Endoscopic ultrasound-guided biliary drainage: A change in paradigm?
2019-06-11EnLingLeungKiBertrandNapoleon
En-Ling Leung Ki, Bertrand Napoleon
En-Ling Leung Ki, Bertrand Napoleon, Department of Gastroenterology, Jean Mermoz Private Hospital, 55 avenue Jean Mermoz, Lyon 69008, France
Abstract
Key words:Endoscopic ultrasound-guided biliary drainage; Endoscopic ultrasound-guided choledochoduodenostomy; Endoscopic ultrasound-guided hepaticogastrostomy; Lumenapposing metal stents; Electrocautery-enhanced lumen-apposing metal stents
INTRODUCTION
Endoscopic retrograde cholangiopancreatography (ERCP) is the current first-line approach for drainage of malignant biliary obstruction (MBO)[1-3]. Although success rate is high, difficult cannulation or access due to surgically altered anatomy, prior duodenal obstruction or stenting, periampullary diverticulum, and large tumors account for a failure rate of 5%-10%[1-3].
Percutaneous transhepatic biliary drainage (PTBD) is the conventional salvage procedures for failed ERCP. However, it is associated with significant morbidity,discomfort, and re-interventions[1-3].
ERCP and PTBD have proven their usefulness over 40 years of experience. Since the first report by Giovanniniet al[4]in 2001, endoscopic ultrasound-guided biliary drainage (EUS-BD) has been developed as an alternative means of biliary drainage.Several methods have been described. Rendez-vous technique and antegrade stenting(AGS) are alternative means to achieve trans-papillary drainage. However,choledocoduodenostomy (EUS-CBD), and hepaticogastrostomy (EUS-HGS) are newer approaches which achieve extra-papillary drainage by trans-mural stenting.
Initially, EUS-BD was considered an advanced technique performed by experts in referral centers. Significant morbidity limited its indications despite its efficacy.
The last published guidelines accept the following indications for EUS-BD drainage[1-3,5]: (A) failed ERCP performed by a referral center with high expertise; (B)altered anatomy or malignant obstruction precluding papillary access; (C) failed cannulation due to occluding tumor; and (D) contraindication to percutaneous access such as large volume ascites.
Expert consensus and guidelines agree that specialized pancreaticobiliary endoscopists should perform EUS-BD[1-3,5]. Surgical and interventional radiology back up must be available due to potential severe adverse events (AE).
With the growing experience in EUS-BD and new EUS specific tools, overall improvement in efficacy, and safety are apparent. A growing body of evidence suggests that EUS-BD may not only be feasible as salvage to failed ERCP but also as a first-line technique for biliary drainage in MBO[6]. Compared to ERCP it confers two important theoretical advantages: (1) it avoids papillary trauma and subsequent risk of pancreatitis; and (2) it does not traverse the malignant stricture hence reducing the risk of tumor ingrowth that ultimately leads to stent dysfunction and re-intervention.
Our review aims to present the evolving data on EUS-BD that could potentially change the current algorithm by making it the first-line technique for biliary drainage.As data in benign conditions remains scarce and its role is uncertain[7], we will focus on extra-papillary drainage in MBO.
TECHNIQUES, EFFICACY AND ADVERSE EVENTS OF EUSBD
Techniques and material
EUS-BD can be performed through intra-hepatic (transgastric-transhepatic) or extrahepatic (transenteric-transcholedochal) approaches. For the intrahepatic route, the echoendoscope is positioned in the distal esophagus, gastric cardia or lesser curvature, which enables left intra-hepatic access. For the extra-hepatic route, the echoendoscope is frequently positioned in the duodenal bulb and sometimes the prepyloric antrum.
Until recently, EUS-BD was performed using devices borrowed from ERCP. It was first demonstrated using a plastic stent for EUD-choledocoduodenostomy (EUSCDS)[4]. Plastic stents present the risk of bile leak, bile peritonitis, and occlusion[1,2,5].SEMS have largely superseded them. Partially covered (PC) and fully covered (FC)SEMS are preferred over uncovered (UC) SEMS to prevent bile leak[1-2], however,conventional designs still lack anti-migratory property.
Device-related shortcomings have led to the development of specifically designed EUS-BD stents including lumen-apposing metal stents (LAMS), hybrid metal stents(distal covered and proximal UC portions with anti-migratory properties), and one-step dedicated devices with pre-mounted hybrid stents[8].
The most data and extensive experience are on LAMS. LAMS are a recently developed, revolutionary device, designed for EUS trans-luminal drainage[9]. They are short dumbbell-shaped FC metallic stents with wide flanges to allow anchoring across non-adherent structures (Figure 1). LAMS were initially designed for drainage of pancreatic fluid collections. Indications have expanded to EUS-CDS for distal MBO.The newer version of dedicated LAMS have integrated an electrocautery-enhanced delivery system (ECE-LAMS) to allow puncture and release of the stent in a single step procedure hence decreasing the number of accessory exchanges, and reducing the potential of complications[10-12]. There are several different LAMS available with different lengths and diameters. The AXIOS stent with diameters of 6 and 8 mm and saddle length of 8 mm is custom designed for EUS-CDS.
Efficacy and AE
Four meta-analyses reported a technical success rate of EUS-BD of 90%-94.7%, clinical success rate of 87%-94%, and AE rate of 16%-29%[7,13-15]. MBO was the most frequent indication.
AE of EUS-BD depend on the route, the device used, type and extent of disease and operator experience. Overall AE rate for EUS-BD is 16.5%-23.3%[7,13,14]. The most frequent AE are bleeding, bile leak, pneumo-peritoneum, cholangitis, stent migration,abdominal pain, and peritonitis. Although these complications are often self-limited and can be treated conservatively or with endoscopic re-intervention, some complications such as stent migration into the peritoneal cavity may be fatal[8].
In EUS-CDS, the most frequent complications are pneumo-peritoneum and biliary leak predominantly occurring with plastic or UCSEMS[16]. In EUS-HGS, needle puncture into the peritoneal cavity increases the risk of pneumo-peritoneum and bile leak. Smaller intra-hepatic duct caliber precluding the placement of a wider metallic stent may also predispose to these complications due to incomplete sealing of the bilio-enteric fistula. Finally, the movement of the liver during respiration may lead to stent migration, resulting in biliomas and trauma to the bilio-enteric tract.
AE progressively decrease as experience grows and with the development of new stents. In a more recent prospective international multicenter study on efficacy and safety of EUS-BD, by Khashabet al[17](n= 96), 10.5% (10/96) AE occurred: 2 pneumoperitoneum, 1 sheared wire, 1 bleeding, 3 bile leaks, 2 cholangitis, and 1 perforation. 4 AE were graded mild, 4 were moderate, 1 was severe, and 1 was fatal due to unintended perforation. 91.3% of inserted stents were SEMS (44 FC, 26 PC, 14 UC) as oppose to plastic stents. The necessity for track dilation with the use of plastic stents or SEMS was a likely predisposing factor for bile or air leakage.
The advent of LAMS for EUS-CDS confers the theoretical advantage of decreasing migration and bile leak. ECE-LAMS also removes the need for tract dilation, and numerous guide-wire exchanges, potentially reducing complications. Data on LAMS show excellent efficiency and safety profile in short series[6]. Two larger trials have been published for the use of LAMS in distal MBO: (1) A multicenter, retrospective study by Kundaet al[10](n= 57) showed that EUS-CDS with LAMS or ECE-LAMS, had a technical success rate of 98.2% (56/57) and clinical success rate of 94.7% (54/57).Mean procedure time was 22.4 min. Overall AE rate was 7% with 2 duodenal perforations, 1 bleed, and 1 transient cholangitis. During follow-up, 9.3% (5/54) with clinical success required re-intervention for 1 stent migration and 4 sump syndromes;(2) A recent multicenter, retrospective study by Jacqueset al[11](n= 52), showed that EUS-CDS with ECE-LAMS had a technical success of 88.5% (46/52), and clinical success rate of 100% (46/46). Mean procedure time was 10.2 min. 3.8% (2/46) patients presented short-term complications (1 bleed and 1 cholangitis due to obstructive bezoar). Long-term AE were 13.5% including 6 (11.5%), recurrent jaundice due to 4 tumor obstructions and 2 sump-syndromes. One patient experienced stent migration at 6 wk. In univariate analyses, a small common bile duct diameter and not following the recommended procedure technique were significant risk factors for technical failure. Median survival time without biliary complications was 135 d. Interestingly,expert and non-experts performed the procedure however no difference in technical or clinical success was found in the two groups. Finally, 2 patients underwent pancreaticoduodenectomy with no interference of the stent on the procedure.
Choice of EUS-BD technique
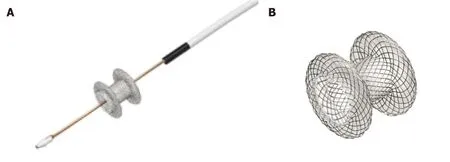
Figure 1 Hot AXIOS deployed.
Currently, there is no established consensus for the choice of EUS-BD technique, and data remains conflicting[1,7,18]. Subgroup analyses from 2 meta-analyses compared extra-hepatic and intra-hepatic routes for EUS-BD[7,13]. Technical and clinical success rates were similar although AE were less frequent with the extra-hepatic compared to the intra-hepatic approach (OR = 0.40, 95%CI: 0.18-0.87,P= 0.022)[13]. A multicenter retrospective study by Dhiret al[18]compared success and complication rates in patients undergoing EUS-BDviadifferent methods. This study showed that success rates of different techniques were comparable, but that AE rates were higher for transhepaticvstrans-duodenal route (30.5%vs9.3%,P= 0.03). A systematic review by Alvarez-Sanchezet al[16]also showed that AE rates were higher for intra-hepatic (18%)compared to the extra-hepatic (14%) approach. On the other hand, a systematic review and meta-analysis by Uemuraet al[19]of 10 studies (n= 434), concluded that EUS-HGS and EUS-CDS had equal efficacy and safety.
In summary, AE in EUS-BD are non-negligible. The more recent data shows an overall lower rate of AE in EUS-BD compared to older publications, which could reflect increasing experience and the development of EUS-BD specific devices.
In choosing which technique to use for EUS-BD, a combination of factors appears to be important in decision making; technical expertise, the risk of AE, and anatomy[12]. It is also generally admitted that EUS-HGS is technically more challenging than EUSCDS. In general, in patients with distal common bile duct obstruction and adequate duct dilatation, the trans-duodenal and trans-hepatic approaches for EUS-BD have similar efficacy, but extra-hepatic route may be a safer option. Future trials will probably rapidly confirm that LAMS specifically designed for EUS-CDS further reduce complications of this route of drainage and simplify the technique. Hence EUS-HGS will probably be reserved to patients where EUS-CDS is not possible.
COMPARISON EUS-BD WITH PTBD
PTBD has a high success rate (87%-100%). However, the external drainage catheter causes discomfort to the patient, and AE are non-negligible, reaching 30%, including pneumothorax, bleeding bile leak, and infection. PTBD is also contraindicated in the presence of ascites or multiple liver metastases[20-23]. EUS-BD offers drainage in a single session in the event of failed ERCP; provides internal drainage with less physical discomfort; allows better nutritional absorption; and avoids electrolyte loss.
The result of randomized controlled trials and meta-analyses comparing EUS-BD to PTBD after failed ERCP show comparable technical and clinical success of 90%-100%with higher complication rates in PTBD[20-23]. Sharaihaet al[23]performed a systematic review and meta-analysis of 9 studies (n= 483), which showed no difference in technical success between EUS-BD and PTBD (OR = 1.78, 95%CI: 0.69-4.59,I2= 22%)after failed ERCP. EUS-BD was associated with better clinical success (OR = 0.45,95%CI: 0.23-0.89,I2= 0%), fewer post-procedure AE (OR = 0.23, 95%CI: 0.12-0.47,I2=57%), and lower re-intervention (OR = 0.13, 95%CI: 0.7-0.24,I2= 0%). There was no difference in length of hospital stay with a pooled standard mean difference of -0.48(95%CI: -1.13-0.16). EUS-BD was more cost-effective.
An interesting multicenter survey by Namet al[24](n= 313) examined patient perception and preference of EUS-BD and PTBD. After explaining the procedure and AE, patients were asked to choose between 2 simulated scenarios. 80.2% of patients preferred EUS-BD. EUS-BD preference declined as AE increased. The authors concluded that technical innovation and improved proficiency to reduce complications of EUS-BD would increase patient acceptability.
In summary EUS-BD must replace PTBD as the standard procedure of choice in failed ERCP in high volume centers with skilled pancreaticobiliary endoscopists.
COMPARISON OF EUS-BD AND ERCP IN DISTAL MBO
Only 6 very recent studies have compared EUS-BD to ERCP[25-30]. These studies were performed in patients with distal MBO and used PC or FC-SEMS for EUS-BD. All 6 trials included patients treated by EUS-CDS, and of this one trial also used EUS-AGS and another EUS-HGS. We hereby discuss the available data, also summarized in Table 1.
Three trials compared a group of patients with EUS-CDS +/- EUS-AGS to a retrospective ERCP control group:
A single-center retrospective study by Kawakuboet al[25](n= 82) comparing the clinical efficacy and safety of EUS-CDS (PCSEMS)vsERCP (PC or FCSEMS) showed that clinical success rates were equivalent between the groups (EUS-CDS 96.2%, ERCP 98.2%;P= 0.54). Mean procedure time was significantly shorter with EUS-CDS than ERCP (19.7vs30.2 min;P< 0.01). Overall AE were not significantly different between the groups (EUS-CDS 26.9%, ERCP 35.7%;P= 0.46). Post-procedure pancreatitis was only seen with ERCP (0%vs16.1%;P= 0.03). Re-intervention rate at 1 year was not significantly different (16.6%vs13.6%,P= 0.5).
A multicenter, retrospective analysis by Dhiret al[26](n= 208) compared the outcomes of EUS-BDvsERCP. Patients in the EUS-BD group underwent EUS-CDS or EUS-AGS with FCSEMS or UCSEMS respectively after 1 or more failed ERCP attempts. Patients in the ERCP group underwent retrograde SEMS placement. In the ERCP and EUS-BD groups respectively; technical success was 94.23%vs93.26%,P= 1;AE were 4.8%vs0%,P= 0.06; and mean procedure time was 30.1vs35.95 min,P=0.05.
Nakaiet al[27]performed a multicenter prospective study (n= 34) evaluating EUSCDS (PC or FC-SEMS)vsERCP (PC or FC-SEMS). For EUS-CDS, technical success rate was 97% and functional success rate 100%, with median procedure time of 25 min.Overall AE were 15% (5/34); 2 with mild abdominal pain and 3 with moderate cholecystitis. Rate of recurrent biliary obstruction (RBO) was 29% (10/34) and nontumor related. Migration occurred in 6, sludge or food impaction in 3, and stent impaction in duodenal wall in 1. Median time to RBO was 11.3 months. In comparison to the ERCP control group, the rate of RBO and cumulative time to RBO of EUS-CDS was comparable to ERCP, which were 36% and 9.1 months respectively. ERCP procedure time was significantly longer (median of 52 min,P< 0.01), and AE rate were comparable.
Three randomized trials compared EUS-CDS +/- HGS to ERCP.
Parket al[28]performed a prospective randomized controlled study comparing efficacy and safety of EUS-CDS (n= 15)vsERCP (n= 15). Both arms used the same PCSEMS. 27 had unresectable pancreatic ductal adenocarcinoma, 1 had distal biliary cancer, and 2 patients had metastatic malignant lymphadenopathy. There were no significant differences for both arms in terms of technical, and clinical success rates(100%vs93%,P= 1.00 and 93%vs100%,P= 1.00 respectively). 4 patients (31%) had tumor ingrowth causing stent dysfunction in the ERCP group. 2 patients had food impaction and 2 patients had stent migration in the EUS-CDS group. There were no significant procedure-related AE in either group. The authors concluded that EUSCDS and ERCP had similar safety and that EUS-CDS was not superior to ERCP in terms of relieving MBO. EUS-CDS had fewer cases of tumor ingrowth but more cases of food impaction and stent migration.
Banget al[29]performed a single center, single-blind, randomized trial to compare EUS-CDS (n= 33)vsERCP (n= 34) as primary treatment for distal biliary obstruction from pancreatic cancer. Both arms used the same FCSEMS. The primary endpoint was the rate of AE for EUS-CDS compared to ERCP, which was not significantly different(21.2%vs14.7% respectively, risk ratio 0.69, 95%CI: 0.24-1.07,P= 0.49). Moderate AE in both groups were around 6%, with no severe AE or procedure-related deaths. For secondary endpoints there were no significant differences between EUS-CDS and ERCP in the rates of technical success (90.9%vs94.1%,P= 0.67), treatment success(97%vs91.2%,P= 0.61), or re-interventions (3.0%vs2.9%,P= 0.99). EUS-CDS did not impede subsequent pancreaticoduodenectomy that was performed in 5/33 (15.2%) of these patients and in 5/34 (14.7%) in the ERCP group (P= 0.99). Median procedure time was similar for EUS-CDS and ERCP (25 minvs21 min respectively,P= 0.178).
In a larger multicenter randomized non-inferiority study by Paiket al[30](n= 125)EUS-BD (EUS-CDS, and EUS-HGS) was compared to ERCP in palliative drainage of distal MBO. In the EUS-BD group a dedicated hybrid PCSEMS pre-mounted on a onestep delivery device was used, whereas in the ERCP group either a PC or FCSEMS was used. Technical success rates were 93.8%vs90.2% (P= 0.003), and clinical success rates 90%vs94.5% (P= 0.49) for EUS-BD and ERCP respectively. EUS-BD had lower rates of overall AE (6.3%vs19.7%P= 0.03) including post-procedure pancreatitis (0vs14.8%), and re-intervention (15.6%vs42.6%). EUS-BD had higher rates of stentpatency (85.1%vs48.9%). There was no difference in patency between EUS-CDS and EUS-HGS. Median procedure time was significantly shorter in EUS-BD 5 min (IQR 3-12)vsERCP 11 min (IQR 7-18),P< 0.001. EUS-BD was associated with higher quality of life (QOL) compared to ERCP at 12 wk post procedure. This study had a notably higher rate of post ERCP pancreatitis and a lower rate of EUS-BD complications compared to other studies. The authors explained these discrepancies by the high number of complex papillary access, and the specific EUS-BD delivery devices used.
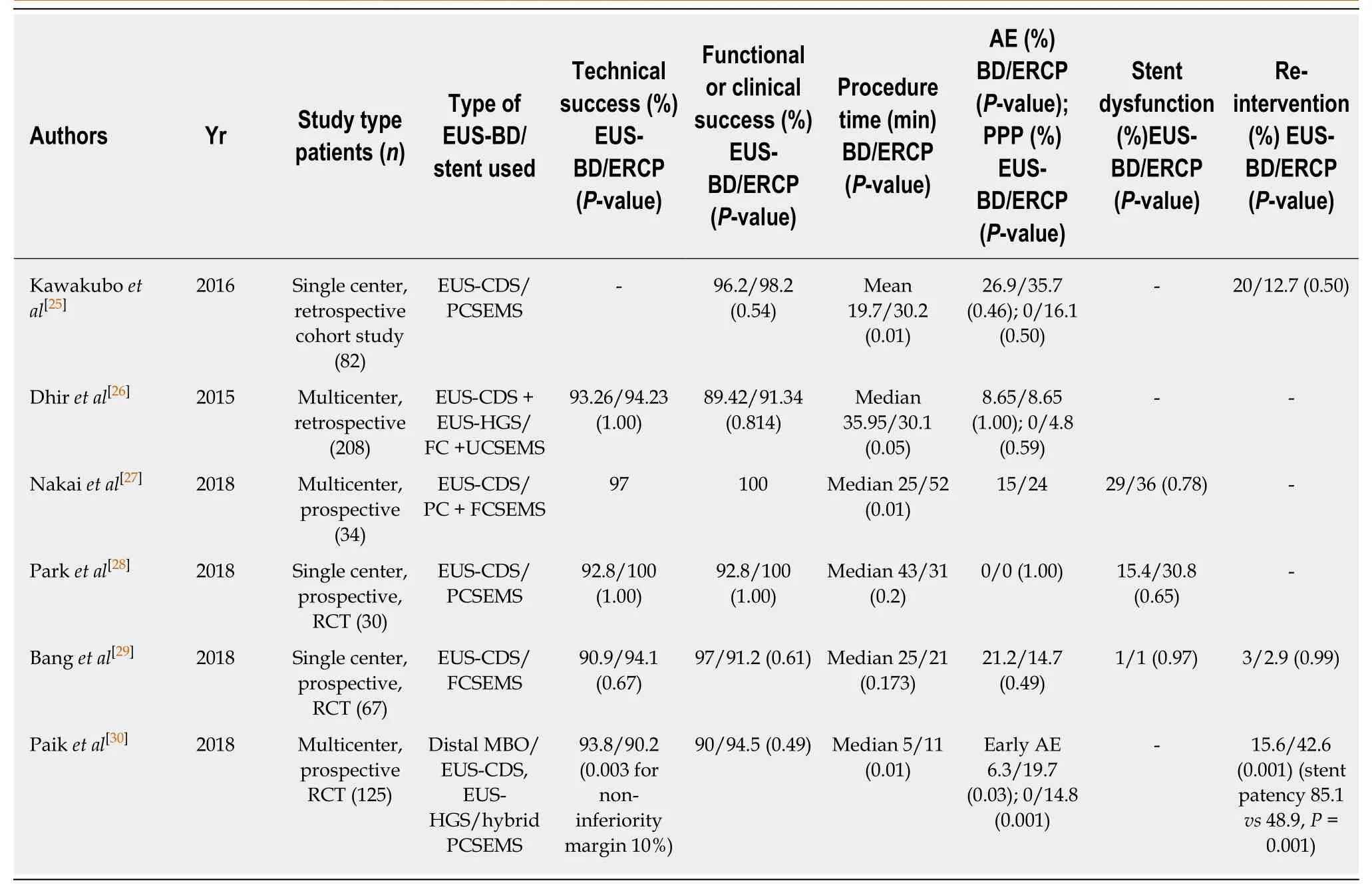
Table 1 Summary of trials comparing Endoscopic ultrasound-guided biliary drainage to endoscopic retrograde cholangiopancreatography in distal malignant biliary obstruction
In summary, recent randomized studies suggest that EUS-CDS is an effective and safe alternative to ERCP that could reduce the re-intervention rate, and risk of pancreatitis without impeding potential curative surgery. Thus EUS-CDS is a practical route of drainage that should be considered in preoperative drainage.
COMPARISON OF EUS-HGS WITH ERCP IN PROXIMAL MBO
ERCP in non-operable hilar stenosis is more challenging than for distal MBO. Bilateral biliary drainage with placement of multiple metallic stent is often required in order to drain ≥ 50% of the liver volume[2,3,31]. The failure rate can reach 27%, with lower clinical response despite successful stent placement. EUS-HGS enables trans-luminal stenting of the left biliary tree without traversing the stricture. It can be combined with ERCP to drain both left and right hepatic ducts. When feasible, the right biliary ducts can also be accessed via EUS-HGS with bridge trans-hilar stenting[31].
Data on EUS-HGS for proximal MBO are limited[7,31], and there is no data comparing EUS-HGS to ERCP in this situation. Furthermore, except for a single-step delivery device only commercially available in Korea, most EUS-HGS specific stents still require a multi-step procedure for adequate positioning.
In summary, the development of new EUS-HGS specific tools, comparative studies between EUS-HGS and ERCP/PTBD, as well as standardization of procedures should be a future goal.
COMPARISON OF EUS-BD AND ERCP IN CASE OF PRIOR DUODENAL STENTING
Gastro-duodenal and biliary obstruction may occur in advanced pancreatic cancer,and double stenting may be required. ERCP is challenging in the presence of prior duodenal stent placement. Yamaoet al[32]performed a multicenter retrospective study(n= 39) to evaluate the outcome of EUS-BD in pancreatic patients with an indwelling gastro-duodenal stent (GDS). This study showed that when a GDS overlay the papilla,EUS-BD technical and clinical success were higher than ERCP (95.2%vs56 %P< 0.01,and 90.5%vs52%P= 0.01 respectively). There was no significant difference in the incidence of AE. The authors concluded that EUS-BD could be a first-line technique for biliary drainage in patients who had a GDS overlying the papilla. In a case series by Anderloniet al[33], single session EUS-CDS with LAMS and duodenal stenting was performed. Results showed 100% technical success with no early or late complications. The short length and design of LAMS did not to interfere with duodenal stenting.
EUS-BD: A PARADIGM SHIFT?
Until recently, EUS-BD was reserved for cases of failed ERCP.
Current data suggests that in multi-disciplinary centers with endoscopic pancreatobiliary expertise EUS-BD is a viable alternative to ERCP and should be favored in cases of prior duodenal stenting. EUS-CDS appears to be a simpler and safer procedure than EUS-HGS, and should be favored when both techniques are possible.
Although recent randomized studies have shown that EUS-CDS is as effective as ERCP with longer stent patency, similar AE profile and reduced risk of pancreatitis precluding early surgery, they also show a higher than expected rate complications and failure of ERCP. In contrast, other studies show a meager failure rate of ERCP in expert hands. A prospective study by Holtet al[34](n= 52) showed that ERCP had a high success rate, in particular when advanced techniques of cannulation were available; hence only 0.6% of native papilla having failed ERCP required EUS-BD.Another retrospective study by Ardenghet al[35](n= 3538), also showed that the failure rate for ERCP was low, 0.68%. In light of the long experience and excellent results with ERCP, this technique should be difficult to replace despite the advantages of EUS-BD.
Nevertheless, the development of ECE-LAMS is a significant milestone in EUSCDS. Growing data suggests it is an efficient and safe tool that reduces procedure time and AE. By virtue of its simple, all-in-one application, ECE-LAMS may reduce the risk of procedural complications such as biliary leakage. Selecting patients with a common bile duct dilation of at least 15 mm diameter, and distal MBO below mid common bile duct appear to be effective measures to reduce procedure-related complications[11,12]. Prospective multicenter, randomized studies are required to compare ECE-LAMS to ERCP in distal MBO. Based on current data it can be hypothesized that such studies would show a comparable efficiency of the two techniques, with reduced pancreatitis and prolonged stent patency in the ECE-LAMS group. Nonetheless, the requirement of EUS and ERCP training to perform EUS-CDS with ECE-LAMS should likely limit the applicability of this technique in a widespread manner. Data are lacking with regards to the learning curve for EUS-BD. A prospective study by Ohet al[36](n= 129) showed that 33 procedures were required to reach a stabilization level in terms of AE and to reduce procedure time. Concerning ECE-LAMS a second follow-up study by Jacques and col[12](n= 61) re-examined the efficacy of ECE-LAMS in distal MBO after a year of further experience. This study under abstract form showed 98.4% technical and clinical success, 1.6% procedurerelated complication (1 bleed during fistulotomy which was self-limited with the expansion of the stent), 0% early complications. Thus, when experience with ECELAMS was acquired for EUS-CDS, this technique was effective and safe for biliary drainage.
Finally, concerning EUS-HGS as an alternative to ERCP, the development of effective all-in-one dedicated devices would reduce AE rates and make it an attractive means of drainage in particular for proximal MBO. Due to the complex nature or proximal MBO, it is likely that ERCP, and EUS-HGS will remain complementary in the future.
CONCLUSION
EUS-BD has enormous potential and has already replaced PTBD in salvage of failed ERCP in expert centers. Several challenges remain before it can fully represent a paradigm shift and replace standard biliary drainage techniques in a widespread manner. The advent of novel EUS-BD specific tools enabling simpler and safer techniques, as well as the growing experience and training of endosonographers, will undoubtedly push the frontiers of its application forward.
杂志排行
World Journal of Gastrointestinal Endoscopy的其它文章
- Role of endoscopic vacuum therapy in the management of gastrointestinal transmural defects
- Should a fully covered self-expandable biliary metal stent be anchored with a double-pigtail plastic stent? A retrospective study
- Role of colonoscopy in diagnosis of capecitabine associated ileitis:Two case reports
- Post-oesophagectomy gastric conduit outlet obstruction following caustic ingestion, endoscopic management using a SX-ELLA biodegradable stent: A case report
- Comprehensive review on EUS-guided biliary drainage
- Endoscopic characteristics of small intestinal malignant tumors observed by balloon-assisted enteroscopy
