Role of endoscopic vacuum therapy in the management of gastrointestinal transmural defects
2019-06-11DiogoTurianiHourneauxdeMouraBrunaFuriaBuzettiHourneauxdeMouraMichaelManfrediKellyHathornAhmadBazarbashiIgorBragaRibeiroEduardoGuimaresHourneauxdeMouraChristopherThompson
Diogo Turiani Hourneaux de Moura, Bruna Furia Buzetti Hourneaux de Moura, Michael A Manfredi,Kelly E Hathorn, Ahmad N Bazarbashi, Igor Braga Ribeiro, Eduardo Guimarães Hourneaux de Moura,Christopher C Thompson
Diogo Turiani Hourneaux de Moura, Kelly E Hathorn, Ahmad N Bazarbashi, Christopher C Thompson, Division of Gastroenterology, Hepatology and Endoscopy, Brigham and Women’s Hospital - Harvard Medical School, Boston, MA 02115, United States
Diogo Turiani Hourneaux de Moura, Igor Braga Ribeiro, Eduardo Guimarães Hourneaux de Moura,Department of Endoscopy of Clinics Hospital of São Paulo University, São Paulo 05403-000,Brazil
Bruna Furia Buzetti Hourneaux de Moura, Michael A Manfredi, Esophageal and Airway Atresia Treatment Center, Boston Children's Hospital - Harvard Medical School, Boston, MA 02115,United States
Abstract
Key words:Gastrointestinal; Endoscopy; Endoscopic vacuum therapy; Negative pressuretherapy; Fistula; Leak; Perforation; Defect
INTRODUCTION AND BACKGROUND
A gastrointestinal (GI) transmural defect is defined as total rupture of the GI wall and these defects can be divided into three main categories including perforation, leaks,and fistulas. Recognition of the specific classification of the defect is essential for choosing the best treatment modality. In the past, many endoscopic techniques,including clips, cap-mounted clips, covered self-expandable metal stents (CSEMS),tissue sealants, endoscopic sutures, cardiac septal defect occluders, septotomies, and internal drainage with pig-tail stents, have been shown to be effective in reducing morbidity and mortality in the treatment of transmural defects. However, the efficacy varies in most studies[1-17]and, thus, endoscopists continue to investigate novel techniques for management of these defects.
Endoscopic vacuum therapy (EVT), also known as endoscopic negative pressure therapy, Endovac therapy, and E-Vac therapy, is an innovative endoscopic option for treating transmural GI defects[18-21]. This endoscopic approach is based on the negative pressure wound therapy for treatment of non-healing wounds. The healing effect of this technique occurs through multiple mechanisms, including changes in perfusion,microdeformation, macrodeformation, exudate control, and bacterial control[22].Although some authors use the term “negative pressure” in their description of this technique[18,19,21], we find this to be misleading, as physical pressure always has a positive value[23,24]. Thus, in this review we will use the term EVT.
The first report of EVT[25]was in the treatment of an anastomotic leak following a rectal surgery in 2003. Since then, EVT has been used in the adult population for closure of esophageal, gastric (most commonly after bariatric surgery), small bowel,pancreatic, and colorectal defects, with success rates above 70%[26-33]. Additionally, one study demonstrated the use of EVT in the pediatric population, with a high success rate in the treatment of upper GI transmural defects[34].
In this article, we review and discuss the mechanism of action, indications,materials, techniques, efficacy, and safety of EVT in the management of patients with transmural defects.
MECHANISM OF ACTION
Vacuum therapy has been commonly used for treatment of non-healing skin wounds.In management of transmural defects, EVT is thought to promote healingviasimilar mechanisms, including macrodeformation, microdeformation, changes in perfusion,exudate control, and bacterial clearance[35,36].
Macrodeformation
Macrodeformation occurs when suction is applied to the sponge resulting in deformational forces being exerted on the defect edges, thus drawing the edges together. Studies showed that a negative pressure of 125 mmHg can decrease the volume of a reticulated open-pore polyurethane sponge by approximately 80%,resulting in substantial shrinkage of the defect[35-39].
Microdeformation
Microdeformation describes the mechanical changes that occur on a microscopic scale when suction is applied. Mechanical strain causes a deformation of the cytoskeleton which initiates signaling cascades leading to release of growth factors which promote cell proliferation and migration, increasing the expression of extracellular matrix components and contractile elements that are necessary for healing. Factors known to affect the efficiency of this mechanism include level of suction, pore size and consistency of the sponge, type of tissue being treated, and deformability of the surrounding tissues[35,40].
Changes in perfusion
Adequate blood flow is essential for healing because it delivers oxygen and vital nutrients to the tissue in addition to removing waste products. Vacuum therapy treatment results in increased microvessel density. Vacuum therapy causes temporary hypoperfusion in the defect edges resulting in localized hypoxia-inducible factor 1α and concomitant modulation of vascular endothelial growth factor expression,leading to increase angiogenesis[22,41,42]. In healthy human skin, suction levels of up to 300 mmHg applied to a reticulated open-pore polyurethane sponge cause a fivefold increase of blood flow[43]. Additionally, other studies have demonstrated that a negative pressure of 125 mmHg considerably increased the blood vessel density,reaching a maximum of 200% in contrast to the vessel density prior to treatment[44].
Exudate control
Fluid accumulation in the extracellular space and tissue edema often occur in chronic defects, inhibiting healing by compressing local cells and tissues. It has been demonstrated that wound healing is improved following fluid removal, and although the exact mechanism for this improved healing is unclear, proposed theories include local alterations in blood flow and removal of harmful substances[22,24,45,46].Additionally, by removing fluid, there is a reduction in the compression forces acting on the microvasculature, which allows increased blood flow and perfusion of the tissue[35].
Bacterial clearance
A high bacterial load may interfere with the process of defect healing; however, there is conflicting evidence regarding the role of vacuum therapy in decreasing bacterial contamination[22]. One randomized study reported that vacuum treatment had a positive effect on wound healing because of a significant decrease in bacterial load compared with non-vacuum-treated wounds[47]. Additionally, a second study including patients with thoracic infections showed improvement in infection control prior to definitive closure[48]. However, other studies have also shown either an increase or no change in bacterial load using this technique[49,50].
INDICATIONS
EVT represents a clinical endoscopic evolution of vacuum-assisted closure therapy, a well-established treatment for open wounds[47,49,51]. Since it is still a relatively new technique, currently no standardized indications for use have been established[51].
All patients with acute or chronic GI defects are candidates for EVT. Endoscopic evaluation is always required prior to treatment to identify the wall defect, to characterize the leak or fistula tract, and to evaluate the contaminated cavity. Larger defects, including perforations, leaks and fistulas, typically associated with fluid collections, are the most common indication for EVT, and studies have shown high efficacy rates of healing associated with this technique[26-34]. When a small defect is associated with a contaminated cavity, dilation of the defect to access the cavity is needed to place the sponge extraluminally. Additionally, small defects, less than 10 mm, without an associated cavity, can be managed with intraluminal placement of the sponge[1,10,52,53].
EVT can be used throughout the GI tract for esophageal, gastric, small bowel,biliopancreatic, and colorectal defects. The most common indications with established data are defects in the esophagus (perforations, leaks and fistulas after anastomoses),stomach (mainly after bariatric surgery), and colorectal areas (anastomotic leaks andfistulas)[26-33,51,54]. Additionally, recent data on early use of EVT in patients with anastomotic ischemic following esophagectomy has been reported with favorable results[55]. The use of EVT in GI ischemia had also been successfully reported in a case of ischemia of the blind end of the jejunal loop after Roux-en-Y gastrectomy[56].
An additional benefit is that EVT can be used in critically ill, hemodynamically unstable patients in need of infectious source control. This technique allows for control of the focus of the sepsis by removing necrotic debris, tissue, and purulent material, while promoting tissue healing and thus hopefully allowing for patient stabilization. It should be noted, however, that if the patient does not clinically respond to EVT therapy, surgical intervention may still be required[48,51,52].
Similar to alternative techniques, EVT has limited efficacy in some clinical scenarios. In defects larger than 5 cm, the sponge size may be insufficient to occlude the defect[52,57,58]. In multiloculated fluid collections, the proper placement of the sponge can be inadequate due to the septations of the collection[57]. In patients with complete dehiscence of a surgical anastomosis, EVT can be used to control sepsis;however, frequently, a second intervention, such as CSEMS or revisional surgery, is needed to restore the anastomosis and preserve continuity of the upper GI tract.Additionally, patients with anastomotic leakage after esophagectomy with necrosis of the gastric conduit usually require surgical revision[51,59]. And finally, another limitation of use of EVT occurs in patients with GI-cutaneous fistula. Mechanistically,EVT relies on the ability to create negative pressure to keep the defect and fistula tract close. Atmospheric exposure prevents this negative pressure system from occurring,and frequently results in dressing malformation and failure. While attempts to plug the fistula at the skin level with occlusive dressings or glue/tissue sealants has been used, this does not maintain an ideal negative pressure seal, which can lead to moisture buildup and eventual failure[52].
To date, contraindications to EVT remain unclear. However, it is recommended that EVT should be avoided in patients with defects in close vicinity of major vessels or those on therapeutic anticoagulants due to the risk of major bleeding[26,60-62].Additionally, it should be avoided in patients with defects in connection to the tracheobronchial system[18].
PROCEDURE
The procedure can be performed in the operating room, endoscopy suite, or at the bed side. In those patients with upper GI defects, anesthesia with endotracheal intubation is recommended for safe airway management during the passage of the sponge.However, during exchanges, deep sedation may be preferred in certain patients. In those patients with lower GI defects, deep sedation is likely safe depending on other clinical factors. Once the patient is adequately sedated, endoscopic evaluation is required to identify and characterize the wall defect and to evaluate the contaminated cavity. Once adequately evaluated, endoscopic irrigation and debridement is recommended.
A meticulous evaluation of the cavity (with or without fluoroscopy) is performed to choose the correct sponge size; estimation of the size of the sponge can be based on the size of the endoscope or endoscopist prior experience. After these steps, the endoscope is removed, and the sponge system is prepared[18,34,52,57].
For the purposes of this review, we will explain the detailed technique for use of EVT in upper GI defects. Lower GI defects can be managed with few modifications to this technique. A silicon 16 or 18-Fr (10 to 16 Fr in children) nasogastric tube (NGT) is introduced into the patient's nares and advanced to the posterior pharynx. Then, the NGT is retrieved though the mouth by using a finger or grasper instrument[18,34,52,57].
A custom EVT sponge is assembled using a polyurethane foam (PUF). The custom sponge is cut to size based on the defect size. Of note, the sponge size is limited to the diameter of the esophagus and overestimation of the sponge size may hinder your ability to visualize the perforation, as there is limited working space with the relatively small diameter of the normal esophagus. In general, the standard size of the sponge is 3 to 7 cm in length and 2-3 cm in diameter. After the sponge is cut to the appropriate size and positioned at the tip of the NGT, the sponge is secured using either silk ties or permanent suture (such as 2-0 or greater prolene or nylon). Finally, a stitch is placed through both the tubing and the sponge at both the proximal and distal ends. To facilitate endoscopic placement and retrieval, a permanent suture is driven to the distal part of tube and tied into a small loop[18,34,52,57].
After the customized sponge system is created, a grasper should be placed through the working channel of the endoscope before insertion into the patient mouth. Then,the short suture loop is grasped with the device. Some authors like to soak the spongewith water-soluble contrast to allow fluoroscopic-assisted placement, however, this is an optional technique. Then, the sponge and the endoscope are lubricated and inserted into the mouth. Due to the size of the system and the endoscope, introduction into the upper esophageal sphincter can be difficult and careful attention should be paid to avoid trauma during insertion[18,34,36,57].
Depending on the size of the perforation, the endoscope should either be driven to the perforation site (if smaller than 10 mm) or should be driven through the perforation into the cavity (if larger than 10 mm) (see topic below: intracavitary and intraluminal EVT). Once inside the cavity, the grasper can be advanced while the endoscope is withdrawn to the GI lumen. Then, the suture loop is released from the grasper. After placement, under endoscopic visualization, the sponge can be pushed or pulled with the grasper to ensure proper position[18,34,36,57].
Once the sponge is in proper position, the NGT is secured to the nose. The suction tubing is hooked up to the vacuum therapy unit and canister. The NGT with the sponge is then attached to the canister tubing using a custom adapter. The vacuum therapy setting frequently used in the GI tract is 125 mmHg of pressure at continuous moderate intensity, however, some authors also describe the use of a higher pressure,to 175 mmHg. If the patient is uncomfortable, or if the patient experiences pooling of secretions above the sponge on the continuous suction setting, the settings can be changed to intermittent suction (5 min on, 2 min off) at the same pressure[18,34,52,57]. It should be mentioned that in patients with a gastrostomy, the procedure described above can be performedviaa retrograde fashion through the gastrostomy[34].
There is limited data regarding oral fluid intake in patients during EVT treatment.While the administration of oral fluids may be controversial, in our experience, low volume of clear fluid (for example, 50 cc of water) administered four times daily for comfort need did not impact treatment course.
Intracavitary and Intraluminal EVT
The two techniques of EVT placement, intraluminal (Figure 1A) and intracavity(Figure 1B), are based on where the sponge system is placed[53,58]. In intracavitary placement, a short sponge is typically placed into the extraluminal cavity as a long sponge would be more likely to fold on itself rendering it less effective. With continuous EVT, the cavity ultimately is drained and collapses onto the lumen, which then seals the defect, preventing further contamination. In intraluminal EVT, the sponge system is placed into the GI lumen. In this approach, frequently a long,cylindrical sponge systems is used. When the vacuum is applied, the lumen collapses over the defect zone, and the EVT system keeps the tract dry by draining GI secretions, allowing the defect to seal avoiding contamination[20,53,58]. Independent of where the sponge system is placed, the most important mechanisms of action of EVT are the simultaneous drainage and closure of the defect.
Sponge system exchanges
The sponge system should ideally remain in place for approximately 3 to 5 d at a time.No more than 7 d is recommended. The sponge embeds into the surrounding tissue,and thus, the longer the sponge remains in place, the more difficult it will be to remove. To exchange the sponge, continuous suction should first be turned off. Then,the endoscope is used to drive between the tissue and the sponge interface to dislodge the sponge from the granulation tissue. If the sponge does not dislodge easily with gentle traction, water or saline can be infused into the NGT to disconnect the sponge from the tissue.
It is important to understand that NGT manipulation should be performed carefully because if the NGT is dislodged from the sponge, retrieving the sponge becomes very challenging. This can drastically increase procedure time and risks associated with prolonged procedures. A grasper can also be used to manipulate the sponge and to grab the loop suture in the distal part of the sponge system to remove it. Similar to insertion, the diameter of the sponge is too large to be removed through the nares with the NGT. Thus, the sponge must be removed from the mouth. Once the sponge is outside the mouth, the NGT can be cut with a blade or scissors[18,34,52,57].
Open-pore polyurethane sponge and open-pore film drains
Several open-pore polyurethane sponge drains (OPDs) (Figure 2) and open-pore film drains (OFDs) (Figure 3) have been developed with different advantages[19,20,53,63-67]. In general, short systems (< 5 cm) are used for intracavitary EVT and long systems (> 5 cm) are used for intraluminal therapy[53]. OPDs are more frequently used in EVT compared to OFDs[19,20,53].
The only commercially available OPD for EVTis the Endosponge® (B. Braun Melsungen AG, Melsungen, Germany) which is marketed for use in the esophagus.However, no electronic pump system has been approved for GI endoscopy therapiesyet[53].
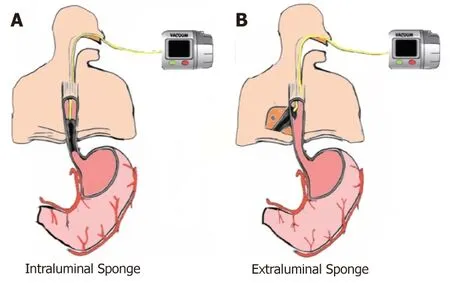
Figure 1 Sponge placement. A: Intraluminal endoscopic vacuum therapy (EVT); B: Intracavitary EVT.
OFDs are newer compared to OPDs and have been developed using a very thin open-pore, double-layer, drainage film (Suprasorb®CNP Drainage Film, Lohmann and Rauscher International GmbH and Co; Rengsdorf, Germany), which is approved for vacuum therapy in wound skin defects[19]. The film is wrapped around the openings in the NGT instead of the PUF[53]. These new drains have the advantage of a very small diameter facilitating their introduction through the nares and placement into small wall defects[65]. These drains also have the advantage to adhere well to the intended defect but adhere less tightly to the normal mucosa surrounding the defect during EVT[53]. A combination of the tools, with PUF wrapped with the open-pore film was also reported in some studies[64,66]. Nutritional support is imperative to wound healing, and thus, for EVT in upper GI defects a double lumen drain has been developed with an additional jejunal feeding tube to allow for enteral feeding access[67,68].
Notably, in our experience, we used gauze coated with perforated sterile plastic drain instead of OPDs or OFDs. This technique, described by Dr. Flaubert Sena de Medeiros, is feasible with a lower cost and non-inferior results to other drains systems[69](Figure 4).
Timing and costs
The initial endoscopic vacuum system placement takes approximately 30 to 60 min,including diagnostic endoscopy, evaluation (with or without dilation), irrigation, and placement of the sponge system. Subsequent sponge system exchanges take approximately 30 min of procedural time[57]. One study evaluated the cost of EVT use and demonstrated that for an average treatment span of 25 d, including 8 sponge exchanges per patient, the total cost per patient was approximately $10118.00[57].
EFFICACY
EVT efficacy in the treatment of transmural GI defects is well reported in case series,cohort studies and systematic reviews. To date, no randomized control trials have been published comparing EVT versus other surgical or endoscopic techniques. In this section, the efficacy of EVT will be discussed with regards to management of transmural GI defects, including those involving the esophagus, stomach (postbariatric complications), small bowel, biliopancreatic, and lower GI tract.
Upper GI defects
The successful use of EVT in upper GI defects was first published in 2008[70]. In this report, two patients with intrathoracic anastomotic leaks after esophagectomy and gastrectomy were successfully treated with a mean of 5 sponge exchanges over a mean of 15 d, without adverse events. After this report, different centers published on the use of EVT in upper GI transmural defects. To date, the most common use of EVT in the upper GI tract has been for closure of esophageal defects[20,57-59,62,71-74]. The inspiration and expiration respiratory movements associated with EVT facilitate the extraluminal transport of even small amounts of fluids[53].
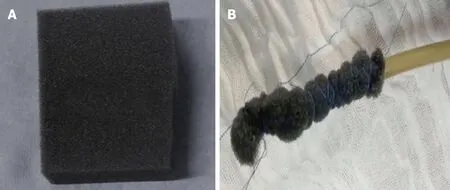
Figure 2 Open-pore polyurethane sponge drain.
In acute perforations, EVT has shown satisfactory results in several studies. Loskeet al[75]demonstrated in a series with 10 patients, including iatrogenic perforations from the cricopharyngeal to the gastroesophageal junction, that all patients were successfully treated within a median of 3 to 7 d without any associated adverse events or need for adjunctive therapy. Kuehnet al[60]demonstrated a similar clinical success rate of 100% in a separate series including 10 patients with acute perforation (8 iatrogenic and 2 Boerhaave). And finally, Heitset al[76]published their study which evaluated the efficacy of EVT in esophageal acute perforations (iatrogenic,spontaneous, and foreign body-associated), showing a primary clinical success of 90%with a mean sponge exchange of 5.4 (2 to 12) and a period of 19 ± 14.26 d.
The majority of studies on the use of EVT in upper GI endoscopy are related to the treatment of intrathoracic leaks, including the use of EVT as primary or as a rescue therapy (Figure 5). In these studies, the efficacy rate of EVT varies from 66.7% to 100%[58,59,73,77,78], with two of these studies demonstrating an efficacy of 100% without any adverse event[77,78].
There are several cohort studies comparing the use of EVT with other techniques in the management of esophageal leaks[27,79-83]. In one retrospective analysis comparing EVT versus self-expandable stents (metal and plastic stents), overall closure rate was 84.4% for EVT versus 53.8% for the stent group. Additionally, a multivariate analysis showed successful defect closure was independently associated with EVT[79]. The superiority of EVT compared to SEMS was confirmed in two other comparative studies[81,82]. Additionally, Manfrediet al[34]showed the superiority of EVT compared to stents in pediatric patients (mean age 24 mo) showing successful closure in 88% of patients who underwent EVT versus 63% of patients who had stent placement. The largest series comparing EVT versus other approaches in the management of leak after esophagectomy showed that EVT is superior to surgical revision, stent placement, and conservative management[80]. These results were confirmed in a recent systematic review and meta-analysis[83], showing that the esophageal defect closure rate is significantly higher in EVT than SEMS, with a shorter treatment duration,lower major complication rate, and lower in-hospital mortality.
The indications for use of EVT in the upper GI tract are expanding to different applications. A recent series[55]demonstrated the use of EVT in the management of anastomotic ischemia, without active leak, after esophageal resections. This study showed interesting results; 75% of the patients developed complete mucosal recovery,while the other 25% of patients developed a leak during the use of EVT. However,these leaks were ultimately successfully treated with EVT. With the increase in the use of EVT, a recent study[28]evaluating patients who underwent EVT in the treatment of esophageal transmural defects concluded that EVT is well tolerated with a satisfactory long-term quality of life.
Post-bariatric surgery complications
Obesity is a pandemic and bariatric and metabolic surgery is the most effective treatment. Despite satisfactory clinical results, the number of adverse events,including leaks and fistulas, after bariatric surgery has increased[1,84-90]. Therefore, the use of EVT in the post-bariatric surgery setting is increasing. While older management algorithms published in 2015 and 2016, did not cite the EVT approach[91,92]as a management option, those from more recent years have proposed the use of EVT in both early and chronic settings[1,93].
A recent study[94], demonstrated the use of EVT in patients with early infradiaphragmatic leakage after bariatric surgery, including laparoscopic sleeve gastrectomy (LSG) and RYGB. In this series, some cases were performed with EVT alone and others with EVT with stent (stent-over-sponge). In 80% of patients, the leakwas connected to abscess cavities. Clinical success, defined as no signs of persistent leakage, was achieved in all patients studied.
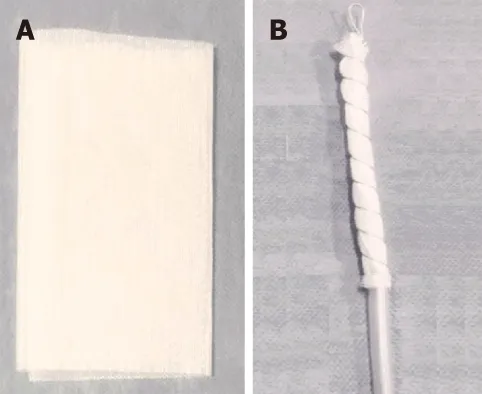
Figure 3 Open-pore film drain.
In a study including patients with acute, early, late, and chronic leaks after sleeve gastrectomy, the use of EVT was associated with 100% resolution of leaks confirmed by upper GI series, with an average of 10.3 sponge exchanges over an average of 50 d[95]. The satisfactory results of EVT in the management of post-LSG leaks was confirmed in other reports[30,96]. However, in contrast to those results, one report demonstrated a case in which the EVT failed to heal a staple line leak after a revisional bariatric surgery (adjustable gastric band to LSG)[97].
In terms of the RYGB subgroup, one group performed a study in a porcine model,performing 10 RYGB. The gastrojejunal anastomoses were fashioned, and a 2 cm defect was created across the staple line. Seven of the ten pigs received EVT and three were included in the control group that did not receive any therapy. All porcine treated with EVT had complete healing of the defect and all control porcines had persistent leak, demonstrating that EVT can be effective in the management of gastrojejunal anastomotic leaks[98]. In humans, while there is limited data for the use of EVT for gastrojejunal leaks, one case report demonstrated the successful use of EVT in the treatment of a post-RYGB leak which had failed prior endoscopic attempt with CSEMS[99]. Additionally, a case report[56]showed a complete reperfusion and epithelization of an ischemic blind jejunal loop after RYGB with EVT management.
Small bowel and biliopancreatic defects
There are several reports of the use of EVT in the management of duodenal wall defects[100-103], including leaks and perforation[29,64,100-103]. Depending on the location of the defect, the sponge system can be placed eithervianasal/oral orviapercutaneous stoma, such as gastrostomy and jejunostomy, in cases where the defect is located distal to the duodenum[92,100,104].
The use of EVT has been successfully reported in treatment of duodenal iatrogenic perforations during endoscopic procedures such as ERCP[100]and post argon plasma coagulation, after endoscopic mucosal resection of an adenocarcinoma[102], and in the management of post-surgical complications[29,101,102].
The successful use of EVT has also been reported in the management of postsurgical duodenal leaks[64,103]. Loskeet al[64]reported the treatment of a duodenal leak with EVT using the pull-through technique along an intestinal-cutaneous fistula. In this case, the sponge was placed in the internal opening of the duodenal fistula. The EVT application resulted in closure of the defect next to the tube and internal drainage of the GI/pancreatobiliary secretions, immediately stopping external drainage. After 3 sponge exchanges over the course of 14 d, the EVT was removed,and at 3-mo follow-up, the defect was completed healed.
The use of EVT has also been reported in the treatment of biliopancreatic conditions including infected pancreatic fluid collections and post-pancreatic surgery[32,66,105-107].Several case reports[66,105,106]have described the successful multi-step use of EVT in infected pancreatic collections. First, an endoscopic drainage with stent is performed.Then, after at least 1 wk, the stent is removed, followed by dilation of the tract and placement of the EVT system. However, despite the favorable results of EVT in the management of pancreatic fluid collections shown in these reports, there is a theoretical risk of massive hemorrhage when performing this technique in the region of the celiac trunk and portal venous system[66]. Due to this risk, we recommend endoscopic drainage with stents as a first approach and EVT as a rescue therapy in selected cases[108-110].
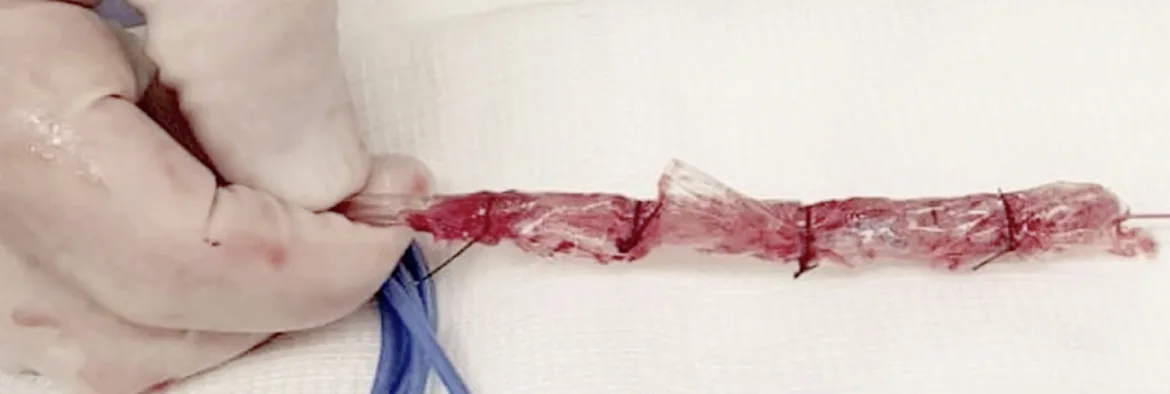
Figure 4 A low cost modified endoscopic vacuum therapy drain system made with a gauze coated with perforated sterile plastic.
EVT has also been described in the management of complications after biliopancreatic surgery[32,106,107]. Loskeet al[107]described the treatment of a dehiscence of the biliojejunal and pancreaticojejunal anastomoses with EVT in a patient with a previous gastroenterostomy. A separate report[106]showed the feasibility and efficacy of EVT using a long sponge (12 cm in length) placed in the stomach for the treatment of a pancreatic-gastric anastomosis dehiscence. Additionally, a third case report demonstrated the successful use of EVT with a two-sided sponge using the pullthrough technique in the treatment of a pancreaticgastrostomy[32].
Lower GI defects
Anastomotic leak is the most significant adverse event after colorectal surgery, with a range of occurrence between 1.5% to 23%, and is considered the major cause of postoperative morbidity and mortality[111,112]. The best approach for the treatment of anastomotic leaks has not been identified yet, especially in lower anastomoses[113]. The management decision in this population must be based on the clinical condition of the patient, including operative intervention for unstable patients (i.e., those with peritonitis), and more conservative modalities for stable patients[111,112].
Endoscopic modalities, including stents, fibrin glue, clips, cap mounted clips, and double pigtail catheter drainage show variable success in the management of lower GI defects[112-117]. In 2003, Weidenhagenet al[25]described the first use of EVT in the lower GI tract for sepsis control caused by an anastomotic leak after a rectal surgery,showing a successful outcome. After this favorable report, the use of EVT in the management of lower GI defects increased and several studies were published showing a high efficacy and safety profile[113,118-120].
The first study evaluating EVT in the treatment of anastomotic leak after low anterior resection (LAR)[113]included 29 patients and showed 90.3% successful closure with a mean of 11.4 ± 6.3 sponge exchanges and a duration of 34.4 ± 19.4 d. In this study, most of patients had a protected stoma created at the primary surgery. In a retrospective study[118]including anastomotic leak after rectal resection, Hartmann's stump insufficiency, and rectal perforation, EVT demonstrated an 83% closure rate overall. For those patients with anastomotic leak, the closure rate success was 90%,similar to several other studies[121-123]. The German multicenter study[120]using EVT in the treatment of anastomotic leakage after colorectal surgery, including patients with rectal cancer and ulcerative colitis, analyzed the use of EVT after anastomotic leakage after colorectal surgery in two groups. One group were those patients whom underwent treatment within 6 wk post-operatively and the second group after 6 wk post-operatively. Patients whom underwent the procedure within 6 wk post-surgery had a higher closure rate (75%vs38%). In this study, closure was achieved in a median of 40 d with a mean of 13 sponge exchanges.
One concern in the use of EVT in lower GI tract is that the feces may block the vacuum system, and thus, in some centers, physicians limit the use of EVT to those patients with fecal diversions. However, several studies have included patients without fecal diversion, and have shown efficacy of the method, suggesting that the lack of fecal diversion is not an exclusion criteria for EVT[119,124-127]. A study comparing the use of EVT in patients with and without stoma is needed to confirm this hypothesis.
Recently, a systematic review[112]including 14 studies (case series and cohort studies) with a total of 197 patients with anastomotic leakage treated with EVT showed an overall successful closure rate of 88.8%, with very low rates of adverse events.
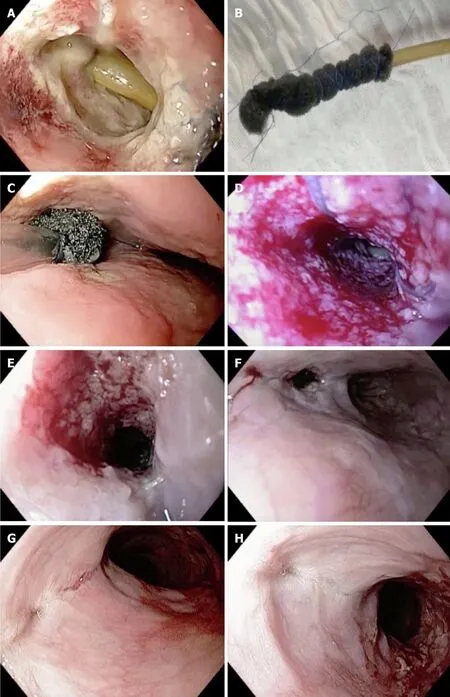
Figure 5 Endoscopic vacuum therapy in the management of an esophageal defect.
SAFETY
In general, EVT is a safe procedure with a low rate of adverse events. The most common complaint from patients during EVT treatment is related to the NGT, as this can cause significant patient discomfort, including pain, nausea, and emesis,especially in those patients with an additional nasoenteral tube. Additionally, patients have reported distress over having to undergo numerous repeat procedures forsponge exchanges[26,51,62].
The most frequent adverse events are sponge dislocation, minor bleeding after sponge exchange due to ingrowth of granulation tissue into the sponge, and anastomotic strictures. However, major bleeding events have also been reported[26,51,60,62].
One major concern regarding EVT in the upper GI tract is the risk of major bleeding, due to the risk of development of a fistula between the cavity and the aorta(or aortic branches), as well as formation and rupture of pseudoaneurysm involving vessels or heart chambers due to the ongoing inflammatory process of EVT[51,62].Unfortunately, several studies have reported major bleeding events. A prospective study[26]including 52 patients with upper GI defects treated with EVT reported 4.1%minor adverse events, including sponge dislocations and minor bleeding after sponge removal. Minor bleeding was usually self-limited and more frequent sponge exchanges could potentially mitigate this risk.
However, more notably, in this study, two patients died due to major bleeding related to EVT. One patient died from acute hemorrhage 56 d after initial EVT placement. The other patient died 12 d after initial EVT placement due to a nonmanageable hemorrhage after sponge removal during the third sponge exchange. In this case, authors believe that a rupture of the descending aorta occurred. In a case series[60]including 5 patients that were successfully treated with EVT, two anastomotic strictures were reported. In both cases dilation with bougies were performed. One of these patients had two dilations without adverse events. The other patient had severe bleeding after dilation and unfortunately died, with cause of death on autopsy being identified as an aortoesophageal fistula leading to hemorrhagic shock. In a retrospective study[62]including 21 patients, two bleeding events (10%) were reported.One bleeding event occurred from the pancreas during treatment of a posterior gastric perforation and the other bleeding event occurred from an aortic branch during treatment of an esophageal anastomotic leak. In these two cases, fresh blood was seen in the EVT output fluid and the EVT was terminated immediately. Both patients underwent surgery for aortic stenting.
Based on these major bleeding reports, if a significant bleed occurs during treatment, EVT should be stopped and a triple-phase CT performed to direct possible management. Additionally, the CT scan should be reviewed prior to starting EVT in the upper GI tract to exclude vascular issues.
CONCLUSION
EVT is a new option in the management of GI transmural defects. EVT use has been increasing and appears to be effective in the treatment of this condition as a first line therapy, as well as a salvage procedure when other options have failed. The most experience with EVT is in the treatment of esophageal transmural defects, showing better results than any other therapy. However, due to the major bleeding risks associated with this technique, patients should undergo this procedure in experienced centers and be monitored closely for adverse events.
杂志排行
World Journal of Gastrointestinal Endoscopy的其它文章
- Endoscopic ultrasound-guided biliary drainage: A change in paradigm?
- Should a fully covered self-expandable biliary metal stent be anchored with a double-pigtail plastic stent? A retrospective study
- Role of colonoscopy in diagnosis of capecitabine associated ileitis:Two case reports
- Post-oesophagectomy gastric conduit outlet obstruction following caustic ingestion, endoscopic management using a SX-ELLA biodegradable stent: A case report
- Comprehensive review on EUS-guided biliary drainage
- Endoscopic characteristics of small intestinal malignant tumors observed by balloon-assisted enteroscopy
