Imaging biomarkers for the treatment of esophageal cancer
2019-07-10KoichiHayanoGakuOhiraAtsushiHirataTomoyoshiAoyagiShunsukeImanishiToruTochigiToshiharuHanaokaKiyohikoShutoHisahiroMatsubara
Koichi Hayano, Gaku Ohira, Atsushi Hirata, Tomoyoshi Aoyagi, Shunsuke Imanishi, Toru Tochigi,Toshiharu Hanaoka, Kiyohiko Shuto, Hisahiro Matsubara
Abstract Esophageal cancer is known as one of the malignant cancers with poor prognosis.To improve the outcome, combined multimodality treatment is attempted. On the other hand, advances in genomics and other “omic” technologies are paving way to the patient-oriented treatment called “personalized” or “precision”medicine. Recent advancements of imaging techniques such as functional imaging make it possible to use imaging features as biomarker for diagnosis,treatment response, and prognosis in cancer treatment. In this review, we will discuss how we can use imaging derived tumor features as biomarker for the treatment of esophageal cancer.
Key words: Esophageal cancer; Computed tomography perfusion; Dynamic-contrastenhanced magnetic resonance imaging; Texture analysis; Diffusion-weighted imaging;Positron emission tomography
INTRODUCTION
Esophageal cancer is the seventh most common cancer, and sixth leading cause of death in the world[1]. Surgical resection is the only curative method for esophageal cancer, but is limited to early stage disease, and the recurrence rate after radical resection of esophageal cancer is reported to be approximately 50%, and most cases of recurrence occur within two years after surgery[2-4].
In this context, personalized or precision medicine, which enables the best choice of treatment based on certain biomarkers, is highly desirable, preventing side-effect and extra expenses, leading to more effective multidisciplinary treatments. Angiogenesis,tumor stroma, hypoxia, heterogeneity, and metabolism are known as typical biological features of malignancies, and these have been investigated to be biomarkers for diagnosis, prognosis, and treatment response. These biological features are usually investigated by the cell and molecular biology, but recent advances in imaging technique enable non-invasive assessment of various tumor functions, which have been investigated their biomarker value in malignancies. Imaging derived markers have the advantage of being non-invasive, spatially resolved and repeatable,compared to bio-specimen biomarkers which are obtained by removing a sample from a patient. Recent increasing interests in “Radiomics”, which is an emerging field that converts imaging data into a high dimensional mineable feature space using a large number of automatically extracted data-characterization algorithms, makes imaging derived biomarkers more valuable. In these contexts, this review will discuss how we can use various imaging derived biomarkers including perfusion analysis using computed tomography (CT) or magnetic resonance imaging (MRI), texture analysis, diffusion-weighted imaging (DWI), and positron emission tomography(PET) in terms of prediction of treatment response or prognosis to improve outcome of esophageal cancer patients.
PERFUSION ANALYSIS
Perfusion analysis using dynamic contrast-enhanced CT (DCE-CT/CT perfusion) and DCE-MRI (MR perfusion) can quantify tissue hemodynamics by measuring the temporal changes in tissue attenuation after administration of intravenous contrast media[5-7]. Since “angiogenesis” plays an important role in almost all types of cancer progression[8-10], quantification of vascular physiology using CT or MR perfusion techniques may reflect tumor angiogenesis, and has a potential to be a biomarker in cancer treatment[11]. These perfusion techniques are readily incorporated into the existing CT and MRI protocol, and enables in vivo quantification of tissue hemodynamics using the modeling tracer kinetics on the imaging workstation (Figure 1)[5,6]. In esophageal cancer, several previous papers reported associations of CT or MR perfusion parameters with treatment response to chemotherapy or chemoradiotherapy (Table 1). In MR perfusion studies, it was reported that higher Ktrans value (a parameter related to vessel permeability and tissue blood flow) before chemoradiation therapy (CRT) was associated with better treatment response[12,13]. In CT perfusion studies, Hayano et al[14]reported that higher blood flow of the tumor before CRT was associated with better treatment response and overall survival in esophageal squamous cell carcinoma patients. Makari et al[15]also reported that high tumor BF measure by CT perfusion might predict good response to neoadjuvant chemotherapy and CRT. Interestingly, all these reports suggested that a higher blood flow/perfusion of the tumor was associated with a better outcome of CRT or chemotherapy. It is reasonable because a higher tumor blood flow/perfusion leads to a better drug delivery and a higher oxygenation, resulting in better chemo- and radiosensitivity. There are a few paper reporting perfusion change in esophageal cancer during chemotherapy or CRT. Sun et al[12]demonstrated that the complete response group showed a significant decrease in Ktrans. Similarly, Hayano et al[16]reported that a significant decrease of blood flow in the tumor was observed from CT perfusion study of esophageal cancer, and they reported that patients with a greater reduction in tumor blood flow during CRT survived significantly longer than those with lower tumor blood flow reduction. It is speculated that the tissue fibrosis due to CRT leads to compression of tumor capillaries and increased flow resistance, results in decrease of blood flow/perfusion after CRT. In fact, it was reported that patients who achieved pathological complete response (pCR) after neoadjuvant CRT had tumors with lower blood flow than non-pCR[17].
Regarding relationship between CT/MR perfusion and angiogenesis, published results are controversial. For example, Chen et al[18]demonstrated that tumor blood volume measured by CT perfusion was significantly correlated with micro-vessel density in esophageal cancer, while Sato et al[19]reported that there was no significant correlation of tumor blood flow with the micro-vessel density in CT perfusion study of gastric cancer. Sato et al[19]speculated that blood flow assessed with perfusion imaging reflected only the functional vessels with a lumen, and not the functionless tumor vascularity; and therefore, micro-vessel density studied immunohistochemically in vitro using surgical specimens might be inadequate for “in vivo tumor vascular physiology. These factors may affect controversial results on relationship of CT/MR perfusion with immunohistochemically evaluated angiogenesis.
This perfusion technique using CT and MRI is very interesting and exciting technique with a potential to be a useful biomarker, but this technique is still considered a research tool in the realm of oncology. A consensus and standardization of data acquisition and analysis methods have yet to be established. The definition of the tumor region of interest (ROI) is subject to similar consideration, because the method used to draw the ROI clearly influences the perfusion parameters. Relatively high radiation dose and complicated procedure should be improved to be more common examination in clinical practice of esophageal cancer.
TEXTURE ANALYSIS
Analysis of texture within tumor on medical imaging such as CT, MRI, and PET,which reflects structural abnormality or heterogeneity in the tumor, is emerging as a potential biomarker to predict prognosis and treatment response in patients with cancer[20], because most malignant tumors show a striking amount of intratumor heterogeneity, which has implications for diagnosis, treatment efficacy, and the identification of drug targets[21]. There are various methods including statistical-,model-, and transform-based methods with various texture parameters[22]. Common texture parameters are entropy (a measure of irregularity), uniformity (a measure of uniform distribution of grey-levels), skewness (a measure of asymmetry of the histogram), kurtosis (a measure of peakedness and tailedness), and fractal dimension(a measure of complexity)[23-25]. Ganeshan et al[26]reported that tumor heterogeneity(uniformity) assessed on unenhanced CT was correlated with 18F-fluorodeoxyglucose(18F-FDG) uptake, and was an independent predictor of survival in 21 patients with esophageal cancers. Yip et al[27]reported that post-treatment uniformity and entropy of the tumor measured on contrast-enhanced CT were correlated with overall survival in esophageal cancer patients treated with CRT. In fractal analysis of PET imaging,Tochigi et al[28]reported that the low fractal dimension of tumor 18F-FDG uptake was associated with favorable survival, and they concluded that metabolic heterogeneity measured by fractal analysis can be a novel imaging biomarker for survival in patients with esophageal squamous cell carcinoma.
These texture analysis is a post-processing mathematical technique, which can apply to any medical imaging with no additional radiation exposure, special protocol,and cost, and maximizes the information obtained from current standard medical imaging (Figure 2). This technique still needs further investigation and standardization to be used in clinical practice, but has a potential to be a valuable clinical tool in the management of esophageal cancer.
DIFFUSION-WEIGHTED IMAGING
In 1905, Einstein described molecular diffusion or Brownian motion formally on the basis of the random translational motion of molecules[29]. Recent advances in magnetic resonance gradient technology have allowed acquisition of the apparent diffusion coefficient (ADC) value, which can be calculated by the DWI measurements acquired with a different gradient duration and amplitude (b-values)[30]. DWI has been discussed in terms of its biomarker value for cancer treatment in a consensus meeting,and a publication on consensus and recommendations for DWI as a cancer biomarker has been published highlighting the potential of this technique in the management of cancer patients (Figure 3)[31].
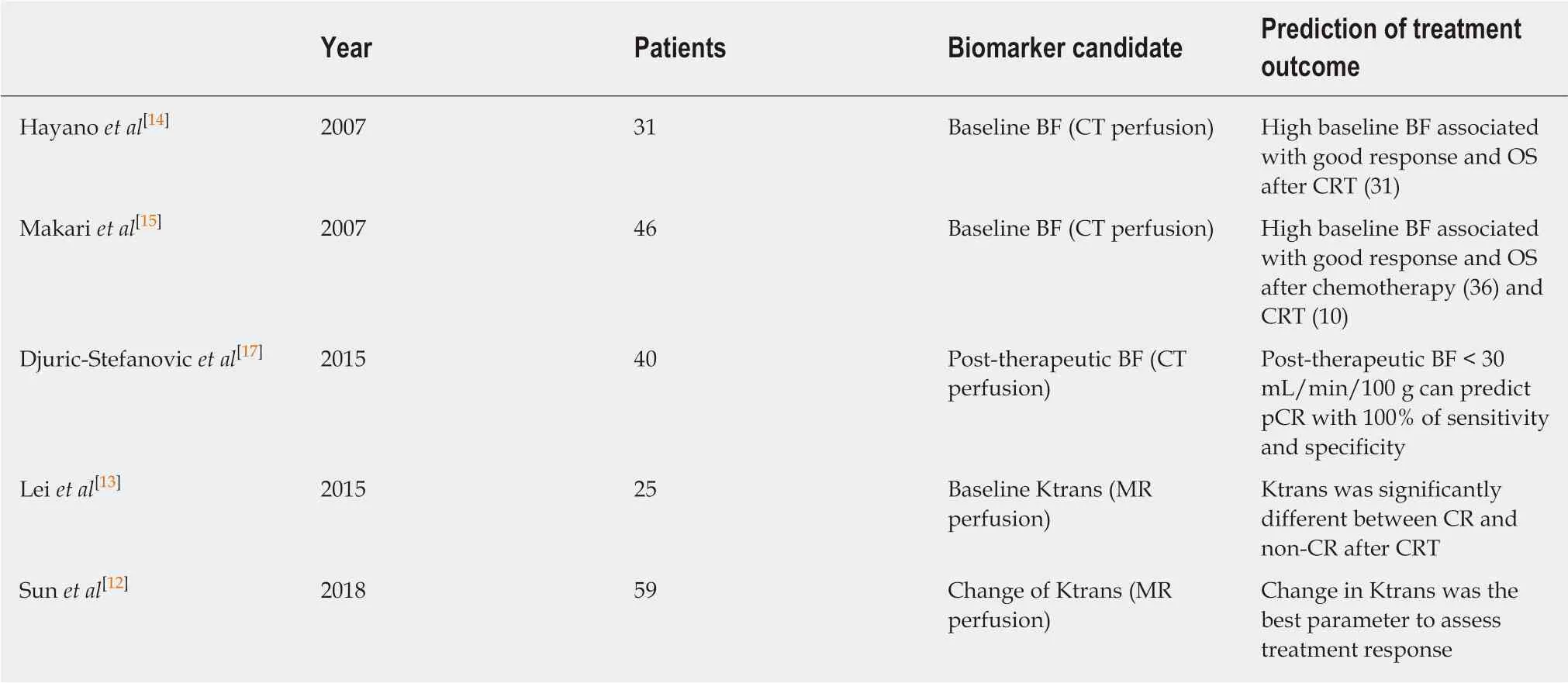
Table 1 Summary of reports on computed tomography or magnetic resonance perfusion in esophageal cancer
In esophageal cancer, there are seven papers evaluating DWI for prediction of CRT response and prognosis[32], but the results are controversial (Table 2). In 2011, Aoyagi et al[33]reported that higher baseline tumor ADC was associated with better survival.Another study also suggested that high baseline tumor ADC was associated with good response to CRT[34], while De Cobelli et al[35]reported that the high baseline tumor ADC was associated with poor response to CRT. Because of these conflicting results, it is still unclear whether pre-therapeutic tumor ADC can really predict response or survival after CRT. Cheng et al[32]performed meta-analysis, and reported that change of ADC and post-therapeutic ADC of the tumor were promising reliable and valuable predictor for the response to CRT, rather than pre-therapeutic ADC.Interestingly, Imanishi et al[36]reported that early increase of tumor ADC (> 15% after 20 Gy) could predict treatment response with 85% of accuracy and 100% of positive predictive value. Similarly, three studies suggested importance of post-CRT ADC and the change of ADC after 2-3weeks of CRT in terms of prediction of response to CRT in esophageal cancer[37-39].
Because DWI does not need radiation exposure and contrast agents, it can be an ideal biomarker. However, standardization of data acquisition and analysis methods have yet to be established for DWI. Low spatial resolution, especially in high b-value image, should be improved for accurate detection and quantification of the tumor.
POSITRON-EMISSION TOMOGRAPHY
PET is a quantitative imaging technique with use of various types of tracers. 18F-FDG,which can quantify glucose metabolism of the tissue, is the most common clinical PET tracer. Theoretically, malignant tumor cells exhibit strongly enhanced energy consumption, and lead to increased 18F-FDG uptake due to the increased number of glucose transporters and the increased hexokinase activity (Figure 4). The standardized uptake value (SUV) is generally used to quantify the tissue glucose metabolism, which has been reported its biomarker value in the treatment response and prognosis in various types of malignancies.
Regarding the biomarker value of pre-therapeutic PET in surgically treated esophageal cancer, Fukunaga et al[40]reported that a high SUV of the tumor before surgery had a poorer prognosis compared with those with low FDG uptake in esophageal cancer patients who received curative surgery without neoadjuvant therapy in 1998. After this paper had been published, seven papers on this subject were published[41-47], and all those papers suggested that high tumor SUV before surgery was associated with poor survival in surgically treated esophageal cancer (no neoadjuvant therapy)[48]. On the other hand, interestingly, pretherapeutic tumor SUV may not associate with survival in patients who received neoadjuvant chemotherapy or CRT[49,50], while the tumor SUV after neoadjuvant therapy can be a biomarker for survival. Swisher et al. reported that the tumor SUV after CRT is the most accurate test to predict survival in esophageal cancer patients (87% is adenocarcinoma) who were treated CRT followed by curative surgery[51]. Higuchi et al[52]also demonstrated that post-CRT SUV uptake in the tumor (cut-off 2.5) was the preoperative prognostic factor in esophageal squamous cell carcinoma patients who were treated neoadjuvant CRT or chemotherapy. Regarding change in tumor SUV during neoadjuvant therapy,early decrease (after 2 wk of neoadjuvant therapy) in FDG uptake is reported to be a predictive marker for response and survival[53-59]. However, some studies included patients with a wide range of disease (adenocarcinomas and squamous cell carcinomas, stage I through IV), and studies used different neoadjuvant treatment regimens.
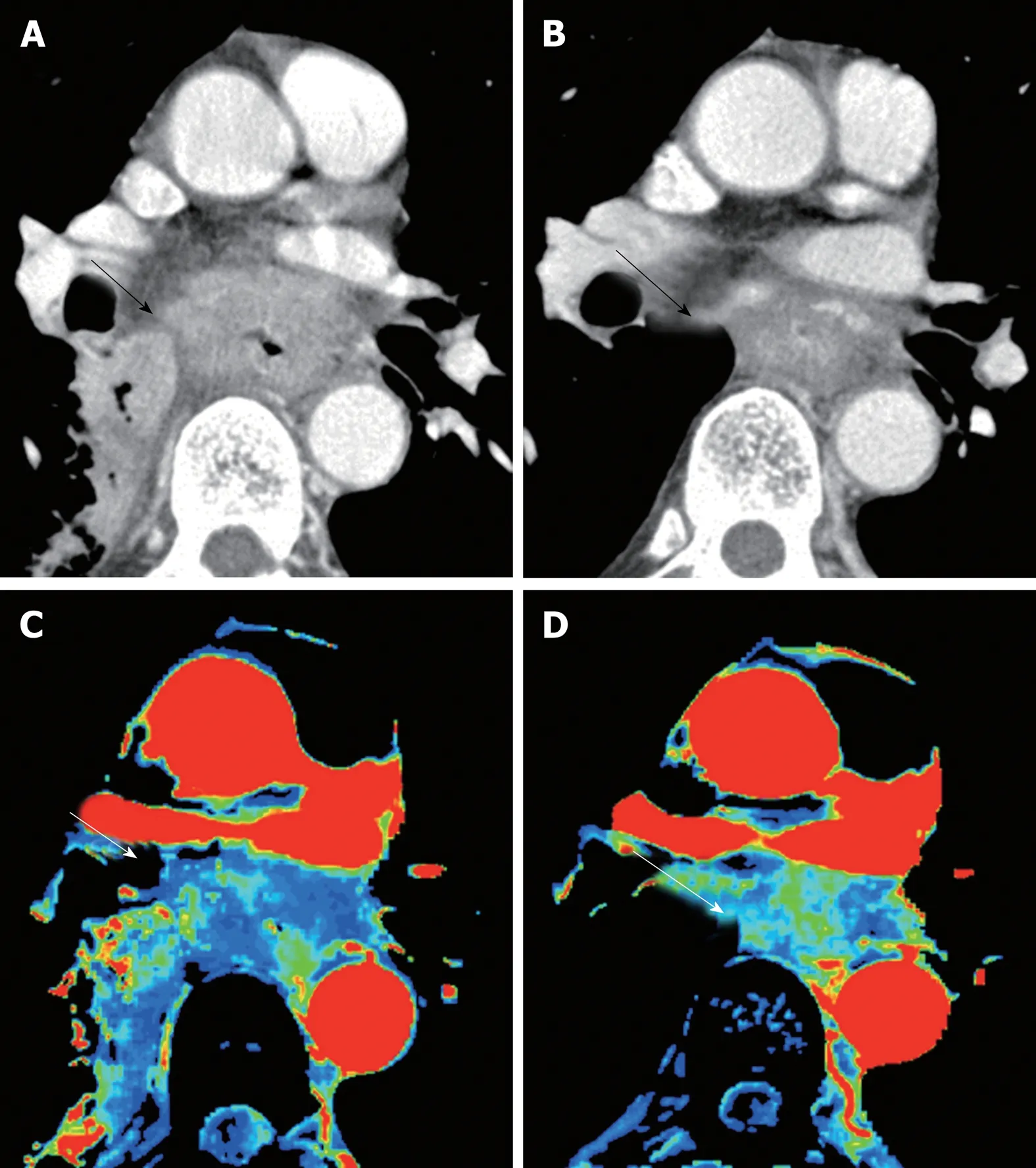
Figure 1 Perfusion change of esophageal cancer during chemoradiation therapy. A: Computed tomography(CT) image at baseline; B: CT image at post-chemoradiation therapy (CRT); C: Blood flow map by CT perfusion at baseline; D: Blood flow map at post-CRT. Baseline blood flow of this tumor was low, and this patient was diagnosed as no-responder. CRT: Chemoradiation therapy; CT: Computed tomography.
Nevertheless, FDG-PET seems to be served as a useful biomarker for treatment response and prognosis in various types of treatments, and we need further investigation with a large multicenter prospective trial to confirm usefulness of FDGPET in the management of esophageal cancer.
CONCLUSION
Ideal biomarker should be simple, non-invasive, reproducible, and widely available.Given the wide availability and the less invasiveness, imaging has a big potential to be an ideal biomarker. As we reviewed, various imaging biomarkers showed interesting results, and some of them are ready to use in clinical practice of esophageal cancer patients, which would provide patients more personalized and effective treatment, leading better outcome.
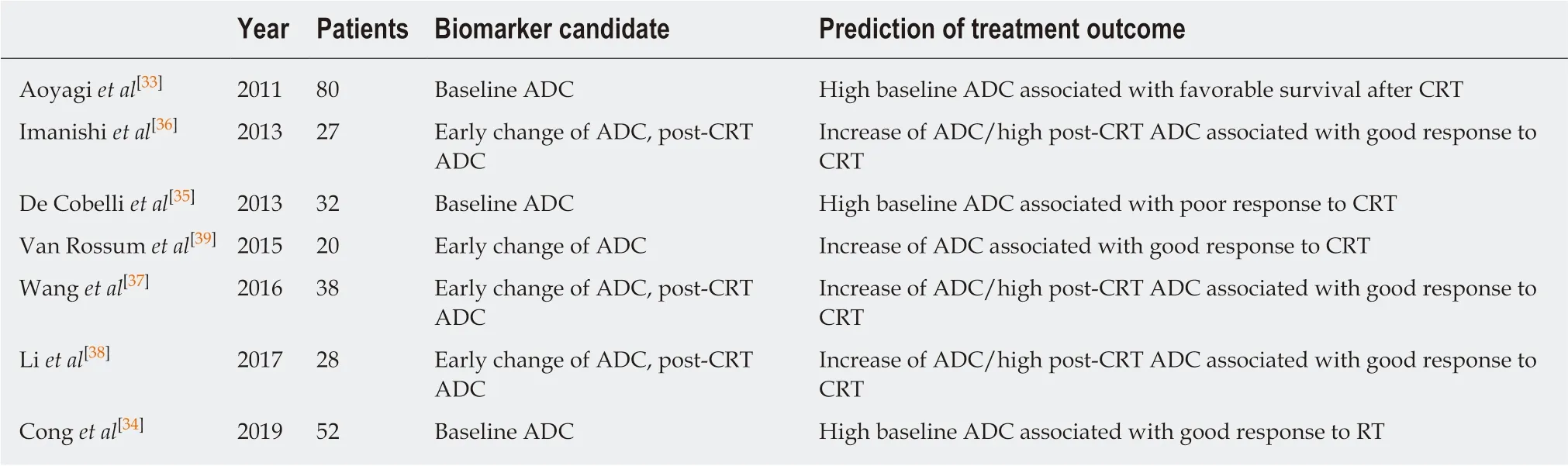
Table 2 Summary of reports on diffusion-weighted imaging in esophageal cancer
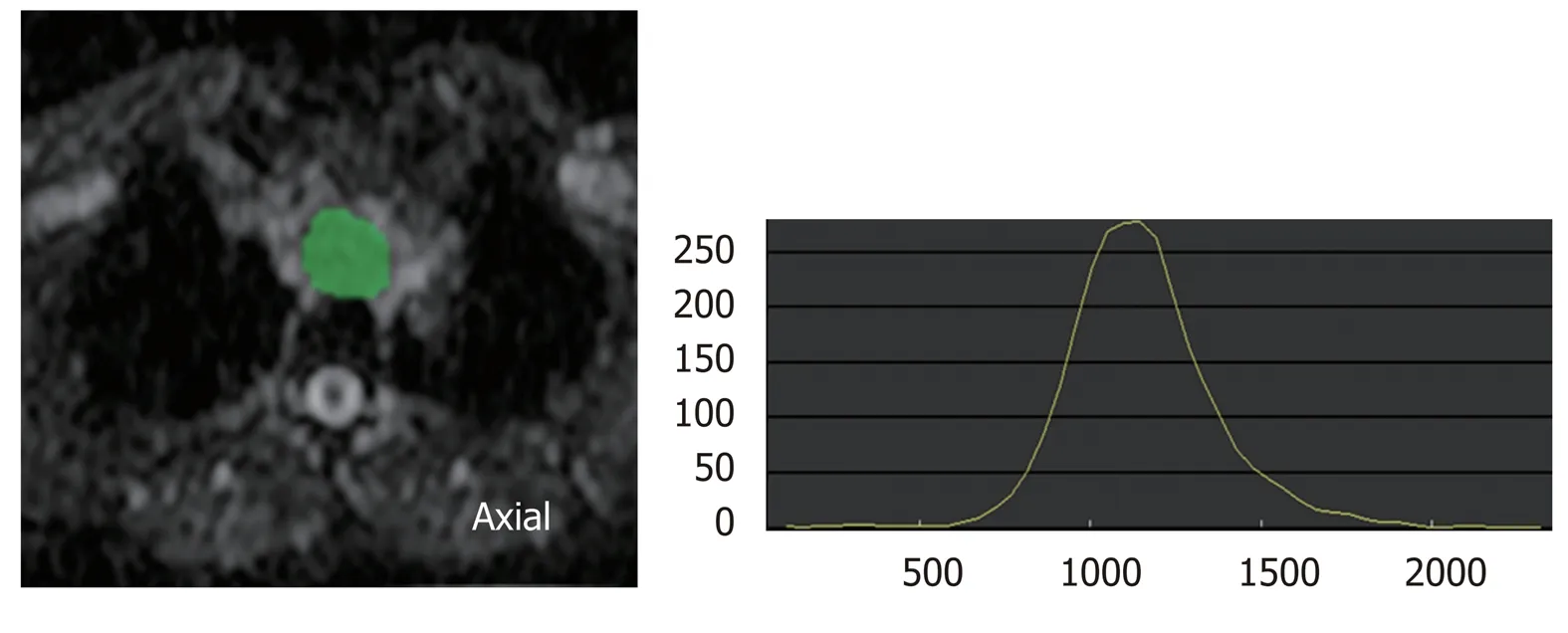
Figure 2 Histogram analysis of diffusion-weighted imaging. Histogram analysis is one of the texture analyses. This is the histogram analysis of apparent diffusion coefficient (ADC) map. Region of interest (ROI) for the tumor is drawn on ADC map, and distribution of pixels in the ROI is quantified as histogram parameters such as kurtosis and skewness. ADC: Apparent diffusion coefficient; ROI: Region of interest.
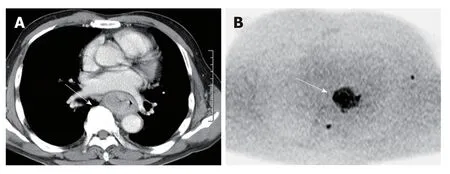
Figure 3 Advanced esophageal squamous cell carcinoma in contrast enhanced computed tomography image and diffusion-weighted imaging at b = 1000.A: Contrast enhanced computed tomography image; B: Diffusion-weighted imaging. The tumor showed conspicuous high signal intensity on high b-value diffusionweighted imaging.
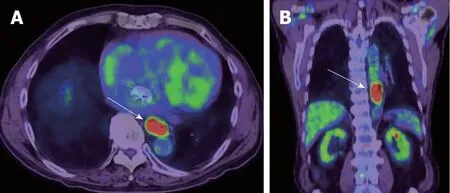
Figure 4 18F-fluorodeoxyglucose positron emission tomography image of esophageal cancer. A: Axial image; B: Coronal image. Malignancies exhibit strongly enhanced energy consumption, resulting in increased 18F-fluorodeoxyglucose uptake.
杂志排行
World Journal of Gastroenterology的其它文章
- Immunotherapy for hepatocelluiar carcinoma:Current and futrure
- Application of Big Data analysis in gastrointestinal research
- Biomarkers and subtypes of deranged lipid mettabolism in non-alcohlic fatty liver disease
- Development and in vitro study of a bi=specific magnetic resonance imaging molecular probe for hepatocellular carcinoma
- Effect of NLRC5 on activation and reversion of hepatic stellate cells by regulating the nuclear factor-κB signaling pathway
- Freeze-dried Si-Ni-San powder can ameliorate high fat diet-induced non-alcoholic fatty liver disease
