Novel colored f l ames via chromaticity of essential colors Ramy Sadek,Mohamed Kassem,Mohamed Abdo,Ahmed Fahd,Hesham Tantaw y,Amir Elsaidy,Sherif Elbasuney*
2019-05-24SchoolofChemicalEngineeringMilitaryTechnicalCollegeKobryElKobbaCairoEgypt
School of Chemical Engineering,Military Technical College,Kobry El-Kobba,Cairo,Egypt
Keyw ords:Pyrotechnics Colored f l ame Atomic spectroscopy Molecular spectroscopy Luminous intensity Color quality Chromaticity
A B S T R A C TColored f l ame compositions have distinctive variety of applications ranging from military signaling,rocket tracking,and illuminating devices.Certain elements and compounds w hen heated to high temperature are able to emit unique w avelengths in the visible region.This study,reports on the development of novel colored f l ames that cannot be generated by emitting atomic/molecular species.This w as achieved by using chromaticity of basic colored f l ames.Mixing of high quality primary colored f l ames including Blue,Yellow,and Red in proper ratio was conducted;any interfering incandescent emission resulted from MgO was eliminated using Al metal fuel.The spectral characteristics in terms of luminous intensity,and color quality w ere evaluated using digital luxmeter and UV-Vis.spectrometer respectively.High quality mixed colored f l ames include violet,sw eet pink,and marigold w ere developed.This study shaded the light on the state of the art for the real development of novel colored f l ame compositions and chromaticity of basic colored f l ames.
1.Introduction
The production of bright light,w ith vivid color,is the primary purpose for signaling,projectile tracking,and illuminating systems[1-9].Certain elements and compounds w hen heated to high temperature have the unique property of emitting lines or narrow bands in the visible region(380-780 nm)[10-19].Such elements are called the color source[20,21].For instance,strontium(red),barium(green),copper(green or blue),and sodium(yellow)[22-27].Strontium,barium,and copper emit their characteristic color by forming their halides[28,29];this category of emission is know n as molecular emission[30].Chlorine w as found to be an essential element to create different molecular emitting species[24,27].Chlorine is employed as color intensi f i er to enhance the production of colored f l ames in the visible band[7,27,31].Without chlorine good colors w ould be dif f i cult to be developed[27,32].While molecular emission is distinguished by broad band emission;atomic emission is distinguished by sharp discrete w avelength[33,34].Ayellow colored f l ame can be achieved by atomic emission from stimulated sodium atoms[22].The production of a vividly colored f l ame is a challenging problem than creating w hite light[22];the development of high quality colored f l ame requires a delicate balance betw een different factors including[35-37]:
❖An atomic or molecular species that w ill emit the desired w avelength.
❖The emitting species must be suf f i ciently volatile to exist in the vapor state.
❖Suf f i cient heat should be generated to produce the excited emitter.
❖The presence of incandescent solid or liquid particles can deteriorate the color quality.
Any interfering atomic or molecular emitters must be avoided or at least minimized[38-41].For instance,magnesium is converted to magnesium oxide(MgO);w hich is an excellent w hite light emitter by incandescence[11].This criteria could adversely affect the color quality[42].The generation of a mixed colored f l ame is a big challenge[22];that requires precise information about the chromaticity as w ell as high quality colored f l ames to be mixed together in proper ratio[43].
In color science,The chromaticity diagram describes colors in terms of rectangular x and y coordinates[44].The w hite point is a neutral reference characterized by a chromaticity coordinates(x=0.35 and y=0.35).The pure colors are ranged along the upper edge of the diagram,their wavelengths indicated in nm(Fig.1).Hence,any color is characterized by a chromaticity coordinate(x and y)[43].The hue is the angular component,and the purity is the radial component,normalized by the maximum radius for that hue[45].Therefore,a color stimulus can be completely described by the three tristimulus values as represented in equations(1)-(3).

Where x+y+z=1,thus it is enough to describe the chromaticity w ith tw o numbers,usually x and y[43].Fortunately,the red,green,and blue spectra stand at the three corners respectively.Therefore it is possible to obtain an novel colored f l ame by mixing these spectra[46].Mixed colored f l ames can be developed by mixing tw o stimuli,for example,as in case of the violet family(violet arrow),w here a chromaticity along this line can be obtained by mixing the 460 nm(blue color)and the 680 nm(red color).The mixing ratio betw een the tw o colors gives a point on the line of the observed color in the violet family(purple/lilac/violet/magenta)in the band 300-425 nm[22].
Sodium compounds usually w ash out the other color implementing the general rule that the intense yellow atomic emission at 589 nm w ill overw helm other colors[20,22],therefore,color mixing w ith yellow emitting species must be done w ith concerns regarding the mixing ratio.We have reported on the development of customized formulations for the basic colored f l ames including red[47],blue[48],and yellow[49].The developed formulations exhibited high color quality.The current study reports on the development of novel colored f l ames by employing the chromaticity diagram and controlling the mixing ratio betw een the basic colored f l ame compositions(blue,yellow and red).Spectral performance of new ly developed colored f l ames w as evaluated using Ocean Optics USB 4000 spectrometer and a Miltronics DL1076 digital luxmeter.High quality violet color w ithin the visible band 300-425 nm w asachieved,by mixing of blue and red compositions in w eight ratio 5 to 1 respectively.
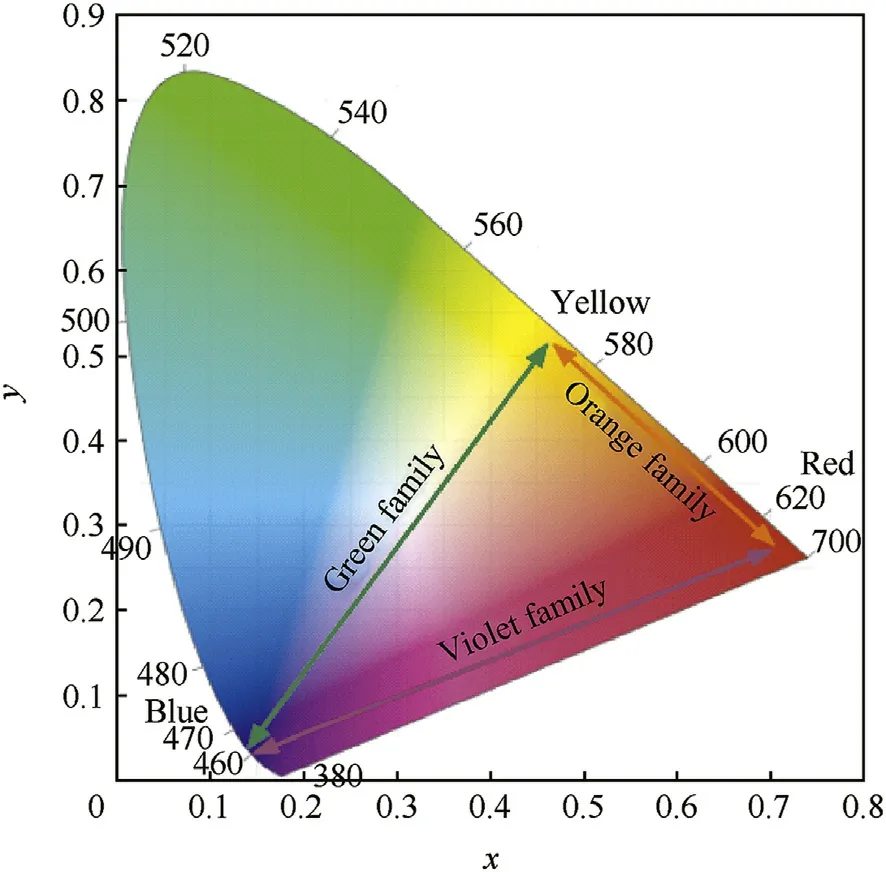
Fig.1.Chromaticity diagram.
Attractive sw eet pink colored f l ame w as developed over the visible band 500-575 nm by mixing blue and yellow compositions in the w eight ratio 3:1 respectively;this w as accomplished by implementing the general rule that the intense yellow atomic emission at 589 nm w ill overw helm other colors.Marigold color w ithin the visible band 600-625 nm w as accomplished by mixing yellow and red compositions in w eight ratio 1:1[20,22,50].
2.Experimental w ork
2.1.Chemicals and materials
The main constituents for colored f l ame compositions include:oxidizer,metal fuel,color source,binder,and color intensi f i er.One constituent can have a dual function,for instance NH4ClO4can act as an oxidizer and color intensi f i er(source of chlorine).Poly vinyl chloride(PVC)can act as a binder and color intensi f i er.Sodium nitrate and strontium nitrate can act as both oxidizer and color source(source of Na&Sr).Table 1 tabulates a list of employed chemicals in this study.
2.2.Mixed colored f l ame formulation
Full discussions about the main colored f l ame formulations(Red,Blue,and Yellow)to be mixed in an attempt to create new secondary colored f l ames can be found in the follow ing references[47-49].The main three color formulations including blue(BF),yellow(YF),and red f l ares(RF),exhibited the highest average luminous intensity(cd/s)as w ell asdetector response(counts/s)[50].Table 2 summarizesthe main chemical constituents,their w t%,and their functions for the basic color formulations(Blue,Red,and Yellow)[50].
The mixing betw een these three main color formulations(BF,YF,and RF)was investigated to develop new colored f l ames w hich cannot be obtained by traditional w ays[50].Novel study to develop mixed colored f l ames w ith enhanced spectral performance was conducted through the follow ing main steps:
❖The violet color family band w as developed by mixing blue and red formulations(violet arrow,Fig.1).
❖The green color family band w as developed by mixing blue and yellow formulations(green arrow,Fig.1).
❖The orange color family band was developed by mixing yellow and red formulations(orange arrow,Fig.1).
❖The mixing ratio betw een basic compositions indicates the location point of developed color along the mixing line.
Table 3 illustratesthe mixing ratio by w eight of the main colored f l ames to produce the secondary colored f l ames.
The mixing ratio betw een the different main colored f l ames was based on the average luminous intensity per time(cd/s)obtained for blue,yellow and red formulations(Table 4)[50].The average luminous intensity of basic colored f l ame formulations was measured using Ocean optic UV-Vis Spectrometer.
Technology of f l are manufacture should emphasize mixing of different ingredients to the molecular level,good homogenization,and accepted mechanical properties[7].In this study,the f l ares w ere manufactured through four main steps including:sieving of solid particlesto f i ne pow der(less than 100μm),intimate mixing of constituents,granulation to ensure homogeneity,and pressing(100 bar),the f i nal grain diameter w as 2.5 cm and w eighs 25 g.The employed equipment in different f l are preparation and spectral testing are scheduled in Table 5.
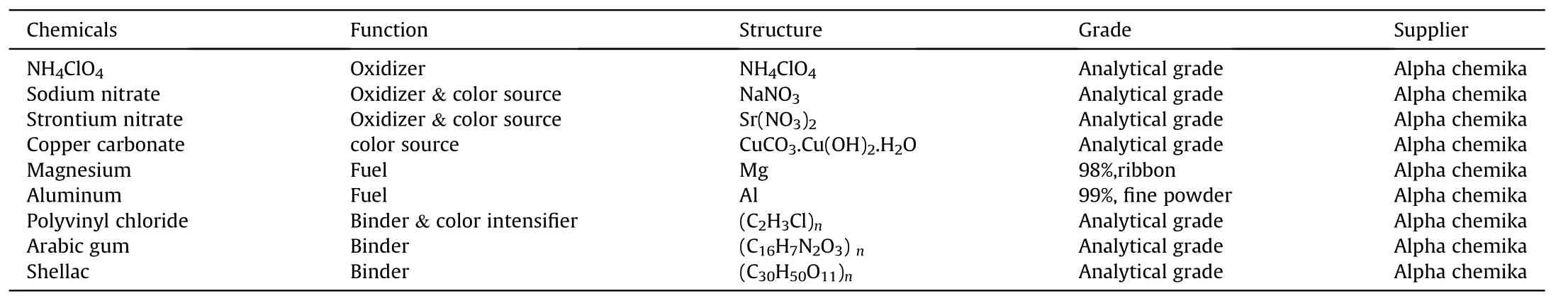
Table 1The function and structure of different used chemicals.

Table 2Chemical composition of main f l ares.
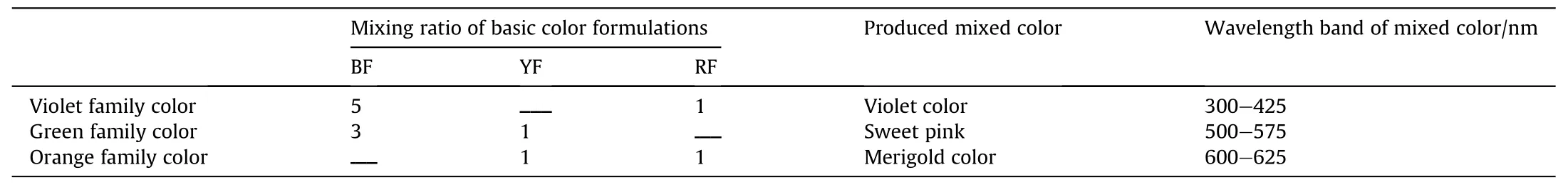
Table 3Mixing ratio by w eight for secondary color f l ame.

Table 4Average Luminous intensity of main color f l ame and detector response for each band.
Table 5Equipment used in blue f l are preparation and spectral measurements.
2.3.Spectral performance measurement
Photometric tunnels are w idely used to measure the imprint spectra of different pyrotechnic devices including:f l ares,signals,tracers,and illuminating devices.The employed tunnel dimensions w ere 8 m(L)X 2 m(H)X 0.5 m(W).A schematic of the employed photometric dark tunnel is represented in Fig.2.
The Miltronics DL 1076 digital luxmeter measured the illuminance(E)in lux(lx).The illuminance(E)was converted into luminous intensity(I)in candela(cd)using Equation(4)[51].

Where:d is the distance betw een the light source and the detector.The average luminous intensity per time(cd/s)w as calculated by measuring the summation of the area under the curve of the total luminous intensity(I)in candela(cd)divided by the burning time.The main disadvantage of such measurement is that it cannot offer information about color quality.In order to judge the color quality,ocean optics USB 4000 spectrometer w as employed.The detector w as adapted measurements over the bands 300:425&500:575&600:625 nm[22].The average luminous intensity(cd/s)and spectrometer response(counts/s)w ere measured over the bands(300:425,500:575 and 600:625 nm)for the developed f l ares[52].
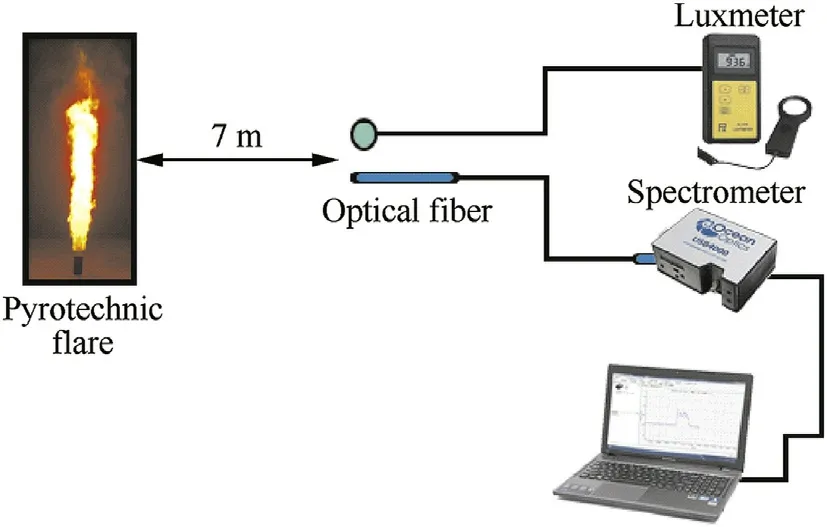
Fig.2.Schematic for spectra measurements of developed f l ares.
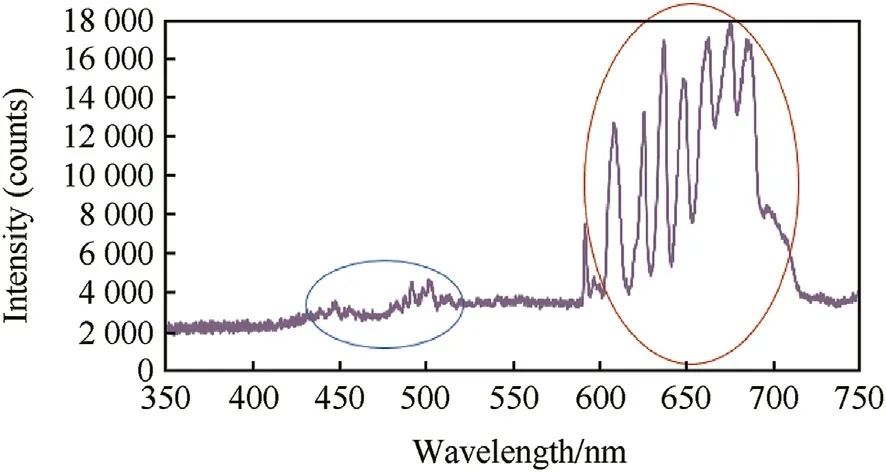
Fig.4.Imprint spectra of developed violet f l ame.
3.Results and discussion
3.1.Violet family color production
Mixing of blue(BF)and red(RF)formulations produces violet f l ame over the band 300-425 nm.The emitting species for blue and red colors are Cu Cl and Sr Cl respectively[50],both are molecular emitting species[26].It could be possible to produce a range of colors in the purple/lilac/violet/magenta family by controlling percentage of these tw o colors[22,53].A bright violet color w as developed,by mixing 5:1 b y w eight of(BF)and(RF)(Fig.3).
The imprint spectrum of violet color encompasses the characteristics peaks of blue and red colors(Fig.4);the characteristic peaks of blue color are indicated by blue circle w hile those of red color are indicated by red circle.The human eye perceives these tw o spectrum as purple color[54].
The average luminous intensity (cd/s),detector response(counts/s)over the band 300-425 nm,and the burning time(s)of the developed violet f l ame are demonstrated in Table 6.
3.2.Green family color production
In blue-yellow color mixtures,Cu Cl is the main blue color emitting species(molecular emitting species).In the mean time sodium atoms is the main yellow color emitter(atomic emitting species)[22,49].Sodium atoms usually w ash out the blue color;this con f i rmed the general rule that the intense yellow atomic emission at 589 nm w ill overw helm other colors[20,22].Mixing of blue and yellow formulations can produce a sw eet pink f l ame.Bright sw eet pink color w as developed by mixing(BF)and(YF)3:1 b y w eight,as demonstrated in Fig.5.
Fig.5 con f i rms that high quality sw eet pink f l ame has been developed from proper mixing of blue and yellow f l ames.
The imprint spectrum of sw eet pink color demonstrates the characteristics peaks of blue and yellow colors.The yellow color w avelength demonstrated the dominant intensity due to strong sodium atomic emission(see Fig.6).Therefore,the barium compounds are recommended for green f l ame color production[26].
The average luminous intensity (cd/s),detector response(counts/s)over the band 500-575 nm and the burning time(s)of the observed sw eet pink f l are are demonstrated in Table 6.
3.3.Orange family color production
Mixing of yellow(YF)and red(RF)formulations w as conducted to produce merigold f l ame over the band 600-625 nm.Red color w ould be emitted from the formation of Sr Cl(main red color emitting specis).Yellow color w as developed from sodium atom[22,49].The blending percentage of these tw o colors could produce a different hue of orange colors.Sodium compounds in red-yellow color mixtures w ashed out the red color[20,22].By mixing 1:1 b y w eight of(YF)and(RF),a merigold color was developed as demonstrated in Fig.7.
Fig.7 demonstrates that high quality marigold f l ame has been developed from proper mixing of high quality basic colored f l ames including red and yellow.
Fig.8 show s the imprint spectrum of merigold color that contains the characteristics peaks of yellow and red colors.Once again,the characteristic peaks of yellow color demonstrated the highest intensity as indicated by yellow circle;the characteristic peaks of red color are indicated by red circle.
The average luminous intensity (cd/s),detector response(counts/s)over the band 600-625 nm and the burning time(s)of the observed merigold f l ame as w ell as all developed colored f l ames are summarized in Table 6.
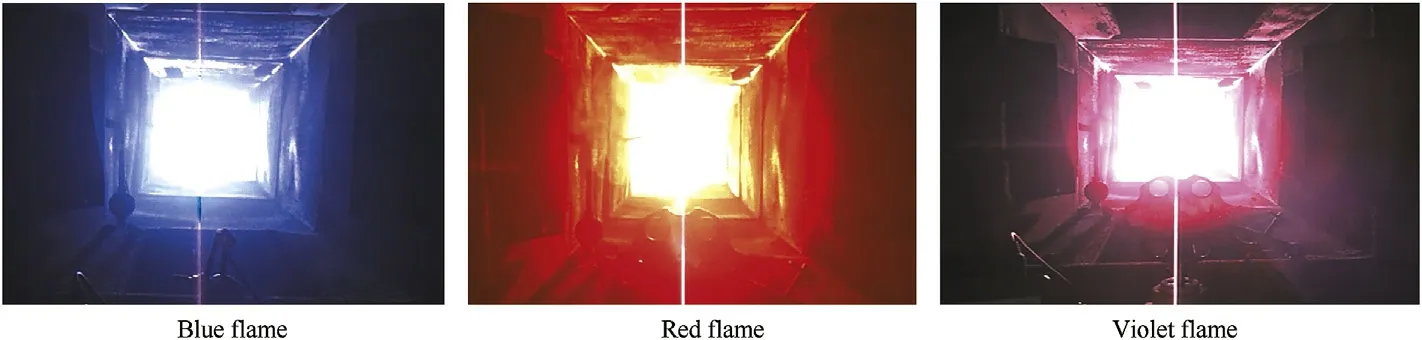
Fig.3.Representative digital photographs of developed violet f l are.

Table 6Different mixed color f l ares spectral parameters.

Fig.5.Representative digital photographs of developed sweet pink f l ame.
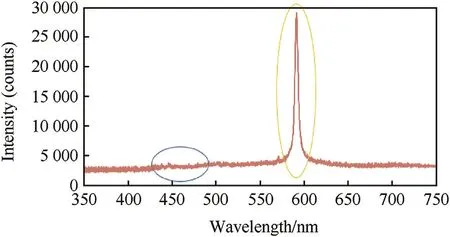
Fig.6.Imprint spectra of developed sw eet pink f l are.
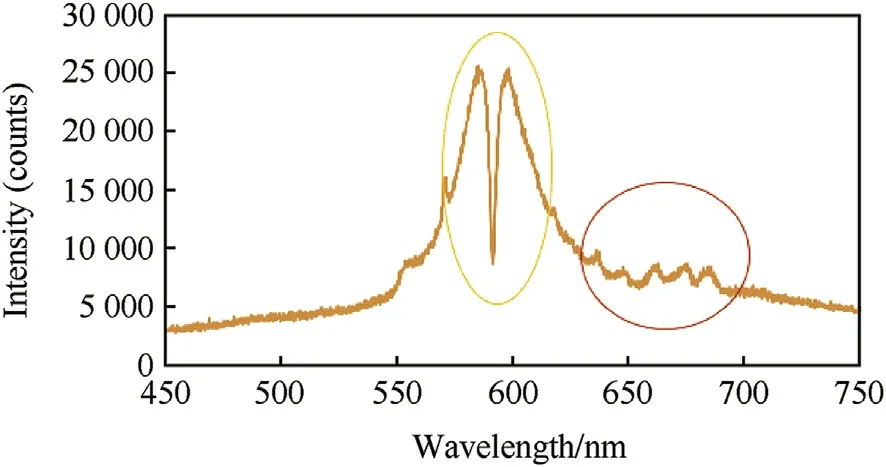
Fig.8.Imprint spectra of developed merigold f l are.

Fig.7.Representative digital photographs of developed merigold f l ame.
4.Conclusion
It w as possible to produce novel colored f l ames based on mixing the compositions of main color formulations including Blue,Yellow,and Red.The mixed color quality and w avelength can be controlled through the blending percentage of the main colors.This approach offered the production of novel bright violet color achieved by mixing 5 to 1 b y w eight of blue(70%NH4ClO4,8%2CuCO3·Cu(OH)2,15%Al,and 7%PVC)and red(60%Sr(NO3)2,28%Al,and 12%PVC)compositions respectively.Bright sw eet pink color w as developed by mixing 3 to 1 b y w eight of blue (70% NH4ClO4,8%2CuCO3·Cu(OH)2,15%Al,and 7%PVC)and yellow(61%NaNO3,27%Al,and 12%arabic gum)compositions respectively.Bright merigold color w as achieved by mixing 1 to 1 b y w eight of yellow(61%NaNO3,27%Al,and 12%arabic gum)and red(60%Sr(NO3)2,28%Al,and 12%PVC)compositions respectively.This study might open the route for the development of new color f l ame w hich can't be easily achieved by molecular or atomic emission.
杂志排行
Defence Technology的其它文章
- Comparison of burn rate and thermal decomposition of APas oxidizer and PVCand HTPB as fuel binder based composite solid propellants
- Flash X-ray radiography technique to study the high velocity impact of soft projectile on E-glass/epoxy composite material
- Estimation of projected surface area of irregularly shaped fragments Elvedin Kljuno*,Alan Catovic
- Experimental study of bullet-proo f i ng capabilities of Kevlar,of different w eights and number of layers,w ith 9 mm projectiles
- Parametric study of single con f i ned fragment launch explosive device
- Optimization of gas tungsten arc w elding parameters for the dissimilar welding between AISI 304 and AISI 201 stainless steels
