen as the heat source for caprolactam sweating to remove 80% of the impurities at a relatively low molten solid content (20%). Guardani, et al.[37]investigated the static melt crystallization process,in which the layer growth rate reached a higher value and the sweating temperature could more significantly in fluence the separation effect on the caprolactam-water system. Based on the results of static melt crystallization,they built a melt crystallization model to calculate the number of separation stages needed to achieve the target purity.
Solvent evaporation and caprolactam crystallization occur concurrently during evaporation crystallization of caprolactam. Caprolactam crystal has low melting point(68 °C), evaporation and crystallization often occur under vacuum in order to control the crystallization temperature below melting point of caprolactam crystal[38]. Diepen,et al.[39]investigated this crystallization method and controlled the temperature in the crystallizer by controlling the evaporation rate of water. They determined the solid-liquid equilibrium phase diagram and the optimum evaporation conditions covering a water content of 5%, a pressure of 4.2 kPa, and a temperature of 53 °C.Shiqin Boyuan, et al.[40]disclosed a method for crystallization of the benzene-caprolactam solution by vacuum evaporation. This method could simplify the refining process by combining benzene distillation with caprolactam crystallization.
2.6 Other separation processes
The caprolactam refining process is not fixed. In some studies, some cases have proposed that the sequence of some steps is adjusted or some steps should be added.For example, Wu Jian, et al.[41]proposed the catalytic hydrogenation should be followed by ion exchange in order to improve the operating cycle of ion exchange resin. Lu Shijian, et al.[42]disclosed the refining method composed of alkali washing and dehydration followed by water extraction in their patent.
Another refining process, which has received an increased attention, is benzene distillation, in which the crude caprolactam after benzene extraction will be distilled directly without water stripping. After benzene removal, the crude caprolactam is distilled several times in order to remove water and various light and heavy components. DSM, BASF and Shiqin Boyuan[40]have published similar patents[43-45].
3 Refining by Gas Phase Beckman Rearrangement
Crude caprolactam obtained via the gas-phase Beckman rearrangement contains methanol, water, and various light and heavy components[46]. The distinct difference in caprolactam product obtained from the liquid phase rearrangement process is that the refining process of gas phase rearrangement products is greatly shortened without using extraction and ion exchange processes. The gas phase rearrangement process has been industrialized only by the Sumitomo Corporation of Japan, and its refining process mainly includes distillation, crystallization, and hydrogenation[47]. Xie Li, et al.[48]tried to refine the crude caprolactam by distillation, benzene dissolution, water extraction, ion exchange, hydrogenation, and distillation that are similar to liquid phase rearrangement, but the UV and VB values of the product did not meet the quality specifications.
3.1 Refining by distillation
The refining of gas phase Beckman rearrangement product by distillation often occurs at the beginning of the crystallization process. The distillation process can mainly remove water and some light and heavy components.Fan Yingqi, et al.[49]introduced a method for removal of heavy components from crude caprolactam, which was obtained via the gas phase Beckman rearrangement,and distillation. In this method, crude caprolactam after removal of water and light by-products is subject to distillation under an absolute pressure of 0—50 kPa and a tower kettle temperature of 100—200 °C to remove heavy components, while caprolactam is collected at the top of the tower. The heavy components in the tower kettle are further evaporated by a scraped thin film evaporator under conditions covering a temperature of 130—170 °C and a pressure of 10 kPa. The vapor is condensed before being re-circulated in the tower for removing the heavy components.
3.2 Refining by crystallization
Crude caprolactam refining via crystallization is based on the difference in partition coefficient between caprolactam and its impurities in liquid and solid phases. The impurity molecules are not easily embedded in the lattices, so the product refined by crystallization has a higher purity. The refining process of gas-phase Beckman rearrangement product by crystallization usually includes several steps.Usually the first step is cooling crystallization, to which fresh solvent needs to be added, followed by evaporating crystallization to ensure the yield of caprolactam[47,50].
The cooling crystallization process is used to refine caprolactam based on difference in solubility between caprolactam and impurities in the solvent. Guo, et al.[51]determined the solubility of caprolactam in isopropyl ether, methyl tert-butyl ether, n-butanol, isopropanol and n-propanol used as solvents at 5—40 °C, respectively, in order to provide a reference for cooling crystallization of caprolactam. Generally speaking, the solubility of caprolactam increases with an increasing polarity of solvent[52].
Shimazu, Yasumoto, et al.[46]introduced a method for refining of crude caprolactam through gas phase Beckmann rearrangement by direct cooling crystallization. Methanol, water, and some light and heavy impurities were removed from crude caprolactam by distillation before the melted caprolactam was directly mixed with cold solvent for rapid crystallization, with a crystallization residence time of 20—40 min. Cold solvent includes n-heptane or aliphatic hydrocarbons with greater polarity than n-heptane. The product was washed twice until the required purity was achieved. The cold solvent not only could achieve cooling crystallization of caprolactam, but also could dissolve some impurities in the liquid phase. Subsequently, the impurities were removed from caprolactam by solid-liquid separation.After crystallization, the mother liquor still contained residual caprolactam, and therefore must be concentrated by evaporation before going back to the crystallization unit. Due to the absence of cold wall in the crystallization process, the above patented method can effectively avoid scaling in the crystallizer. In this direct cooling operation,the temperature of the system is related to the amount of solvent added, i.e. a bigger amount of solvent needs to be added as required by a lower operating temperature, so an increase of yield in the cooling process at a decreased temperature needs to be calculated by solubility. In this patent, a relatively short residence time in the crystallizer always leads to more serious crystal nucleation, maybe resulting in solvent inclusion, difficulty in filtration, etc.In order to overcome the above problems, Tu Chunyan,et al.[53]developed a method of crystallization by addition of crystal seeds. This method aims at optimization of the grain size by controlling the crystallization process in the metastable zone to prevent the formation of polycrystalline nuclei. In this patent, aliphatic hydrocarbons were still used as solvents, which may lead to poor separation effect on some polar impurities.Therefore, Cheng Shibiao, et al.[54-56]subsequently invented the method for caprolactam crystallization by halogenated hydrocarbons or ether-containing mixed solvents. The use of these solvents provided a good refining effect by controlling the crystallization process in the metastable zone.
The above crystallization methods aim at refining by addition of solvent to crude caprolactam for cooling crystallization. Similar to refining by extraction, an increase of solvent consumption can improve the refining effect, but may increase the cost of subsequent solvent recovery, so it is necessary to calculate the energy consumption in practical use.
3.3 Hydrogenation
The process for hydrogenation of gas-phase Beckman rearrangement products also aims at removal of the unsaturated substances with properties that are similar to caprolactam. The Sumitomo Corporation disclosed the use of zeolite catalysts for hydrogenation of gaseous rearrangement product in a patent in 2005[57]. Cheng Shibiao, et al.[58]disclosed that the noncrystalline nickelbased catalysts also had good hydrogenation effect on caprolactam prepared by gas-phase Beckmann rearrangement reaction. Xie Li, et al.[48]pointed out that the optimized hydrogenation conditions included a reaction temperature of 90 °C, a reaction pressure of 0.7 MPa, and a stirring rate of 360 r/min in their paper.
4 Conclusions
With gradual expansion of domestic caprolactam production capacity, the caprolactam producers will compete more violently. As an important link of caprolactam production, the refining process will also affect the selection of overall production process.Although the liquid phase rearrangement process has higher selectivity, its refining process has such problems as long process scheme, serious pollution, etc. Compared with the refining process of liquid phase rearrangement product, the refining process of gas phase rearrangement product includes a crystal refining means, which makes the product with higher impurity content comply with the quality standard, and the refining process of gas phase rearrangement product is more energy-efficient and environmentally friendly. Therefore, the refining of gas phase rearrangement product by crystallization perhaps will become a subject of major concern in the future research.
杂志排行
中国炼油与石油化工的其它文章
- Optimization of Dividing Wall Column with Heat Transfer Process Across the Wall for Feed Properties Variation
- Numerical Simulation of Optimization of Mixing Tank for Residue Upgrading Reactor
- Biodegradability and Tribological Properties of Mineral Base Oil Enhanced by Caprylic Methyl Diethanolamine Phosphate Ester
- Enhanced Anti-Wear Property of Low Viscosity Engine Oil for Sequence IVB Engine Test Meeting the GF-6 Specification
- Study on Flue Gas Desulfurization Process with Selective SO2 Removal by N-formylmorpholine
- Research and Application of Image Recognition Technology in Microscopy Diagnosis of Catalytic Cracking Catalysts
