Immunotherapy for hepatocelluiar carcinoma:Current and futrure
2019-07-10MichaelJohnstonSalimKhakoo
Michael P Johnston, Salim I Khakoo
Abstract Hepatocellular carcinoma (HCC) arises on the background of chronic liver disease. Despite the development of effective anti-viral therapeutics HCC is continuing to rise, in part driven by the epidemic of non-alcoholic fatty liver disease. Many patients present with advanced disease out with the criteria for transplant, resection or even locoregional therapy. Currently available therapeutics for HCC are effective in a small minority of individuals. However,there has been a major global interest in immunotherapies for cancer and although HCC has lagged behind other cancers, great opportunities now exist for treating HCC with newer and more sophisticated agents. Whilst checkpoint inhibitors are at the forefront of this revolution, other therapeutics such as inhibitory cytokine blockade, oncolytic viruses, adoptive cellular therapies and vaccines are emerging. Broadly these may be categorized as either boosting existing immune response or stimulating de novo immune response. Although some of these agents have shown promising results as monotherapy in early phase trials it may well be that their future role will be as combination therapy,either in combination with one another or in combination with treatment modalities such as locoregional therapy. Together these agents are likely to generate new and exciting opportunities for treating HCC, which are summarized in this review.
Key words: Adoptive cell therapy; Cancer vaccine; Checkpoint inhibitor; Hepatocellular carcinoma; Immunotherapy; Liver cancer; Oncolytic virus
INTRODUCTION
Hepatocellular carcinoma (HCC) is the predominant form of primary liver cancer,constituting 75%-85% of cases. It presents a significant health burden as the sixth most commonly diagnosed cancer worldwide in 2018. In addition, reflecting its poor outcome, it was the fourth leading cause of cancer death[1]. The incidence of HCC varies country by country depending on the relative prevalence of key risk factors.These include chronic infection with either hepatitis B virus (HBV) or hepatitis C virus(HCV), as well as aflatoxin exposure[2]. These are more common in lower human development index countries. Although vaccination against HBV is recommended to reduce HCC development and has been used successfully in countries such as Taiwan, problems such as logistics of delivery and vaccine availability are significant factors which limit this approach[3].
HCV and HBV related cirrhosis are associated with the highest incidences of HCC.However, other aetiologies of cirrhosis, including non-alcoholic fatty liver disease(NAFLD), alcohol related liver disease and hereditary haemochromatosis are also strongly associated with an increased incidence of HCC[4]. Furthermore, any optimism about the revelatory impact new HCV drugs will have on HCC burden is forestalled by the global rise in NAFLD, type 2 diabetes mellitus and the “metabolic syndrome”as endemic risk factors for HCC development[5,6]. In particular the incidence of HCC is rising, particularly in countries with a high socio-demographic index, and consistent with this HCC may arise on the background of a non-cirrhotic liver in NALFD[7,8].
Surgery is the most successful treatment for HCC, either liver transplantation or liver resection depending on liver function, the presence of portal hypertension and tumour burden. Selection for surgery remains based upon Barcelona clinic liver cancer (BCLC) criteria for the most part, although “extended criteria” may be used by experienced centres[9]. Unfortunately given that liver cancer usually occurs on the background of cirrhosis, the residual liver post-resection still presents an environment predisposing to the development of subsequent tumours. Thus recurrence is a significant problem[10]. Transplantation obviates this concern to an extent by removing the background liver but patients may be outwith criteria for transplant at presentation or subsequently become ineligible while waiting for a suitable donor organ. Post-liver transplantation HCC recurrence appears to occur in 10%-20% of patients[11].
Locoregional therapy is the main alternative therapy depending on the stage of the underlying liver disease. This largely comprises two major types: (1) Percutaneous ablation such as microwave ablation or radiofrequency ablation (RFA); (2) Intraarterial chemoembolotherapy, namely transcatheter chemoembolization[9]. Ablation may even be a first line option over surgery in selected early stage tumours with comparable mortality rates, albeit higher recurrence rates[12]. Nonetheless, locoregional therapy is for the most part not curative treatment with recurrence being common.
Unfortunately the majority (> 70%) of patients present with advanced disease outwith the criteria for transplant, surgery or locoregional therapeutic options[13]. For these patients there remains a paucity of approved therapeutic options. Sorafenib is an oral multi-tyrosine kinase inhibitor (TKI), targeting a number of signaling pathways such as vascular endothelial growth factor (VEGF), and increasing median survival by 3 mo[14]. On this basis it is recommended as the standard first line systemic therapy for patients with Child-Pugh A cirrhosis and BCLC-C[9]. Another oral multi-TKI lenvatinib is now recommended as alternative first line therapy based on noninferiority to sorafenib[9,15]. Based upon survival benefits versus placebo in patients previously treated with sorafenib both oral multi-TKIs regorafenib and cabozantinib have been added as second line systemic therapeutic options[16,17]. Importantly, liver cirrhosis precludes the potential use of many cytotoxic drugs and so, combined with the resistance of HCC to a number of reagents, the development of prospective chemotherapy regimens has been relatively difficult[18].
In recent years cancer immunotherapy has seen a rapid expansion in terms of the number of agents which confer a prognostic benefit by awakening the immune system to mount a response against developing cancers, with particular success in metastatic melanoma[19]. Given the paucity of therapeutic options it is therefore logical that that these immunotherapeutic targets should be explored in HCC, particularly given the correlation between immunological findings and outcomes in HCC[20].Recently, nivolumab was added as the first Food and Drug Administration (FDA)approved immunotherapy for HCC[15]. This expansion in the therapeutic armoury is a welcome one. In this article we review the basis for immunotherapy in HCC, the agents studied to date as well as potential future developments.
LIVER IMMUNOBIOLOGY
Liver immunobiology
In addition to its many metabolic functions the liver has an important immunoregulatory role. Its dual supply of arterial and portal systemic blood makes it a unique recipient for gut pathogen exposure. This anatomy is combined with a honeycomblike vasculature of sinusoids densely laden with specialized immunocytes including macrophages (Kupffer cells), liver sinusoidal endothelial cells (LSECs), natural killer(NK) cells and innate T cells[21].
The LSECs account for roughly 50% of the non-parenchymal cells within the liver[22]. In conjunction with Kupffer cells and dendritic cells (DCs) one of their roles is to act as antigen presenting cells as part of the hepatic reticulo-endothelial system[23].Residing in the space of Dissé between the parenchymal cells and LSECs are hepatic stellate cells which contribute an immune sentinel role in this nuanced interplay[24].Further to the LSECs is an abundance of resident liver lymphocytes, including NK and innate T cells, which serve a number of roles including innate immune response against viruses, intracellular bacteria, tumours and parasites[25,26]. There is thus a rich effector population which needs to be responsive to pathogens, but also immunoregulatory when exposed to the non-pathogenic antigens that flood the liver via the portal vein. These include innocuous nutrient antigens, bacterial degradation products, damaged cells and of course pathogenic or toxic components. It is for this reason that the immune response within the liver requires such precise homeostatic control. The inherent immune tolerogenicity which the liver has developed to adapt to this unique environment of antigen exposure has been well described[23,27]. This manifest immunotolerant capacity is evident in the liver's relatively low rates of allograft rejection compared to other organ transplants[28,29].
Immunobiology in chronic liver disease
There are two aspects to immunobiology in the context of cirrhosis. One is that in cirrhosis there is an active immune-mediated inflammatory process and that as decompensation develops it becomes progressively systemic[30]. However, the precise nature of the immune activity in cirrhosis depends on the underlying liver disease.Combined with a dysregulated immune response that predisposes to infection, this has been elsewhere described as “cirrhosis-associated immune dysfunction”[31]. It is well established the predisposition to bacterial infection and this is most evident in acute-on-chronic liver failure[32,33]. Additionally, the structural damage of cirrhosis compromises reticulo-endothelial function leading to impaired immune surveillance[21]. However, immune dysregulation is also manifest in non-cirrhotic patients, with irregularities such as elevated levels of endogenous cytokines, and a pro-inflammatory environment especially in autoimmune liver disease and viral hepatitis[34].
HCC immunobiology
In the majority of cases HCC is associated with chronic liver disease and in particular cirrhosis. The underlying inflammatory process described above drives hepatocellular DNA damage, endoplasmic reticulum stress and subsequent necrosis of the hepatocyte which leads to regenerative nodular formation, dysplastic nodules and ultimately carcinoma[35]. HCV and HBV also drive an immune-mediated inflammatory response which promotes neoplastic change, the latter also mediating its carcinogenic properties via direct oncogenic transformation following incorporation into host cell DNA[36]. Furthermore, once HCC has developed the tumour can be associated with a rich immune cell infiltrate. Detailed analysis of HCCs indicated that approximately 25% have high inflammatory scores, with high or moderate levels of lymphocyte infiltration[37,38]. As one might expect, tumour infiltrating lymphocytes (TILs) form a large component in solid tumours, in an attempt by the host to mediate an antitumour reaction[39]. Unfortunately this cellular response can be dysfunctional with a higher proportion of CD4+ (helper or T regulatory cells) to CD8+ cells. This promotes immune tolerance and has been shown to confer a worse prognosis[40]. Additionally the innate immune system may be attenuated as evidenced by the hypofunctionality of NK cells in HCC[41,42].
However, although TILs can be identified, within the tumour microenvironment in cirrhosis, they often prove insufficient to control tumour growth[43]. Expansion of myeloid derived suppressor cells (MDSCs) as well as Tregs appears to further enable the evasion of tumour cells from immune detection[44]. This creates an immunosuppressive immune environment through the secretion of transforming growth factor (TGF-β). In addition there are multiple mechanisms of immune evasion including secretion of other immunoregulatory cytokines such as interleukin-10 (IL-10), downregulation of ligands that activate immune cells including MHC class I and NKG2D ligands and expression of ligands that directly inhibit lymphocytes, including both T cells and NK cells[45-48]. Thus HCC is a challenging environment for the immune system. Nevertheless, immunotherapy is one of the most promising avenues for future therapies.
CURRENT AND FUTURE IMMUNOTHERAPEUTIC STRATEGIES IN HCC
Current approaches of immunotherapy were shown in Figure 1. Current immunotherapeutic strategies are based on two fundamental principles: (1) The ability to unmask current immune responses; or (2) The need to stimulate new or different immune responses. Unleashing current immune response relies on there being a preexisting immune reactivity to cancer which is being held in check by microenvironmental factors, such as inhibitory receptors on T cells especially programmed cell death protein 1 (PD-1) and cytotoxic T-lymphocyte associated antigen 4 (CTLA-4),or alternatively immunosuppressive cytokines such as TGF-β. Checkpoint inhibitors fall within this category, and importantly for these therapies to work the precise molecules that the cells are targeting do not need to be known. Conversely, antibodies that directly target molecules expressed on HCC, such as alpha-fetoprotein (AFP) or glypican-3 (GPC-3) are within the second category. These strategies can be enhanced by coupling these antibodies to effector cells, such as T cells or even NK cells. Vaccine therapeutics and the use of oncolytic viruses, discussed below, may straddle these two mechanisms by unmasking pre-existing and inducing de novo T cell responses to antigens expressed by HCC. Additionally, tumour ablation liberates antigens into the periphery and can augment CTL responses, that have been correlated with survival[49-51].
Checkpoint blockade
A rapidly growing list of blocking antibodies to immune checkpoints has been approved by the FDA in recent years for cancer treatment. In general, these are thought to be most effective for tumours with a high mutagenic load, such as melanoma[52]. Although these have been in trials for some time, it was not until recently that the first of these checkpoint inhibitors was approved for use in HCC,when the PD-1 inhibitor nivolumab (Opdivo®) gained FDA approval (Table 1).
Programmed cell death protein 1:PD-1 is a cell surface protein expressed on an extensive number of immune cell types, predominantly CD8+ T cells but also CD4+ T cells, B cells, NKs, Tregs, MDSCs and DCs[53-55]. It is upregulated following activation of T cells and when it binds to PD-L1 (or PD-L2) on target cells this inhibits effector T cell responses. Therefore, blocking its action is an attractive target of immunotherapy.Nivolumab's grading as an approved second line therapy for HCC is supported by evidence from the CheckMate040 trial. This was a phase I/II, open label, noncomparative, dose escalation and expansion trial in advanced HCC of mixed underlying chronic liver diseases (n = 262)[56]. 46 (96%) of 48 patients discontinued treatment in the dose escalation phase, 42 (88%) due to disease progression. However,the objective response rate was 20% in the dose-expansion phase. Incorporation of nivolumab into the AASLD guideline on HCC as second line systemic therapy was made in advance of the first phase III trial results on the basis of CheckMate040[15].CheckMate459 (NCT02576509), is a phase III, randomized, open label trial of nivolumab versus sorafenib which has closed to recruitment and results are awaited at the present time.
There are a number of registered phase III trials looking at PD-1 checkpoint blockade. The ORIENT-32 study (NCT03794440) is a randomized, open-label,multicentre trial in China randomizing patients to a combination of sintilimab (PD-1 inhibitor) and bevacizumab (anti-VEGF antibody) versus a control arm of sorafenib.The RATIONALE-301 study (NCT03412773) is a phase III trial randomizing patients to the PD-1 inhibitor tislelizumab monotherapy versus sorafenib. Pembrolizumab(Keytruda®), another IgG4 isotype antibody targeting the PD-1 receptor of lymphocytes, has been similarly studied as monotherapy. In the 104 patients enrolled in the open-label, phase II trial KEYNOTE-240 (NCT02702401) there were mixed results. An objective response in 17% of patients (complete in 1%, partial in 16%) was offset by serious treatment-related adverse events in 15%, including 1 death associated with ulcerative oesophagitis attributed to treatment[57]. However, based on the promising response rates pembrolizumab was granted accelerated approval for HCC. Unfortunately Merck and Co. has recently announced that the subsequent phase III trial of pembrolizumab versus placebo did not meet its co-primary endpoints of overall survival (OS) and progression-free survival in patients with advanced HCC[58]. These results, although disappointing, would appear to be consistent with the opinion that checkpoint blockade may well be most efficacious as combination therapy[54,59]. Combination of PD-1/PD-L1 blockade may be with VEGF inhibition (NCT03794440, NCT03713593, NCT03764293, NCT03434379), as well as with locoregional treatment or resection (NCT03847428, NCT03755739), or indeed with another checkpoint inhibitor. However, care needs to be taken as combination therapy with checkpoint inhibitors can lead to higher rates of side-effects including an immune-mediated hepatitis[60].
Cytotoxic T-lymphocyte associated antigen 4:CTLA-4 is another membrane bound molecule which keeps the immune response in check. It has a multifaceted role,actively competing for binding to the co-stimulatory molecule CD28, and leading to increased secretion of the immunoregulatory cytokine IL-10, as well as serving as a key mediator by which regulatory T cells (Tregs) dampen immune response[61,62].Inhibition of CTLA-4 is associated with improved clinical outcomes in other malignancies such as metastatic melanoma. In 2011, ipilimumab (YERVOY®) was the first checkpoint inhibitor approved by the FDA. Reports of therapeutic CTLA-4 blockade in HCC have also shown promise. In 2013 there was a reported phase I trial of 20 patients with advanced HCC and a background of HCV who received the CTLA-4 inhibitor tremelimumab[63]. Partial response was 17.6% and disease control was 76.4%. Time to progression was 6.48 mo (95% confidence interval 3.95-9.14).Although intense elevations in transaminases were common, particularly after first dose, no course of steroids were required for hepatotoxicity. The randomized phase III HIMALAYA trial (NCT03298451) is recruiting patients for randomization to a combination of CTLA-4 inhibitor tremelimumab and PD-L1 inhibitor durvalumab versus sorafenib. It leads on from the early clinical data of 40 patients enrolled in the phase I trial of durvalumab and tremelimumab in advanced HCC[64]. Patient selection for combination checkpoint blockade will no doubt be key with 20% of patients having at least one grade 3 adverse event.
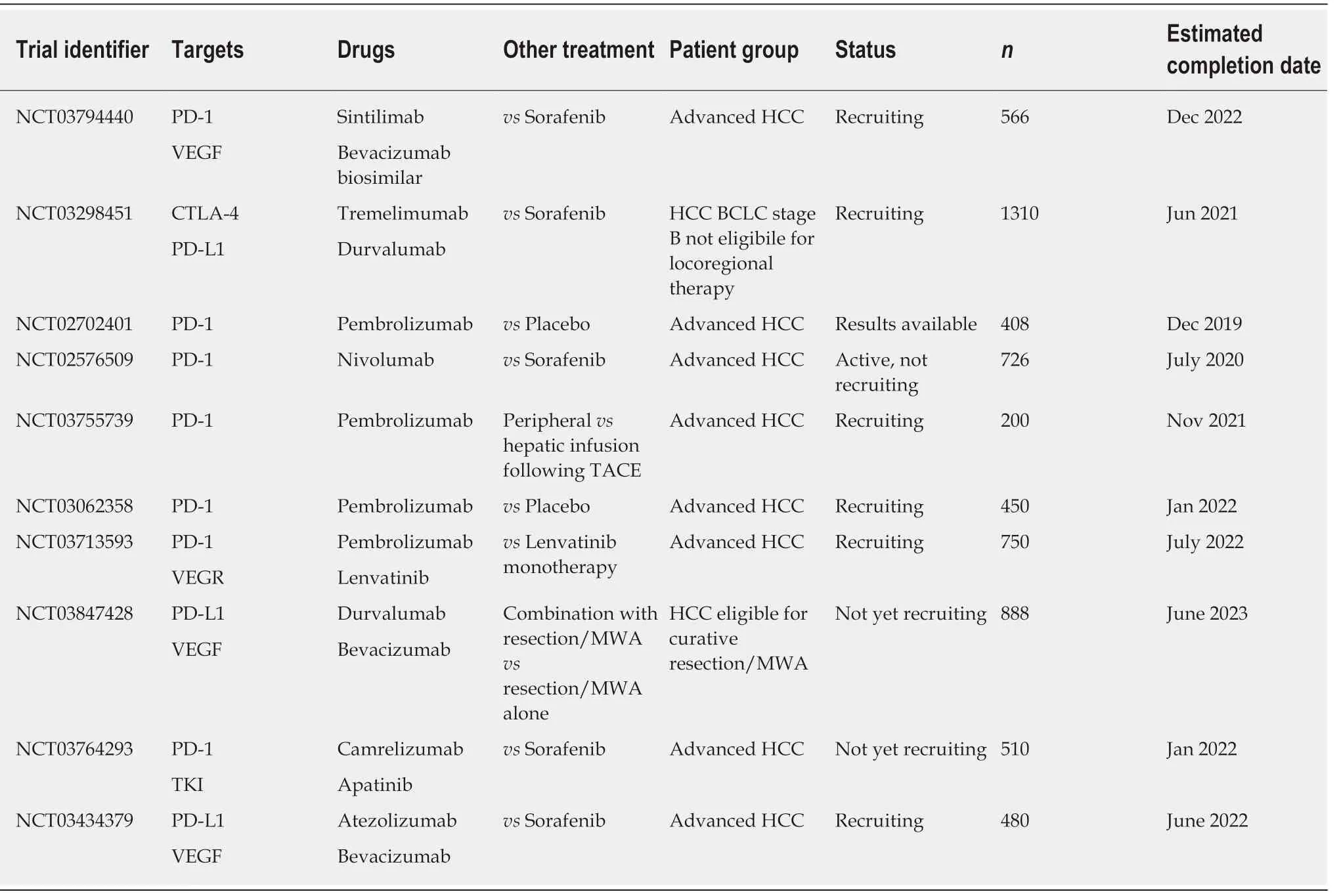
Table 1 Phase lll trials of checkpoint inhibitors
Furthermore, the combination of checkpoint blockade with locoregional therapy is attractive, with the potential for CTLA-4 inhibition to uncouple the systemic immunogenic response which occurs with tumour necrosis. A phase II trial which enrolled 32 patients (predominantly with HCV) treated with tremelimumab and followed by subtotal RFA or chemoembolization, demonstrated that of the 19 patients with lesions evaluable 5 of them (26%) showed partial response[65].
T-cell immunoglobulin and mucin-domain containing-3:TIM-3 is another transmembrane protein which is known to be expressed on CD4+T Helper 1 cells and CD8+cytotoxic cells[66]. Initially identified due to a putative pathogenic association with autoimmune disease, interest in this as a therapeutic has grown due to its role in the ability of tumour cells to evade immunosurveillance. A propensity of CD8+ cells to co-express both PD-1 and TIM-3 seems to contribute to the dysfunctional phenotype of CD8+ T cells[67,68]. We await with interest a phase II trial of dual blockade of anti-TIM-3 and PD-1 in HCC (NCT03680508) which has not yet begun recruitment.
Transforming growth factor-β:TGF-β is a membrane bound molecule expressed on and associated with a Treg subset which suppresses CD4+ T cell response in tumour tissue, promoting progression, in both murine models and HCC patients[69]. Coexpression of PD-1 on these CD4+CD69+ Tregs makes for another potential combination therapy. Results are awaited of a phase I trial assigning patients in parallel to both the anti-TGF-β monoclonal antibody NIS793 and PD-1 inhibitor spartalizumab (NCT02947165) due for completion in April 2021.
Lymphocyte activation gene 3:Closely related to CD4, lymphocyte activation gene 3(LAG-3) is a membrane protein that binds the same ligand, MHC-II[70]. Not only do these proteins suppress T cell activity and cytokine release, but they are also of considerable interest due to their upregulation in T cell exhaustion in the context of chronic viral infection or cancer[71,72]. The synergistic effect of LAG-3 with PD-1 to induce tumour regression raises another further potential combination therapy[73].Although engineered LAG-3 binding therapy for solid tumours remains in early phase trials, given its significant upregulation in tumour infiltrating CD8+ T cells of HCC patients, its potential in liver cancer is eagerly awaited[74].
Adoptive cell transfer
In contrast to the active augmentation of immune response seen with checkpoint inhibition therapy, adoptive cell transfer aims to improve HCC outcomes by passively administering autologous lymphocytes following ex vivo cultivation[75]. This is a longstanding therapeutic strategy starting over 30 years ago, with infusion of TILs leading to improved responses in metastatic melanoma[76]. The broad cell subsets that have been studied in HCC to date include NK cells, cytokine-induced killer (CIK) cells or TILs, and finally chimeric antigen receptor T cells (CAR-T cells).
The first of these, NK cells, form as much as 50% of innate immune cell rich infiltrate within the liver[26]. With their ability to kill cells without prior activation or priming they are best known for forming part of the host defence against infection and tumour development[26]. In a murine model expanded NK cells exert a significant cytotoxic effect against HCC cells, reducing tumour growth and improving OS.Furthermore, they enchanced the effect of sorafenib in the same study[77]. Although clinical data on use is limited, there has been a successfully conducted phase I trial in patients with liver cirrhosis with HCC undergoing liver transplantation. NK cells derived from donor liver perfusate, stimulated with IL-2 and administered showed upregulation of peripheral NK cell cytotoxicity and no adverse events[78]. We await ongoing trials of high affinity NK cells versus sorafenib (NCT03563170) and combination therapy of NK cell transfer with irreversible electroporation (IRE) vs IRE alone (NCT03008343).
Next are the CIK cells which represent another novel immunotherapeutic option.By incubating peripheral blood monocytes with cytokines including IL-1, IL-2, IFN-γ and a monoclonal antibody against the T cell marker CD3, these cells show a significant inhibitory effect on tumorigenesis[79]. These MHC-unrestricted cytotoxic lymphocytes are made up of a heterogeneous group of efficient cytotoxic effector cells comprising predominantly CD3+CD56+ T cells, and some CD3-CD56+ NK cells.Trials into reinfusion of CIK cells have predominantly been studied as an adjunctive therapy following surgical resection, with a theoretical base in murine models showing an effect of these cells on micrometastases[80]. Early trials randomizing postcurative resection patients to adjuvant CIK cell therapy or no adjuvant showed promising results with a significantly reduced risk of recurrence, but without an improvement in OS[81,82]. The largest study to date, involving 230 patients, was a multicenter, randomized, open label phase 3 trial studying CIK cell therapy as adjuvant to RFA, ethanol injection or curative resection. This showed an improvement of 14 mo in recurrence free survival[83]. A systematic review and meta-analysis of CIK cell therapy in HCC in Asia reached similar conclusions that in selected patients,progression free survival and recurrence free survival are improved[84].
Antigen specific T cells have also been studied. These include native TILs and also CAR-T cells. A phase I trial studied administration of autologous TILs in 15 patients with HCC post-resection. This showed successful expansion in 88% and there were no serious adverse events (SAEs) reported[85]. The incorporation of a chimeric antigen receptor into T cells to modulate their antigen selectivity and signaling offers another exciting prospect for immunotherapy in HCC. Although discovered 30 years ago,CAR-T cell therapy for HCC remained relatively in its infancy until more recently[86].The FDA approved the first two CAR-T cell therapies Kymriah®and Yescarta®for lymphoma in 2018 and 2017. A plethora of trials into solid tumours have followed in parallel with these breakthroughs in lymphoma.
HCC has a number of tumour associated antigens (TAAs). Selection of an appropriate antigen for CAR-T cells is integral to their success as a prospective immuno-therapeutic option. Given its high expression and association with poor prognosis in HCC, GPC-3, a member of the glypican family, has been a natural target antigen to study[87,88]. There remains one published phase I trial of 13 patients, 8 of whom had lymphodepletion with fludarabine and cyclophosphamide. These were patients with advanced HCC, portal vein invasion or extrahepatic metastases. This has only been published in abstract form to date, but no dose limiting toxicity was identified and there was one SAE of grade 3 fever found[89]. We await the published results of a further phase I clinical trial (NCT02723942) that was completed in 2017.There are currently five Phase I/II trials recruiting, four of which examining GPC-3 and one EpCAM (NCT03198546, NCT03130712, NCT02715362, NCT03013712,NCT02723942). AFP is another potential target TAA that is being explored[90,91].Unfortunately the propensity of AFP to be found on healthy hepatocytes has stymied its potential as a target antigen of CAR-T cell or other targeted immunotherapies.
Vaccines
Tumour vaccines are agents which increase specific immune responses to tumour antigens. Registered clinical trials for such tumour vaccines in HCC are currently relatively few compared to those studying adoptive cellular therapies and checkpoint inhibitors, in part because of previously disappointing trial results, and also relative lack of efficacy of other tumour vaccines. This may be related to the previous difficulty in identifying the correct tumour antigens, which has now become possible through recent technological breakthroughs allowing massive parallel DNA sequencing. Thus, priming an immune response whether in isolation, or more likely in combination with an immune modulator remains an attractive therapeutic strategy for HCC. A number of agents have been examined to date with regards to this.
Dendritic cells: DCs are professional antigen presenting cells, responsible for a multitude of tasks, including absorption, processing and presentation of TAAs.Allogeneic DCs form one broad subset of vaccines by providing both the antigen and the secondary co-stimulation required to prime an effective T cell response. Isolating DCs from peripheral blood, expanding them ex vivo and stimulating with cytokines such as granulocyte-macrophage colony-stimulating factor (GM-CSF) produces primed DCs for reinfusion. The injection of these cells to induce recruitment of effector cells and provoke a cascade of tumour lysis and further TAA release, is another attractive, targeted mechanism[92]. A number of techniques may be employed to optimize this TAA priming and enhance the efficacy of the vaccine. DCs can be transduced with DNA or RNA encoding known TAAs, or they may be incubated with tumour lysate or fusion of DCs and tumour cells[93].
A recently published phase I trial studied intra-tumoral injection of ilixadencel(pro-inflammatory allogeneic DCs stimulated by GM-CSF and IL-4) either as monotherapy or in combination with sorafenib in 17 patients. The primary objective was to evaluate tolerability. Only one grade 3 adverse event was recorded. 73% of the 15 evaluable patients demonstrated increased tumour specific CD8+ T cells in peripheral blood, suggesting a successful immune provoked response at least[94].
Peptide vaccines:Peptide vaccines constitute an alternative option in terms of generating an effective immune reaction. However, although there has been success in terms of immunological surrogates such as generating GPC-3 reactive cytotoxic T lymphocytes in one phase I trial, this has not translated into clinical successes[95].Despite a plethora of TAAs identified in HCC only trials utilizing AFP, GPC-3 and MRP3 have shown any success inducing a T cell response rate over 70%, with other TAAs such as SSX-2, NY-ESO-1, hTERT and MAGE-A all inducing much lower rates[96].
Oncolytic viruses:A more recent development in the arena of tumour vaccines is the use of oncolytic viruses. These therapeutically useful viruses are targeted to preferentially replicate in cancer cells. To date they have been predominantly introduced by intra-tumoral injection. The modified poxvirus JX-594 remains the lead oncolytic virus of interest in clinical trials with regards to HCC. As an immunotherapeutic agent it piqued considerable interest when it conferred a doserelated survival benefit (median of 14.1 mo compared to 6.7 mo) in a phase II dosefinding trial of 30 patients[97]. The global, randomized, open-label, phase III study of Pexa-Vec (JX-594; an oncolytic vaccinia virus which selectively targets cancer cells) is currently recruiting patients with advanced HCC to two arms of vaccination with sorafenib vs. sorafenib alone[98]. We eagerly await the results of this particularly as a combination therapy.
CONCLUSION
Immunotherapy for HCC is still in its infancy compared to other tumours.Encouraging results with PD-1 inhibitors are emerging, and prospects for combination therapies arising. This makes immunological sense, as the immune system is a multi-faceted and integrated effector system. Optimising this response is challenging, especially because of the immune environment on which HCC arises,and the challenges of treating an individual with cirrhosis, which substantially decreases the therapeutic index of these agents. Nevertheless, the massive interest in immunotherapy, gives hope that better combinations of drugs will be found to treat this challenging disease.
杂志排行
World Journal of Gastroenterology的其它文章
- Application of Big Data analysis in gastrointestinal research
- Biomarkers and subtypes of deranged lipid mettabolism in non-alcohlic fatty liver disease
- Imaging biomarkers for the treatment of esophageal cancer
- Development and in vitro study of a bi=specific magnetic resonance imaging molecular probe for hepatocellular carcinoma
- Effect of NLRC5 on activation and reversion of hepatic stellate cells by regulating the nuclear factor-κB signaling pathway
- Freeze-dried Si-Ni-San powder can ameliorate high fat diet-induced non-alcoholic fatty liver disease
