Comparison of burn rate and thermal decomposition of APas oxidizer and PVCand HTPB as fuel binder based composite solid propellants
2019-05-24ManashKumarDepartmentofMechanicalEngineeringVVITPurnea854301BiharIndia
A.Manash ,P.Kumar Department of Mechanical Engineering,VVIT,Purnea,854301,Bihar,India
b Department of Mechanical Engineering,K N Modi Engineering and Technology,Modinagar,Gaziabad,U.P,India
Keywords:Thermal decomposition Burn rate Thermo-gravimetric analysis AP PVC HTPB Fe2O3
A B S T R A C T In the present investigation an effort has been made to understand the thermal decomposition and burn rate characteristics of APas oxidizer and PVCand HTPBas fuel binder in composite solid propellant.The burning rate study has been carried out at ambient and different pressures of 2.068Mpa,4.760Mpa,6.895Mpa.The mechanism of thermal decomposition of each composition have also been determined by NETZSCH simultaneous thermal analyser,comprising differential scanning calorimeter(DSC)and thermo-gravimetric analyser(TGA).An effort has been made to study the burn rate and decomposition of fuel binder and oxidizer in presence of Fe2O3 and also their overall impact on combustion of propellant.©2018 Published by Elsevier Ltd.This is an open access article under the CCBY-NC-ND license(http://creativecommons.org/licenses/by-nc-nd/4.0/).
1.Introduction
In the modern rocket technology,there is a need for new propellant for better performance of the rocket motor.The performance of the propellant can be increase by increase the oxidizer content,decrease the oxidizer particle size or adding burning rate modi f i er or catalyst to the propellant.How ever the best and most effective w ay to increase burning rate is the addition of catalysts to the propellants.It is w ell know n that the burning rate of these composite propellants w hich consist of ammonium perchlorate(AP)is enhanced,w hen some transition metal oxides(Fe2O3)are added to the compositions[1,3,5].In the present study,the burning rate and thermal decomposition characteristics of AP as oxidizer and PVC[6]and HTPB[7]as fuel binder based propellant w ith and w ithout Fe2O3 as catalysts w ith different oxidizer loading w ere investigated.
Considerable effort that has been devoted to understand the decomposition of ammonium perchlorate and its burning rate,how ever very little attention have been paid to the decomposition of fuel binder.How ever,the fact remains that the fuel binder is responsible to hold the particulate oxidizer or metals in its matrix and any suitable additive w hich may improve the heat of combustion or pyrolysis rate of fuel binder should be in f l uencing the overall burning rate of the propellant.According to the present understanding of modern composite solid propellants also needs the vital inputsdetailing the fuel binder decomposition mechanism and its interaction w ith the oxidizer during the rocket combustion process.
Analytical models of the burning rate and the combustion process do exist,how ever they are useful only for preliminary designs and these analytical models are not much useful for detailed designs or for evaluation of new or modi f i ed propellants because combustion processare substantially undetermined,unpredictable,and unmeasurable to date.Burning rate data are usually obtained in three w ays namely,Standard strand burners,often called Craw ford burners,Small-scale ballistic evaluation motors,or even Full-scale motors w ith proper instrumentation.
In the present investigation,an effort has been made to conduct for experimental studies of determination of pressure dependent burning rate of AP-HTPBand AP-PVCbased solid propellantsw ith various compositions.Three different percentages of AP oxidizer loading viz.70%,75%and 80%by w eight have been employed,using HTPB and PVC as the fuel binder,ammonium perchlorate as the oxidizer,dioctyl adipate for AP-HTPB formalation and DBPfor APPVCformalation,as the plasticizer,TDIas the curing agent for APHTPB formalation and glycerol as cross linking agent for AP-HTPB formalation.In each propellant composition,the effect of ferric oxide,3%by w eight has been investigated in different studies.Burning rate data have been generated for different pressures of 0.101,2.068,4.760,6.895 MPa for each composition.An effort has also been made to analyze the results in light of existing theories and empirical relations.
The objective of the research is to determine the burn rate and thermal decomposition of AP-HTPB and AP-PVCw ith and w ithout Fe2O3and compare the result.
2.Experimental
2.1.Material
Ammonium perchlorate procured from ISRO,Alw aye has been used as an oxidizer.Abimodal particle distribution has been chosen to increase the oxidizer loading i.e.to increase the volumetric eff i ciency.The oxidizer particle size of about 44 and 150μm has been used.The oxidizer w as dried at 60°C for half an hour to remove moisture before using it.For the present study,R-45 M-HTPB prepolymer,supplied by Propellant Fuel Complex,Vikram Sarabhai Space Centre(VSSC),Thiruvananthapuram has and PVC resin in commercial grade was obtained from M/s.Calico chemicals,Plastics and f i bres division,Mumbai,in the form of w hite pow der and w as used after drying in oven been used.Ferric red oxide 101(Fe2O3.αH2O)procured from Howards of Ilford Limited,London.The particle size used is 0.1μm and the scanning electron micrographs of nano Fe2O3incorporated in HTPB revealed a w ellseparated characteristic necklace-like structure ofα-Fe2O3particles at high magni f i cation[11].The composition of the propellant has been given in Table 1 and Table 2.
2.2.Mixing of propellant ingredients
All the ingredients selected for above compositions w ere stored under controlled humidity conditions.The processing variables like mixing time,level of vacuum applied,mixer temperature and sequence of ingredient addition into the mixer w ere kept similar in all the mixings.The quantities of HTPB and DOA w ere mixed in a stainless steel vessel and then ammonium perchlorate and glycerol w ere added in requisite quantities.The catalyst concentration of 3 w t%of total propellant w eight w as added separately in catalyzed compositions.The mixing w as carried out for 30 min and f i nally the TDI w as added to the propellant mix.The mixing w as again done for 30 min to get the f i nal propellant mix.
2.3.Casting of propellant slurry
The propellant slurry w as cast in plastic containers w hich w ere cleaned and greased w ith Metroark grease beforehand.The casting was done under vacuum to avoid blow holes in the strands.
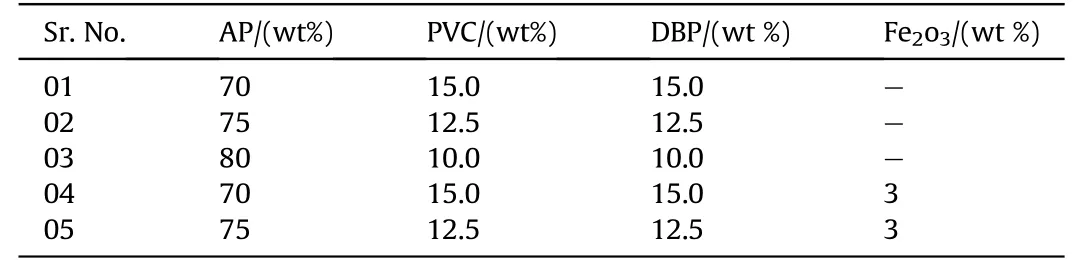
Table 2Formulation of APas oxidizer and PVCas plastisol based composite solid propellants.
2.4.Curing of propellant slurry
The propellant f i lled plastic containers w ere kept in a vacuum electric oven at 60±2°Cfor a period of six days to allow the propellant to cure and attain required mechanical strength.The moulds w ere then removed from the oven and allow ed to cool to room temperature.The propellant samples w ere stored in a desiccator to avoid moisture absorption.
2.5.Composition of propellant formulation
In the present w ork,it has been attempted to use AP-HTPBand AP-PVC composite solid propellant and study their combustion aspects.Three different composition w ere used for experimentally studies w ith prior experience of the mechanical and combustion behavior of AP-HTPB and AP-PVCpropellant at the laboratory.The bimodal AP is taken w ith mess size 150 and 300.The AP are loaded at 70,75 and 80 percents by w eight in PVC plastisol processed w ith equal amount of DBPplasticizer.Similarly,the APare loaded at 70,75 and 80 percents by w eight in HTPBprocessed w ith DOA as plasticizer,TDIas curing agent and glycerol.The basic formulations of the samples consisted of AP-HTPB+catalyst and APPVC+catalyst are show n in Tables 1 and 2.
2.6.Burning rate studies
The burning rates of AP as oxidizer and PVC and HTPB as fuel binder based composite propellants w ith and w ithout catalysts have been determined at ambient condition and at different pressures,2.06,4.76 and 6.89 MPa,using a conventional strand burner[9].Nitrogen gas has been used to pressurize the bomb.The dial type pressure gauges have been used to record incoming pressure and pressures in bomb and line.A surge tank has been provided in the set-up to ensure that a strict pressure level is maintained in the bomb.This has been show n clearly in Fig.1 and Fig.2.
The propellant strands,having tw o f i ne drilled holes at a distance of 5 cm to position the fuse w ires,w ere installed in the bomb and igniter w ire was suitably placed at apex of the strand.The cap,w ith the provision for electrical connections in it w as tightened on to the bomb.The bomb w as then pressurized w ith nitrogen gas to required pressure level.The necessary electrical connections w ere made and the strand w as ignited to record time w ith the help of anelectrical timer.Similar procedure has been follow ed for each strand.The burning rates w ere then determined from the time elapsed betw een the tw o fuse w ires or break w ires.Ferric oxide have been reported to be good burning rate modi f i ers,and have been add at 3%by w eight in all composition of the propellants to investigate the effect of catalyst on the burning rate of AP-HTPB propellant.
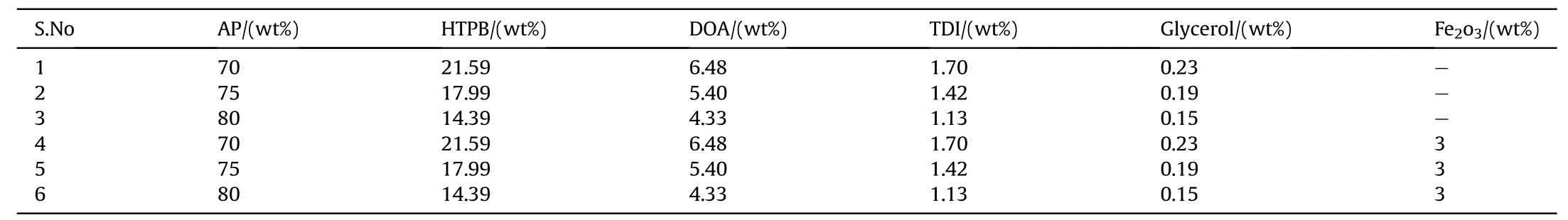
Table 1Formulation of APas oxidizer and HTPB as fuel binder based composite solid propellants.
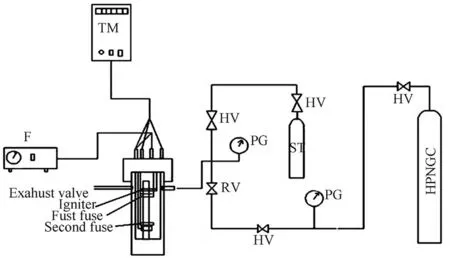
Fig.2.Line diagram of high pressure strand.
2.7.Simultaneous thermal analysis
The simultaneous thermal analysis of the prepared propellant has been carried out on a NETZCH Simultaneous Thermal Analyser(STA 409/PG).The sample w as placed in an alumina crucible and then the lid has been closed.The w eight of the virgin sample w as about 1.0 mg and for catalyzed propellant it was 0.5 mg.Dynamic scans w ere performed at a heating rate of 10°C/min[2]in a temperature range up to 600°C.During the measurement,nitrogen gas w as purged throughout the system at 50 m Lmin-1to maintain an inert environment.
3.Results and discussion
3.1.Burning rate study
3.1.1.Burning rate study of APas oxidizer and HTPB as fuel binder based composite solid propellants
The burning rate results obtain from AP[4,5]as oxidizer and HTPB[7]as fuel binder based composite solid propellant w ith and w ithout Fe2O3[1,3,5]w ith different oxidizer loading has been show n in Table 3.The virgin propellant w ith 70%solid loading having the burning rates in the order of 4.16 mm/s to 9.25 mm/s at the pressure ranges of 0.101 MPa,2.068 MPa,4.760 MPa and 6.895 MPa,at similar pressure range the burning rates have been found in order of 4.26 mm/s to 7.96 mm/s for 75%AP loading.In case of catalyzed propellant for 70%APloading the burning rate is higher than virgin propellant w hich is in the order of 5.55 mm/s to 10.63 mm/s in same pressure condition.For higher oxidizer loading of 75%AP content w ith Fe2O3the burning rates are in range of 4.44 mm/s to 17.54 mm/s,w hich higher burning rate than virgin propellant for same oxidizer loading.The propellant containing 80%AP loading show s unexpected higher burning rate in the order 4.87 mm/s to 33.31 mm/s,this may be due the poor mechanical property of the propellant.So their burning rate has not taken in account.The pressure index value for virgin propellant is 0.098 and 0.433 and for catalyzed propellant is 0.2 and 0.45.
3.1.2.Burning rate study of AP-PVC based composite solid propellants
The burning rate results obtain from APas oxidizer and PVC[6]as fuel binder based composite solid propellant w ith and w ithout Fe2O3[1,3,5]w ith different oxidizer loading has been show n in Table 4.The virgin propellant w ith 70%solid loading having the burning rates in the order of 1.89 mm/s to 6.41 mm/s at the pressure ranges of 0.101 MPa,2.068 MPa,4.760 MPa and 6.895 MPa,at similar pressure range the burning rates have been found in order of 2.01 mm/s to 6.626 mm/s for 75%APloading.In case of catalyzed propellant for 70%APloading the burning rate is higher than virgin propellant w hich is in the order of 2.20 mm/s to 7.93 mm/s in same pressure condition.For higher oxidizer loading of 75%AP content w ith Fe2O3the burning rates are in range of 2.10 mm/s to 9.21 mm/s,w hich higher burning rate than virgin propellant for same oxidizer loading.The propellant containing 80%AP loading show s unexpected higher burning rate in the order 2.164 mm/s to 9.89 mm/s.The main emphasis has been put in the present investigation is higher loading of AP i.e.80 w t%w ith Fe2O3does not sustained,w hich show s brittle nature is not applicable for experimental w ork.So their burning rate has not taken in account.The pressure index value for virgin propellant is 0.301 and 0.376 and for catalyzed propellant is 0.316 and 0.356(see Figs.3 and 4).
3.2.Thermal decomposition study
3.2.1.Thermal decomposition study of APas oxidizer and HTPB as fuel binder based composite solid propellants
The thermal decomposition studies of propellants having different oxidizer loading w ith and w ithout Fe2O3as catalyst at a heating rate of 10°Cper min-1[2]has been show n in Fig.5.The DSCthermograph of various compositions AP-HTPB propellants
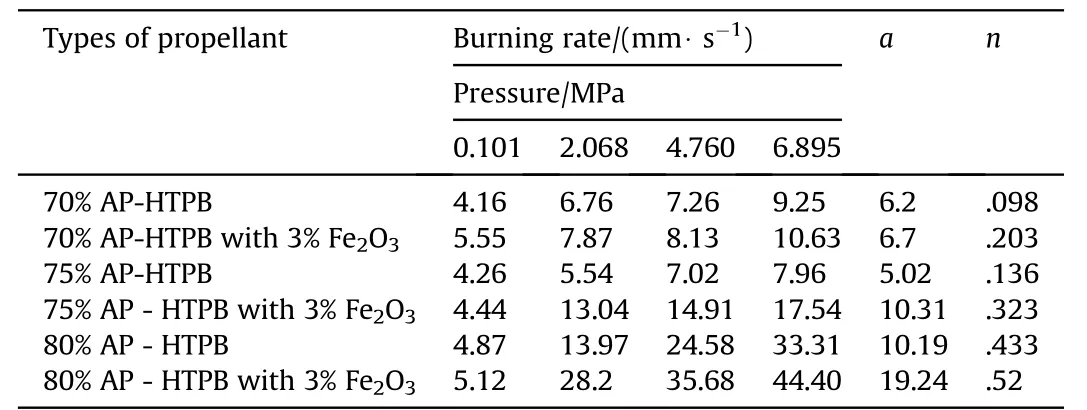
Table 3Burning rate of APas oxidizer and HTPBas fuel binder with Fe2O3 based Composite Solid Propellants at different pressures.
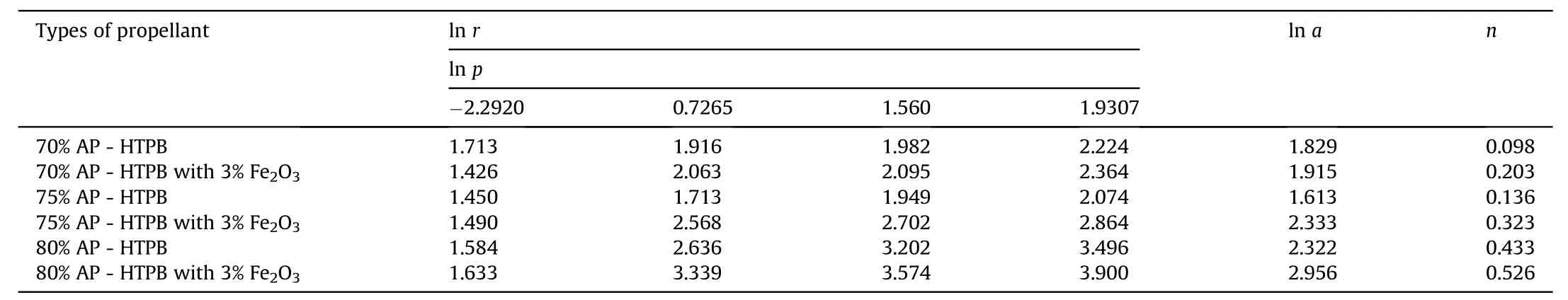
Table 4Pressure dependence of burning rate of APas oxidizer and HTPBas fuel binder w ith Fe2O3 based composite solid propellants at different pressure.
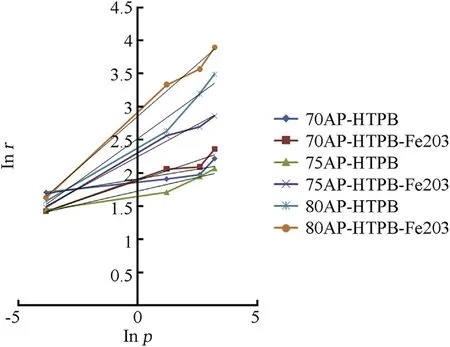
Fig.3.Comparison of burning rate 70,75 and 80%of AP-HTPB composite solid propellant w ith catalyst.
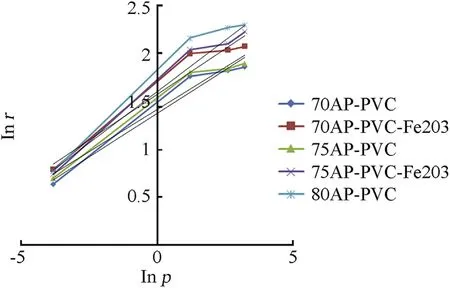
Fig.4.Comparison of burning rate 70,75 and 80%of AP-PVC composite solid propellant.
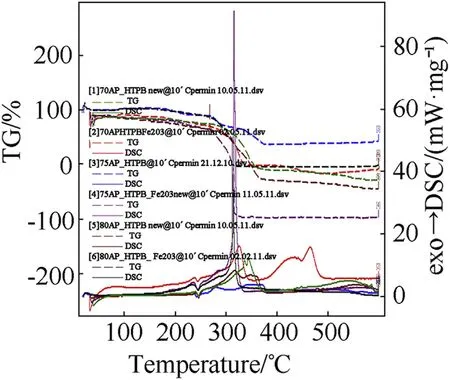
Fig.5.Comparison of STA graph showing DSC/TG of AP-HTPB composite solid propellant.
clearly indicates the endothermic peak is nearly equal at 245°C w ith onset temperature 120-140°C.In the endothermic peak temperature phase transition of AP from orthorhombic to cubic structure takes place.The f i rst exothermic peak of the propellant lies below 360°C and second peaks at below 500°C.DSC characteristics of the propellants w ith Fe2O3have four distinct exothermic peaks.The f i rst exothermic peak lies on 290°C,it represent the low temperature decomposition(LTD)of APand the second exothermic peak is at 365°C,represent the high temperature decomposition(HTD)of AP,f i nally the third and fourth exothermic peak is at 465°Cand 525°Crepresent the depolymerization activity of HTPB.But these peak are not appears in 70%oxidizer loading propellant,it has only tw o peaks.The f i rst and third exothermic peak is due to the combustion of AP[8]and second and fourth peak is due to the decomposition of HTPB[10].From the graph it is clear that Fe2O3marginally lower the phase transition and f i rst decomposition temperature of AP but Fe2O3is observed to in f l uence the second decomposition peak enormously.This indicates that Fe2O3markedly in f l uences the condensed phase combustion reaction.
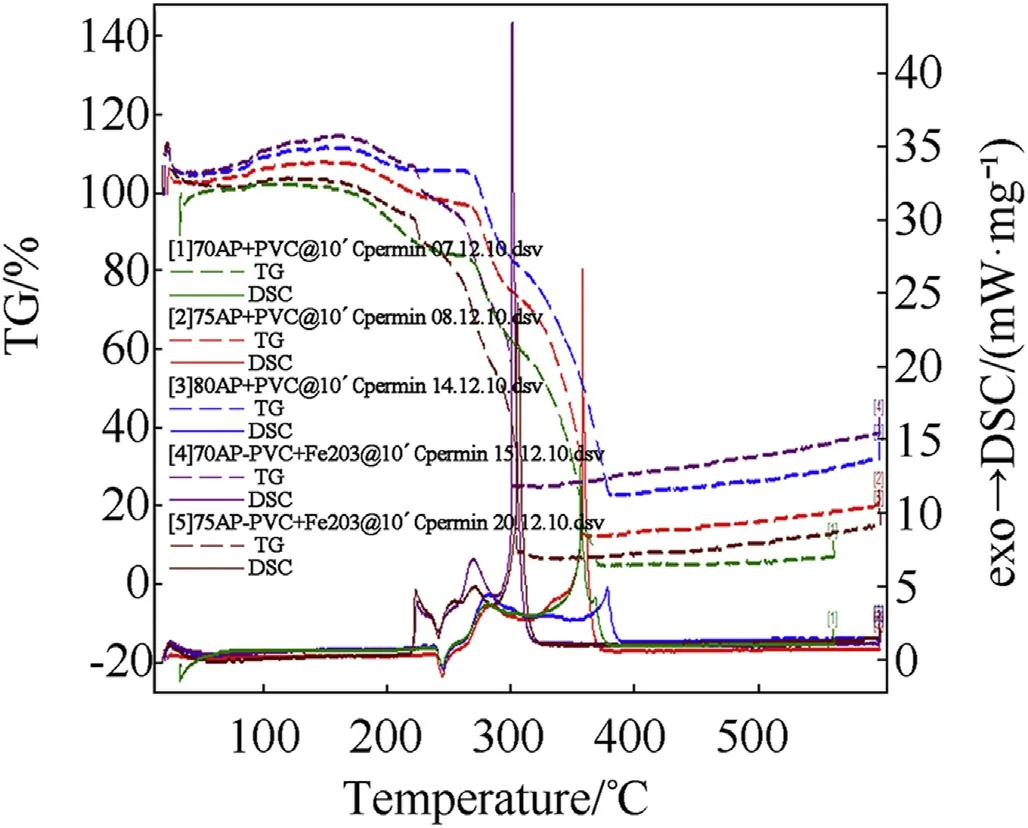
Fig.6.Comparison of STA graph showing DSC/TG of AP-PVC composite solid propellant.
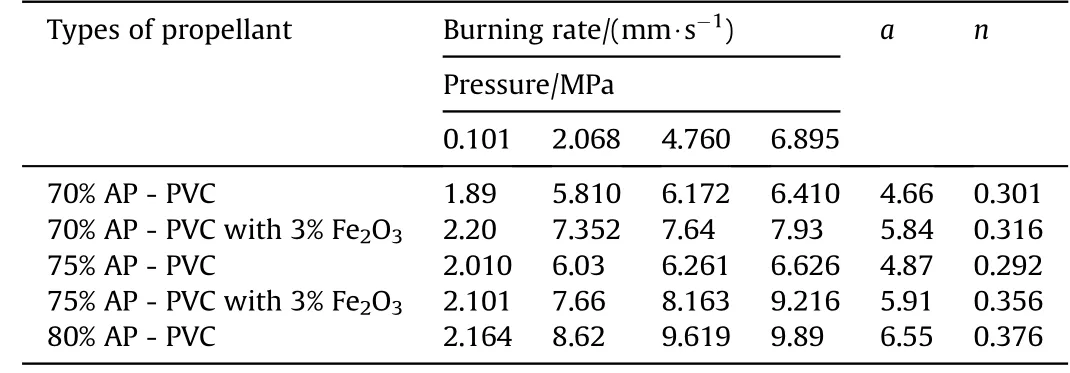
Table 5Burning rate of APas oxidizer and PVCas fuel binder w ith Fe2O3 based Composite Solid Propellants at different pressures.
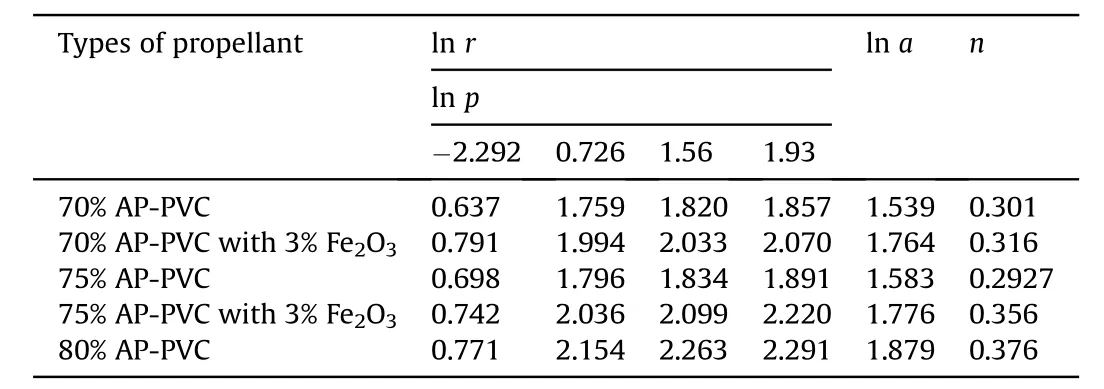
Table 6Pressure dependence of burning rate of APas oxidizer and HTPBas fuel binder w ith Fe2O3 based composite solid propellants at different pressure.
3.2.2.Thermal decomposition study of APas oxidizer and PVCas fuel binder based composite solid propellants
The thermal decomposition studies of propellants having different oxidizer loading w ith and w ithout Fe2O3as catalyst at a heating rate of 10°Cper min-1[2]has been show n in Fig.6.The DSC thermograms of AP-PVC composite solid propellants are given in Fig.6.It is clearly show s that the onset temperature comes in betw een 106±2 except 70 w t%composition.The endothermic peak from orthorhombic to cubic crystal forms at 245±20C and subsequent exothermic decomposition at 378.70C(Table 4).But in presence of Fe2O3the exothermic peaks reduces at 301°C(see Tables 5-8).
3.3.Thermo-gravimetric analysis
The thermo-gravimetric studies of AP-HTPB[12]and AP-PVC[13]based composite solid propellants have also been carried out under nitrogen atmosphere at a heating rate of 10°C/min using Simultaneous Thermal Analyser.The thermograms of all the eleven samples and comparison of the present investigation have been recorded in Figs.5 and 6.The result on the percentage of mass loss and corresponding temperatures for different systems are summarized in Tables 9 and 10.The AP-HTPBdata reveals that all the composite solid propellant are stable up to temperature of 250±30°C.An analysis of result that the 20%mass loss and corresponding temperature reveals that initial decomposition process occurs at higher temperature.The themal decomposition temperature in case of Fe2O3is higher as comparison to uncatalysed APHTPBcomposite solid propellant.The AP-PVC(Fig.6)data reveals that all the composite solid propellant are stable up to temperature of 257±40°C.An analysis of result that the 20%mass loss and corresponding temperature reveals that initial decomposition processoccurs at 257°C.The themal decomposition temperature in case of Fe2O3is lesser as comparison to uncatalysed AP-PVC composite solid propellant.
4.Conclusions
The follow ing conclusions can be draw n from the present investigationson the combustion characteristics of APasoxidizer and HTPB as fuel binder w ith Fe2O3based composite solid propellant.
1)The burning rate of AP-HTPB composite solid propellants results very impressive data w ith expected combustion index values.
2)It was observed that the burning rate increases w ith increase of chamber pressure and also,the combustion pressure rate increases w ith increase in oxidizer loading for all the cases.Further,the phase transition,melting and decomposition processes of the oxidizer are pressure sensitive and associated peaks register a positive temperature shift w ith pressure.
3)Conversely,the addition of catalyst w ith 3 w t%ferric oxide especially in high pressure region show s drastic increase in burning rate w ith increase in AP%loading.
4)The thermal decomposition of AP-HTPB clearly show s an endothermic phase transition peak from orthorhombic to cubic crystal at 245±2°C.
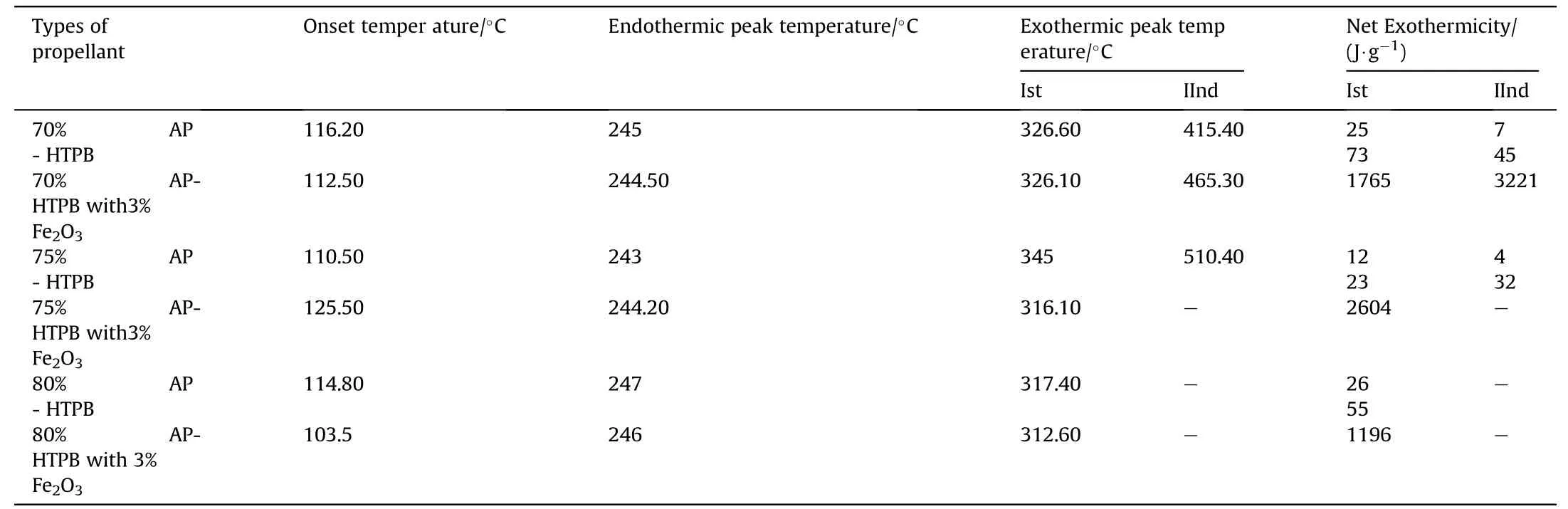
Table 7DSCresults of APas oxidizer and HTPB as fuel binder w ith Fe 2O3 based Composite Solid Propellants.
5)The HTPBused in the propellants is fairly stable upto 300oCand starts slightly decomposing thereafter.But w ith the addition of APthis exothermic decomposition peak of HTPB binder shifted from 375°Cto 326°Cand 450°C-415°C.
6)Similar trend has been found for AP-PVCbased propellants as observed in AP-HTPB based propellants w ith slightly low er data result.
7)The thermal decomposition of AP-PVC clearly show s an endothermic phase transition peak appears at 245°C and subsequent exothermic peak temperature value decreases w ith the addition of ferric oxide.
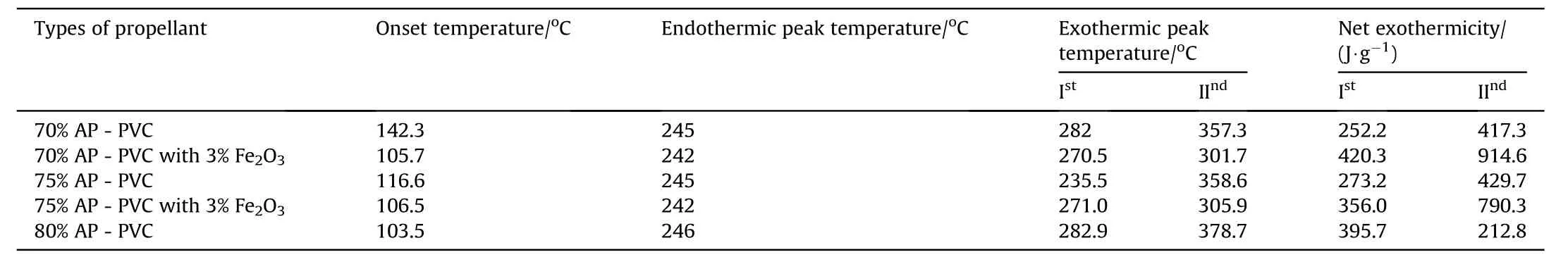
Table 8DSCresults of APas oxidizer and PVCas fuel binder w ith Fe 2O3 based Composite Solid Propellants.
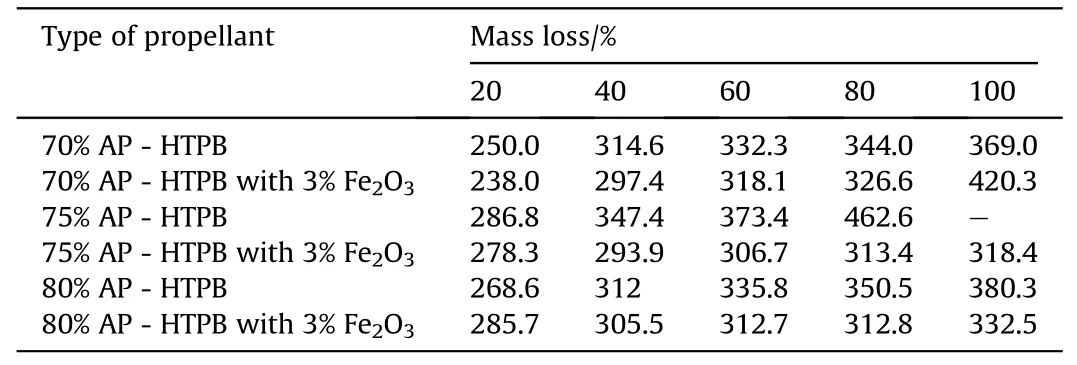
Table 9Percentage of Mass Loss of AP-HTPBversus Temperature(o C)data Obtained from TG Analysis.
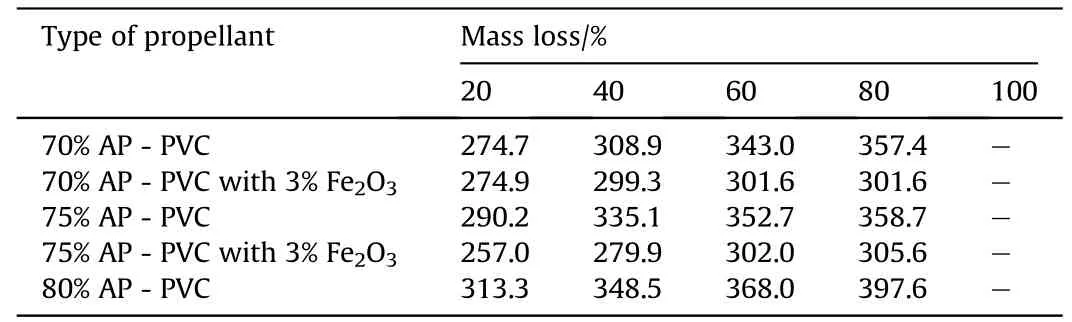
Table 10Percentage of mass loss of AP-PVCversus temperature(o C)data obtained from TG analysis.
8)The resultant data of net exothermicity are correspond to anticipated AP-HTPBthan the AP-PVCbased composite solid propellants.
9)The thermo-gravimetric analysis show s that 100%mass loss in AP-HTPBbased propellants decomposes at 420.3°Cw hereas in case of AP-PVC 80%mass loss occur at 397.6°C.
杂志排行
Defence Technology的其它文章
- Flash X-ray radiography technique to study the high velocity impact of soft projectile on E-glass/epoxy composite material
- Novel colored f l ames via chromaticity of essential colors Ramy Sadek,Mohamed Kassem,Mohamed Abdo,Ahmed Fahd,Hesham Tantaw y,Amir Elsaidy,Sherif Elbasuney*
- Estimation of projected surface area of irregularly shaped fragments Elvedin Kljuno*,Alan Catovic
- Experimental study of bullet-proo f i ng capabilities of Kevlar,of different w eights and number of layers,w ith 9 mm projectiles
- Parametric study of single con f i ned fragment launch explosive device
- Optimization of gas tungsten arc w elding parameters for the dissimilar welding between AISI 304 and AISI 201 stainless steels
