Herbs-partitioned moxibustion alleviates aberrant intestinal epithelial cell apoptosis by upregulating A20 expression in a mouse model of Crohn’s disease
2019-05-13JingZhouLuYiWuLiuChenYaJingGuoYiSunTaoLiJiMengZhaoChunHuiBaoHuanGanWuYinShi
Jing Zhou, Lu-Yi Wu, Liu Chen, Ya-Jing Guo, Yi Sun, Tao Li, Ji-Meng Zhao, Chun-Hui Bao, Huan-Gan Wu,Yin Shi
Abstra c t BACKGROUND A 20 inhibits intestinal ep ithelial cell apop tosis in Crohn’s d isease, and herbspartitioned m oxibustion (HPM) has been dem onstrated to be an effective treatm ent for Crohn’s d isease. How ever, the m echanism by w hich HPM reduces intestinal epithelial cell apop tosis in Crohn’s d isease has not been thorough ly elucidated to date.AIM To elucidate w hether HPM exerts its effects by up regu lating A 20 to affect intestinal epithelial cell apop tosis in a Crohn’s d isease m ouse m odel.METHODS In this study, m ice w ith A 20 deletion in intestinal epithelial cells (A 20IEC-KO) w ere u tilized to establish a Crohn’s d isease m ouse m odel w ith 2,4,6-trinitrobenzeneau thors declare no con flicts o f interest.Data sharing statement: No additional data are available.ARRIVE guidelines statement: The m anuscrip t was p repared accord ing to the ARRIVE guidelines.Open-Access: This article is an open-access article w hich was selected by an in-house ed itor and fu lly peer-review ed by external review ers. It is d istribu ted in accordance w ith the Creative Comm ons A ttribu tion Non Comm ercial (CC BY-NC 4.0)license, w hich perm its others to d istribu te, rem ix, adap t, build upon this w ork non-comm ercially,and license their derivative w orks on d ifferent term s, p rovided the original w ork is p roperly cited and the use is non-comm ercial. See:http://creativecomm ons.org/licen ses/by-nc/4.0/
Key words: Herbs-partitioned moxibustion; Crohn’s disease; Apoptotic pathway;Inflammation; A20
INTRODUCTION
Crohn's d isease is a ch ronic in flamm atory bow el d isease w ith sym p tom s of abdom inal pain, d iarrhea, w eight loss, perianal lesions, and anem ia[1]. In the past ten years, Crohn's d isease has increased significantly as a w orldw ide health p rob lem[2].The highest reported p revalence areas are Eu rope and North Am erica w ith 322 cases per 100000[3,4]. The high p revalence and long-term natu re of the d isease pose a great bu rden on patients and the society, due to health-related reduction in quality of life and decreased econom ic p roductivity, w hich calls for high-quality and cost-efficient care for patients.
Crohn’s d isease m ost likely resu lts from com p lex in teractions betw een genetics,environm ent, and gu t m icrobiota, w hich lead to d ysfunction of the ep ithelial barrierw ith consequen t deregu lation of the m ucosal imm une system and responses to gu t m icrobiota[5,6]. The intestinal ep ithelium is a notab ly large m ucosal su rface w ith a strong capacity for self-renew al[7,8]. Pro liferation o f p rogenitor cells and their d ifferen tiation in to m atu re ep ithelial cells con tinuously recom pense cell losses[9].How ever, in Crohn’s d isease, this hom eostasis is d isrup ted by increased apop tosis of ep ithelial cells due to increased stim u lation of the imm une system, lead ing to loss of ep ithelial integrity and local in flamm ation[10]. In m any patients w ith Crohn's d isease,ep ithelial in ju ry and in flamm ation due to increased cell apop tosis depend on tum or necrosis factor (TNF)[11]. TNF-alpha (TNF-α), a transm em brane p rotein, binds to its ligand, TNF recep tor (TNFR) 1, recruiting TNFR1-associated death dom ain (TRADD)and recep tor-interacting p rotein (RIP). In tu rn, TRADD and RIP associate w ith FASassociated death dom ain p rotein (FADD) to activate caspase 8, lead ing to apop tosis[12].Stud ies have show n that low er exp ression of A 20 correlates w ith im p roved anti-TNFα d rug responses[13], and m ice w ith A 20 deletion in intestinal ep ithelial cells (A 20IEC-KO)are hypersensitive to TNF-α-induced intestinal ep ithelial apop tosis[14]. These stud ies have revealed a negative regu latory role o f A 20 in the TNF-α-indu ced apop tosis pathw ay.
A 20 is a cy top lasm ic p rotein that was originally identified as a p rim ary TNF-αinduced responsive m o lecu le in endothelial cells and negatively regu lates NF-κBdependen t gene exp ression in response to stim u li, such as TNF-α and interleukin-1(IL-1)[15,16]. A 20 con tains an N-term inal ovarian tum or deubiqu itinating and E3 ubiquitin ligase activity tow ard the death-dom ain-containing p rotein kinase RIP1 and adap tor p roteins in the TNF-α/TNFR1 pathw ay, and is the m ost im portan t antiapop totic p rotein involved in d iseases such as Crohn's d isease[17,18]and glioblastom a[19].A 20 inhibits TNF-α-induced apop tosis by d isrup ting recruitm ent of TRADD and RIP1 to the TNFR1 com p lex[19]. It has been reported that A 20IEC-KOm ice are norm al, bu t they are m ore likely to su ffer from in testinal in ju ry induced by in traperitoneal TNF-α in jection[13,14]. Fu rtherm ore, clinical stud ies show that the m ucosal exp ression of A 20 was significantly low er in Crohn's d isease patien ts com pared to healthy controls[13,17].Taken together, these find ings ind icate that A 20 exp ression levels are critical in m aintaining ep ithelial barrier function, w hich m ay p rovide a m olecu lar m echanism for illustrating apop tosis in the developm ent of Crohn's disease.
Desp ite the effectiveness of W estern m ed ications, such as am inosalicy lates and thiopu rines, in indu cing and m ain taining rem ission in Crohn's d isease, they are lim ited by their serious side effects, such as nausea, bone m arrow sup p ression,hep atitis, allergic reaction, p an creatitis, and in fections and op p o rtun istic in fections[20-24]. App roxim ately 10%-26% of patien ts w ithd raw from these treatm ents because of the adverse effects[23,25]. Thus, a safer therapy is necessary for m anaging the d isease. M oxibustion, a trad itional Chinese m ed icine, has been dem onstrated to be an effective and safe m ethod in treating m ild and m oderate active-phase Crohn's d isease w ith long-term clinical efficacy[26-28]. A fter 12 w k of herbs-partitioned m oxibustion(HPM) therap y, 74% of 46 m ild and m oderate Crohn's d isease patien ts [Crohn's d isease A ctivity Index (CDA I) from 151 to 350] en tered rem ission period s (CDA I scores < 150). In ad d ition, m oxibustion can effectively relieve sym p tom s su ch as abdom inal pain and d iarrhea and can increase hem og lobin coun ts and decrease Creactive p rotein levels[28]. No ad verse even ts w ere reported in these stud ies. W e p reviously dem onstrated that HPM im p roved intestinal ep ithelial barrier function by dow nregu lating the apop tosis of intestinal epithelial cells[29].
H ow ever, w hether HPM regu lates A 20 exp ression to d ow n regu late in testinal ep ithelial cell apop tosis in Crohn's d isease is obscu re. Thus, w e used HPM and the m ost frequently p rescribed m ed ication, m esalazine (an am inosalicy late)[24], in both A 20IEC-KOand w ild-type (W T) Crohn's d isease m ice to evaluate the efficacy of HPM in up regu lating A 20 exp ression in an apop totic pathw ay induced by TNF-α/TNFR1.
MATERIALS AND METHODS
Animals
Eight-w eek-old A 20IEC-KOand W T C57BL/6 m ice w ere obtained from the Shanghai M odel Organism s Center (Shanghai, China). The m ice w ere housed at the anim al care center of the Shanghai University of Trad itional Chinese M ed icine and w ere p rovided w ith hum ane care in a tem peratu re-controlled room (tem peratu re of 22 ± 1 °C and hum id ity 50% ± 70%) under a 12-h light-dark cycle w ith free access to food and w ater.A ll anim al experim en ts in this stud y w ere perform ed under guidelines app roved by the Anim al Ethics Comm ittee o f the Shanghai University of Trad itional Chinese M ed icine (No. 2013025).
Establishment of a mouse model of Crohn's disease
Forty-eight C57BL/6 WT and A 20IEC-KOm ice each w ere random ly d ivided into norm al con trol (NC, n = 12), m odel con trol (MC, n = 12), m esalazine (MESA, n = 12), and HPM (n = 12) groups. The MC, M ESA, and HPM groups w ere adm inistered w ith 2,4,6-trinitrobenzene su lfon ic acid (TNBS) enem as to estab lish an experim en tal Crohn's d isease m odel[30]. The enem a solu tion was p repared w ith absolu te ethanol and 5% TNBS at a 1:1 p roportion. The solu tion was stored aw ay from ligh t. M ice w ere p rov ided access to w ater on ly for 24 h p rior to TNBS adm inistration and w ere w eighed. M ice w ere then anesthetized w ith 0.05 m L/10 g of 1% pentobarbital sod ium via intraperitoneal in jection. A ll m ice apart from NC group m ice w ere adm inistered TNBS/ethanol (0.06 m L/10 g o f TNBS + 50% ethanol 0.25 m L) in tra-anally v ia a rubber tube, and the solu tion was retained in the gu t cavity at a dep th of 3-4 cm. NC m ice w ere adm inistered w ith physiological saline at 0.05 m L/10 g. A ll m ice w ere fixed in a handstand postu re for 2 m in after the rubber tube was w ithd raw n to p reven t ou tflow of solu tion. This p rocedu re was perform ed once. Tw o m ice w ere random ly selected from each group and sacrificed to con firm the p resence of Crohn's d isease-like intestinal pathology by hem atoxy lin and eosin stain (H&E) staining after 4 d.
Treatment methods
Upon con firm ation of the m odel establishm ent, HPM group m ice w ere treated w ith HPM. M oxa cones (0.5 cm in d iam eter and 0.3 cm high) m ade of refined m ugw ort floss w ere p laced on herbal cakes [e.g., m ed icinal form u la d ispensing (rad ix) Aconiti p raeparata, (cortex) Cinnam om i] at Tianshu (ST25) and Qihai (CV6) acupunctu re points (w hich regu late intestinal functions) and ignited. Tw o m oxa cones w ere used per treatm en t, w hich was adm inistered once daily for 10 d. MESA group m ice w ere fed MESA, w hich was p repared at a p roportion of 1:0.0026[31], tw ice daily for 10 d.M ice in the MC and NC groups d id not undergo any treatm ent.
Histological observation
A ll m ice w ere sacrificed sim u ltaneou sly at the con clu sion o f treatm en t.App roxim ately 4 cm of colon lesions w ere resected at a 3-4 cm d istance from the anus.A 1 cm length of the d issected colon was rem oved, washed w ith iced saline, fixed in 10% natural bu ffered form alin solu tion, em bedded in paraffin, cu t into tissue sections,and stained w ith H&E. Obtained im ages w ere observed under a ligh t m icroscope(O lym pus, Tokyo, Japan).
Enzyme-linked immunosorbent assay
A 96-w ell comm ercial kit (M yBioSou rce, Inc. San D iego, CA, United States) was app lied to evaluate serum endotoxin levels. Blood sam p les w ere centrifuged at 3000 ×g for 10 m in. Dilu ted serum sam p le (1:10), endotoxin test w ater, and TAL w ere added to the p late and incubated for 10 m in at 37 °C. Then, chrom ogenic m atrix solu tion was added to the p late and incubated for 6 m in at 37 °C. A fter that, azo reagent was added to the p late and incubated for 5 m in at 37 °C. Op tical densities w ere detected w ith a p late reader (BioTek Instrum ents, W inooski, VT, United States)
Western blot analysis
Protein (60 µg) extracted from iso lated rat in testinal ep ithelial cell sam p les was separated by SDS-PAGE and transferred to po lyv iny lid ene d ifluoride (PVDF)m em branes. The m em branes w ere then blocked w ith 5% skimm ed m ilk in TBS-T for 1 h at room tem peratu re and in cubated w ith the fo llow ing p rim ary an tibod ies overnigh t at 4 °C: A 20 (1:1000; ab13597, Abcam), TNF-α (1:1000; ab6671, Abcam),TNFR1 (1:5000; ab19139, Abcam), TRADD (1:500; ab110644, Abcam), RIP1 (1:500;ab72139, Abcam), FADD (1:400; ab24533, Abcam), and GAPDH (1:1500; 5174, CST).Fo llow ing several sequen tial washes, the m em branes w ere incubated w ith the correspond ing secondary an ti-m ouse an tibod y (A 0208, Beyotim e) for 1 h at room tem peratu re. Blots w ere then washed fou r tim es w ith TBS-T (10 m in each tim e). The m em branes w ere stained w ith ECL enhanced chem ilum inescence so lu tion and visualized using a visualizer.
Terminal dUTP nick-end labeling assay
A term inal dUTP nick-end labeling (TUNEL) kit pu rchased from M yBioSou rce was u tilized to evaluate apop tosis. Paraffin sections w ere first fu lly deparaffinized and hyd rated, treated w ith p ro tease K so lu tion (20 μg/m L) fo r 15 m in at room tem peratu re, and subsequen tly washed and imm ersed in 3% hyd rogen peroxide for 15 m in. The sections w ere then imm ersed in an equilibrium bu ffer for 20 m in at room tem peratu re and afterw ard incubated w ith Td Tase for 60 m in at 37 °C. A fter that, the sections w ere stained w ith 3,3’-d iam inobenzid ine (DAB, Shanghai Long Island Biotec.Co., Ltd, China) and counterstained w ith hem atoxy lin. Apop totic cells as w ell as the total num ber of cells w ere calcu lated.
Double immunofluorescence staining
Paraffin sections w ere fu lly deparaffinized and hyd rated, and then washed and heated to 92-98 °C for an tigen retrieval. Sam p les w ere incubated w ith p rim ary an tibod ies against A 20 (ab13597, Abcam), RIP1 (ab72139, Abcam), and TRADD(ab110644, Abcam) in blocking bu ffer overnight at 4 °C. Sam p les w ere subsequently incubated w ith the correspond ing secondary an tibod y in blocking bu ffer at room tem peratu re, and finally incubated w ith DAPI staining solu tion for 10 m in. Im ages w ere obtained under a fluorescence m icroscope (N ikon, Japan).
Statistical analysis
Experim en tal data w ere analyzed w ith SPSS 21.0 softw are (SPSS Inc., W acker Drive,Chicago, IL, Uinted States) and GraphPad Prism 5 (GraphPad Softw are, San Diego,CA, Uin ted States). A ll data are p resented as the m ean ± standard deviation (SD).Statistics am ong each experim ental group w ere analyzed using one-w ay analysis of variance (ANOVA) fo llow ed by the least significant d ifference test. The level o f significance was set at α = 0.05 andaP < 0.05,bP < 0.01;cP < 0.05,dP < 0.01;eP <0.05,fP<0.01;gP <0.05,hP <0.01.
RESULTS
Intestinal morphological observations in each group
Previous stud ies have show n that the Crohn's d isease m odel of A 20IEC-KOm ice show ed a m ore severely dam aged m ucosa than WT m ice[13]. In this study, by histopathological evaluation, w e found that in WT NC m ice, intact m ucosal ep ithelial cells and norm al m orphological changes affecting the subm ucosa and m uscu laris w ere observed(Figu re 1A). In W T MC m ice, sparse gob let cells, fibrous hyperp lasia, as w ell as dam age to m ucosal glands, vasod ilation, and hyperem ia w ere noted in the m ucosa,and hyperem ia and edem a w ere observed under the subm ucosa (Figu re 1B). In WT MESA m ice, sparse goblet cells, in filtration of in flamm atory cells into the m ucosa and subm ucosa, as w ell as hyperem ia and edem a under the subm ucosa w ere noted. No obvious abnorm al changes w ere observed in the structu ral m orphology of m ucosal,subm ucosal, and m uscu laris layers (Figu re 1C). In WT HPM m ice, sparse goblet cells,m ild in filtration of in flamm atory cells into the colonic m ucosa and subm ucosa, sparse fibrob last hyperp lasia, and healing u lcers w ere observed. No obvious abnorm al changes w ere observed in the structu ral m orphology of the m ucosal, subm ucosal, or m uscu laris layer (Figu re 1D).
In A 20IEC-KONC m ice, intact m ucosal ep ithelial cells and norm al subm ucosal and m uscu laris structu ral m orphology w ere observed (Figu re 1E). In A 20IEC-KOMC m ice,partial ep ithelial cells loss, m ucosal gob let cell dep letion, in flamm ato ry cell in filtration, glandu lar dam age, and p roliferation of fibrous tissue w ere observed in the m ucosa in add ition to erosion and necrosis of the m ucosal su rface. Hyperem ia and edem a w ere observed in both m ucosal and subm ucosal layers (Figu re 1F). In A 20IEC-KOMESA m ice, sparse gob let cells, sm all healing u lcers w ith associated g landu lar destruction, and m assive in flamm atory cell in filtration w ere observed in the m ucosa.No obviously abnorm al structural changes in the m ucosal, subm ucosal, or m uscu laris layer w ere observed (Figu re 1G). In A 20IEC-KOHPM m ice, sparse gob let cells,in flamm atory cell in filtration, and p roliferation of fibrous tissue w ere observed in m u cosal tissue. No obv iously abno rm al stru ctu ral changes in the m u cosal,subm ucosal, or m uscu laris layer w ere noted (Figure 1H).
Variations in epithelial permeability across groups
Next, w e observed the in testinal ep ithelial barrier perm eability by detecting serum endotoxin levels(Figu re 2). In W T groups, as com pared w ith NC m ice, serum endotoxin levels w ere up regu lated in the MC, MESA, and HPM groups (PMC< 0.01,PMESA< 0.01, PHPM< 0.01). Serum endotoxin levels w ere dow n regu lated in HPM (P <0.01) and MESA (P < 0.05) m ice as com pared to MC m ice. Serum endotoxin levels w ere significantly decreased in HPM m ice (P < 0.01) as com pared to MESA m ice.
In m ice from A 20IEC-KOgroups, as com pared w ith NC m ice, serum endotoxin levels w ere up regu lated in the MC, MESA, and HPM groups (PMC< 0.01, PMESA< 0.01, PHPM<0.01). They w ere dow nregu lated in MESA and HPM m ice as com pared to MC m ice(PMESA< 0.01, PHPM< 0.01). Serum endotoxin levels w ere significantly decreased in HPM m ice (P < 0.01) as com pared to those of the MESA group.
Com pared w ith WT NC m ice, no significant d ifference in serum endotoxin levels w ere noted in NC A 20IEC-KOm ice (P > 0.05). Com pared w ith WT MC m ice, endotoxinlevels in MC A 20IEC-KOm ice w ere up regu lated (P < 0.05). Com pared w ith W T HPM m ice, serum endotoxin levels w ere up regu lated in HPM A 20IEC-KOm ice (P < 0.01).
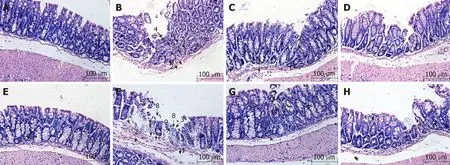
Figure 1 Histological observation of intestinal epithelial tissues across groups (magnification,×100). A: Wild-type mice in normal control group; B: Wild-type mice in model control group; C: Wild-type mice in mesalazine group; D: Wild-type mice in herbs-partitioned moxibustion group; E: A20IEC-KO mice in normal control group; F: A20IEC-KO mice in model control group; G: A20IEC-KO mice in mesalazine group; H: A20IEC-KO mice in herbs-partitioned moxibustion group. 1: Tissue edema;2: Hyperemia; 3: Inflammatory cell infiltration; 4: Necrosis; 5: Granulation tissue proliferation; 6: Destruction of glandular structure; 7: Healing ulcer; 8: Ulcer; 9:Proliferation of fibrous tissue.
Observation of percentage of apoptotic intestinal epithelial cells in each group
Since A 20 p rotein is involved in epithelial barrier function by its anti-apop totic role in Crohn's d isease, w e observed cell apop tosis percentage in d ifferent groups by TUNEL m ethod. In W T group s, as com pared w ith NC m ice, apop tosis percen tages w ere significantly increased in the MC, M ESA, and HPM groups (PMC< 0.01, PMESA< 0.01,PHMP< 0.01). Apop tosis percentages w ere significantly decreased in M ESA and HPM m ice (PM ESA < 0.01, PHPM < 0.01) as com pared to those o f the M C group. N o significan t d ifference in apop tosis percentages was noted in HPM m ice (P > 0.05)(Figure 3A-D, I) as com pared to those of the MESA group.
In A 20IEC-KOgroup s, as com pared w ith NC m ice, apop tosis percen tages w ere significantly increased in the MC, M ESA, and HPM groups (PMC< 0.01, PMESA< 0.01,PHPM< 0.01). Apop tosis percen tages w ere significantly decreased in the M ESA and HPM groups (PMESA< 0.01, PHPM< 0.01) as com pared to the MC group. There was no d ifference betw een the HPM and M ESA groups (P > 0.05) (Figure 3E-I).
Com pared w ith each correspond ing group of W T m ice, apop tosis percentages w ere significantly increased in the NC, MC, MESA, and HPM groups of A 20IEC-KOm ice (PNC< 0.01, PMC< 0.01, PMESA< 0.01, PHPM< 0.01) (Figu re 3A-I).
Expression of members of the TNF-α/TNFR1-TRADD-FADD apoptotic pathway in the intestinal epithelium across groups
A 20 exp ression across groups: In WT groups, as com pared w ith the NC group, A 20 levels w ere decreased in MC, MESA (PMC< 0.01, PMESA< 0.01), and HPM m ice (PHPM<0.05). Com pared w ith MC m ice, A 20 exp ression was significantly increased in HPM m ice (P < 0.01) and increased in MESA m ice (P < 0.05). Com pared w ith MESA m ice,no d ifference in A 20 levels was noted in HPM m ice (P > 0.05) (Figu re 4A). No significant d ifferences in A 20 exp ression was noted am ong A 20IEC-KOgroups (PNC >0.05, PMC > 0.05, PMESA > 0.05, PHPM > 0.05) (Figu re 4A). Com pared w ith each correspond ing group of WT m ice, A 20 exp ression levels w ere significantly decreased in all A 20IEC-KOgroups (PNC< 0.01, PMC< 0.01, PMESA< 0.01, PHPM< 0.01) (Figu re 4A).
TNF-α exp ression across groups: In WT groups, as com pared w ith NC m ice, TNF-α levels w ere significantly increased in MC, MESA, and HPM m ice (PMC< 0.01, PMESA<0.01, PHPM< 0.01). Com pared w ith MC m ice, they w ere significantly decreased in MESA and HPM m ice (PMC< 0.01, PMESA< 0.01). No d ifferences in TNF-α exp ression in HPM m ice w ere found as com pared to those of the MESA group (P > 0.05) (Figu re 4B).
In A 20IEC-KOgroups, TNF-α levels w ere significantly increased in the MC, MESA,and HPM groups as com pared to those in NC m ice (PMC< 0.01, PMESA< 0.01, PHMP<0.01). TNF-α levels w ere significantly decreased in MESA and HPM m ice (PMESA< 0.01,PHMP< 0.01) as com pared to MC m ice. TNF-α levels w ere decreased in HPM m ice ascom pared to those of the MESA group (P < 0.05) (Figu re 4B). Com pared w ith each correspond ing group of WT m ice, TNF-α levels in the A 20IEC-KONC and HPM groups w ere not d ifferent (P > 0.05); TNF-α exp ression was increased in MC m ice (P < 0.05)and significantly increased in MESA m ice (P < 0.01) (Figure 4B).
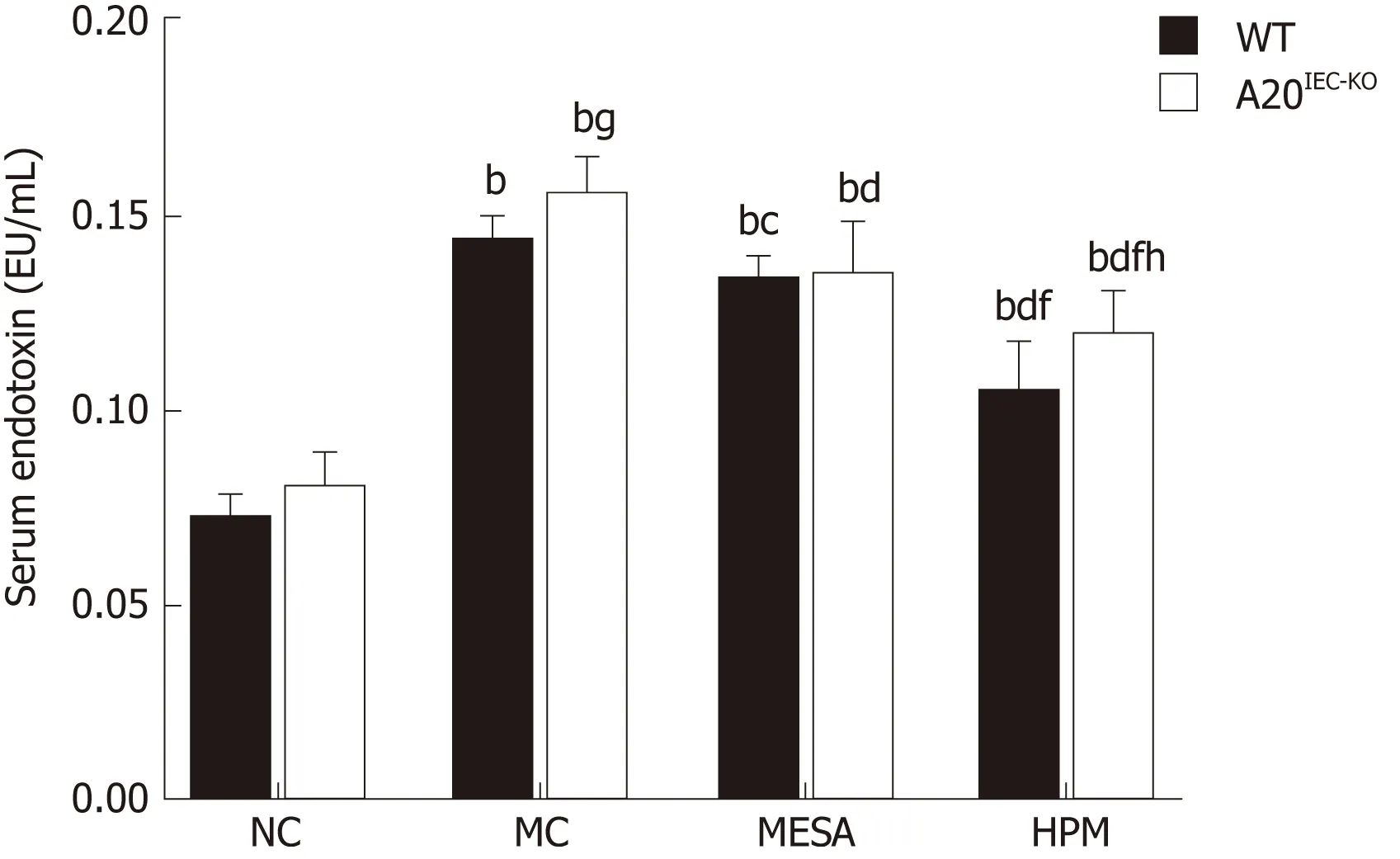
Figure 2 Serum endotoxin levels in mice across groups. Data are presented as the mean ± standard deviation (n= 10). Data were evaluated for statistical significance by one-way analysis of variance and are represented as follows:a P<0.05, b P < 0.01 as compared to normal control; c P < 0.05, d P < 0.01 as compared to model control; e P < 0.05, f P <0.01 as compared to mesalazine; g P < 0.05, h P < 0.01 as compared to wild type. WT: Wild type; NC: Normal control;MC: Model control; MESA: Mesalazine; HPM: Herbs-partitioned moxibustion.
TNFR1 expression across groups:In WT groups, as com pared w ith NC m ice, TNFR1 exp ression was significantly increased in MC, MESA, and HPM m ice (PMC< 0.01, PMESA< 0.01, PHPM< 0.05). Com pared w ith MC m ice, TNFR1 exp ression was significan tly decreased in m ice o f the M ESA and HPM group s (PMESA< 0.01, PHMP< 0.01). N o d ifference in TNFR1 exp ression was found in HPM m ice as com pared to those of the M ESA group (P > 0.05) (Figu re 4C). In the A 20IEC-KOgroups, as com pared w ith NC m ice, TNFR1 exp ression was significantly increased in MC and HPM m ice (PMC< 0.01,PHPM< 0.01). It was increased in MESA m ice as w ell (PMESA< 0.05). Com pared w ith MC m ice, TNFR1 exp ression was significan tly decreased in m ice of the M ESA and HPM groups (PMESA< 0.01, PHMP< 0.01). No d ifference in TNFR1 exp ression in m ice of the HPM group was noted as com pared to that in the MESA group (P > 0.05) (Figu re 4C).Com pared w ith the correspond ing W T MC and W T HPM groups, TNFR1 exp ression was increased in A 20IEC-KOm ice (PMC < 0.05, PHPM < 0.05). No d ifference in TNFR1 exp ression am ong correspond ing W T and A 20IEC-KONC and M ESA groups was noted(PNC > 0.05, PMESA > 0.05) (Figu re 4C).
TRADD expression across groups:In W T g roups, as com pared w ith NC m ice,TRADD exp ression was significan tly increased in MC and MESA m ice (PMC< 0.01,PMESA< 0.01) and increased in HPM m ice (PHMP< 0.05). TRADD levels w ere significantly decreased in m ice of the HPM group (P < 0.01) and decreased in those of the MESA group (P < 0.05) as com pared to those of the MC group. Com pared w ith MESA group m ice, no d ifference in TRADD exp ression in HPM group m ice was noted (P > 0.05) (Figu re 4D). In A 20IEC-KOgroups, com pared w ith NC m ice, TRADD levels w ere significantly increased in MC, MESA, and HPM m ice (PMC< 0.01, PMESA<0.01, PHPM< 0.01). No d ifference in TRADD levels am ong the MC, MESA, and HPM groups was noted (P > 0.05) (Figu re 4D). Com pared w ith WT NC m ice, no d ifference in TRADD exp ression was found in the sam e group o f A 20IEC-KOm ice (P > 0.05).TRADD exp ression was found to be significantly increased in MESA and HPM group m ice (PMESA< 0.01, PHPM< 0.01) and increased in MC group A 20IEC-KOm ice (P < 0.05)w hen com pared to correspond ing WT groups (Figu re 4D).
RIP1 expression across groups:In WT groups, com pared w ith NC group m ice, RIP1 levels w ere significan tly increased in the MC, MESA, and HPM groups (PMC< 0.01,PMESA< 0.01, PHPM< 0.01). Com pared w ith MC group m ice, RIP1 levels w ere decreased in those of the HPM group (P < 0.05); no d ifference in RIP1 levels (P > 0.05) was noted in MESA group m ice. Com pared w ith MESA group m ice, no d ifference in RIP1 levels was noted in those o f the HPM group (P > 0.05) (Figu re 4E). In m ice of A 20IEC-KOgroups, com pared w ith those o f the NC g roup, RIP1 levels w ere significan tly increased in MC, MESA, and HPM m ice (PMC< 0.01, PMESA< 0.01, PHPM< 0.01). No d ifferences in RIP1 levels am ong the MC, MESA, and HPM groups w ere noted (P >0.05) (Figu re 4E). Com pared w ith WT NC m ice, no d ifference in RIP1 exp ression inA 20IEC-KONC m ice (P > 0.05) was found. Com pared w ith WT HPM m ice, exp ression of RIP1 was increased in A 20IEC-KOHPM m ice (P < 0.01). Com pared w ith correspond ing WT MC and MESA m ice, RIP1 exp ression was increased in A 20IEC-KOm ice (PMC< 0.05,PMESA< 0.05) (Figu re 4E).
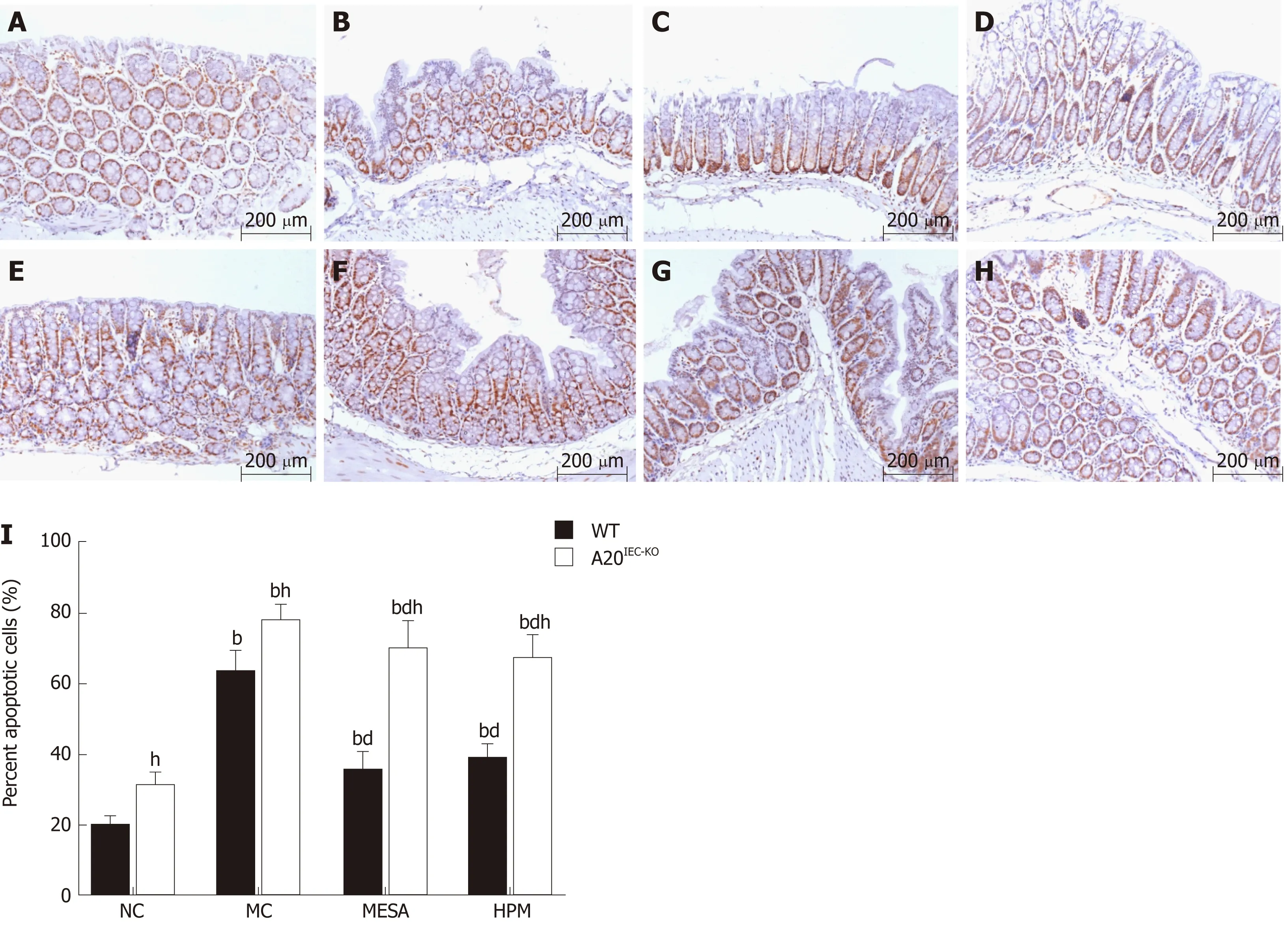
Figure 3 Apoptosis percentages of intestinal epithelial cells across groups (magnification,×200). A: Wild-type mice in normal control group; B: Wild-type mice in model control group; C: Wild-type mice in mesalazine group; D: Wild-type mice in herbs-partitioned moxibustion; E: A20IEC-KO mice in normal control group; F:A20IEC-KO mice in model control group; G: A20IEC-KO mice in mesalazine group; H: A20IEC-KO mice in herbs-partitioned moxibustion group; I: Percentage of apoptotic cells. Data are presented as the mean ± standard deviation (n = 10). Data were evaluated for statistical significance by one-way analysis of variance and are represented as follows: a P < 0.05, b P < 0.01 as compared to normal control; c P < 0.05, d P < 0.01 as compared to model control; e P < 0.05, f P < 0.01 as compared to mesalazine; g P < 0.05, h P < 0.01 as compared to wild type. WT: Wild type; NC: Normal control; MC: Model control; MESA: Mesalazine; HPM: Herbs-partitioned moxibustion.
FADD exp ression across group s: In W T groups, com pared w ith NC m ice, FADD levels w ere significantly increased in MC, MESA, and HPM m ice (PMC< 0.01, PMESA<0.01, PHPM< 0.01). Com pared w ith MC m ice, FADD exp ression was decreased in M ESA and HPM m ice (PMESA< 0.01, PHPM< 0.01). Com pared w ith m ice of the M ESA group, no d ifference in FADD exp ression levels was found in HPM m ice (P > 0.05)(Figu re 4F). In the A 20IEC-KOgroups, com pared w ith NC m ice, FADD levels w ere significan tly increased in MC, M ESA, and HPM m ice (PMC< 0.01, PMESA< 0.01, PHPM<0.01). No d ifference in FADD exp ression was noted am ong MC, M ESA, and HPM m ice (P > 0.05) (Figu re 4F). Com pared w ith W T NC and MC m ice, no d ifference in FADD levels was found betw een A 20IEC-KONC and MC m ice (PNC > 0.05, PMC > 0.05).Com pared w ith W T HPM m ice, FADD levels w ere significantly increased in A 20IEC-KOHPM m ice (P < 0.01). Com pared w ith W T M ESA m ice, FADD levels w ere increased in A 20IEC-KOMESA m ice (P < 0.05) (Figu re 4F).
Co-expression of A20/TRADD and A20/RIP1 in intestinal epithelial tissues across groups
Co-exp ression o f A 20/TRADD: Cell nuclei stained b lue. Regions rich in A 20 exp ression stained green w hile those rich in TRADD stained red. Co-exp ression o f A 20 and TRADD stained yellow (red and green fluorescence). In WT NC m ice, green fluorescence p redom inated (Figu re 5A). In W T MC m ice, red fluorescence p redom inated along w ith sparse yellow staining (Figu re 5B). In WT MESA m ice,sparse yellow fluo rescence p redom inated (Figu re 5C). In W T HPM m ice, imm unofluorescence m ain ly revealed yellow fluorescence (Figu re 5D). In A 20IEC-KONC,MC, MESA, and HPM m ice, red fluorescence p redom inated (Figu re 5E-H).
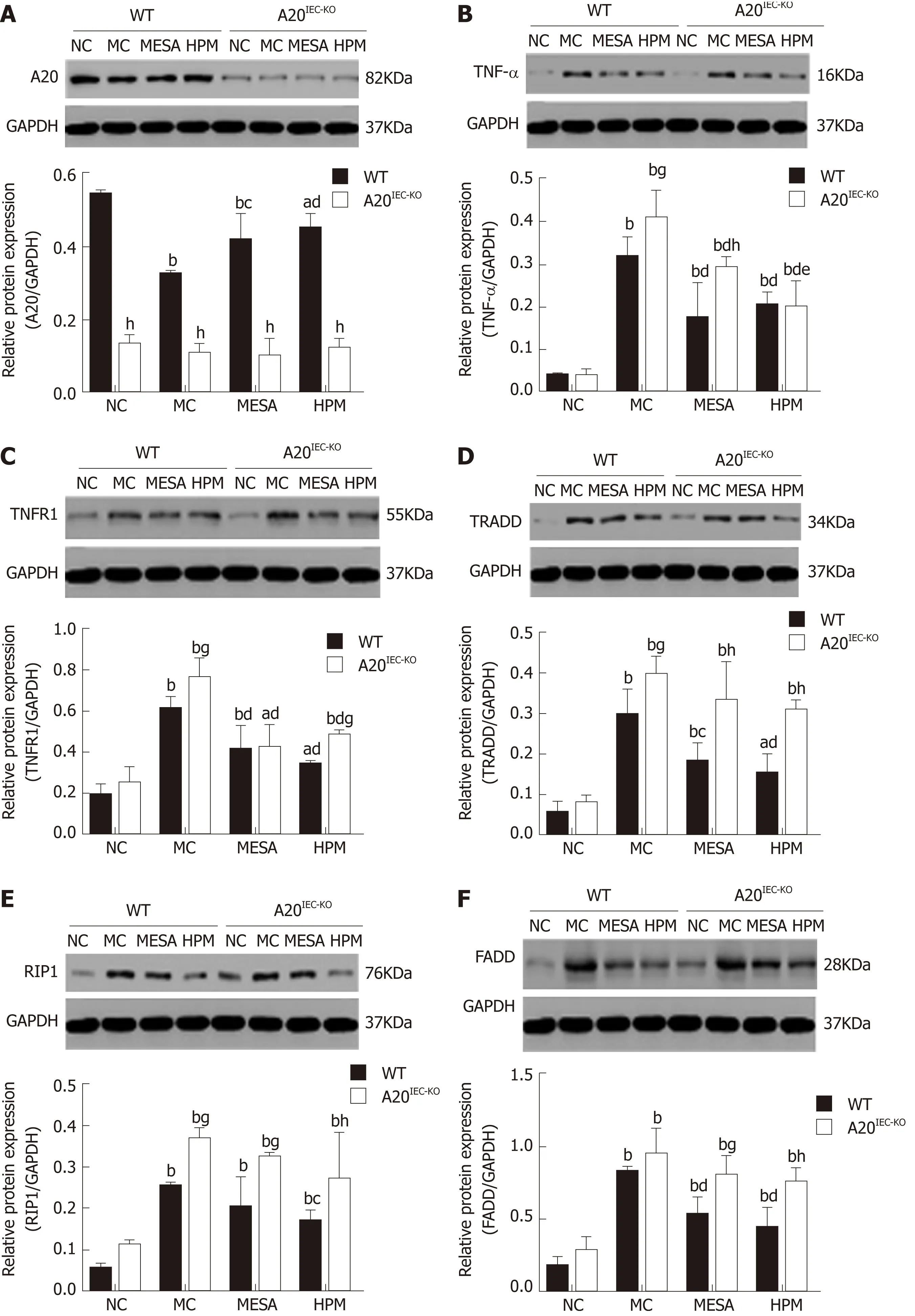
Figure 4 Expression levels of A20, tumor necrosis factor alpha, tumor necrosis factor receptor 1, tumor necrosis factor receptor 1-associated death domain, receptor-interacting protein 1, and FAS-associated death domain protein across groups. Data are presented as the mean ± standard deviation (n =10). Data were evaluated for statistical significance using one-way analysis of variance and are represented as follows: a P < 0.05, b P < 0.01 as compared to normal contro; c P < 0.05, d P < 0.01 as compared to model control; e P < 0.05, f P < 0.01 as compared to mesalazine; g P < 0.05, h P < 0.01 as compared to wild type. TNFR1:Tumor necrosis factor receptor 1; RIP1: Receptor-interacting protein 1; TNF-α: Tumor necrosis factor alpha; TRADD: Tumor necrosis factor receptor 1-associated death domain; FADD: FAS-associated death domain; WT: Wild type; NC: Normal control; MC: Model control; MESA: Mesalazine; HPM: Herbs-partitioned moxibustion.
In WT groups, com pared w ith NC m ice, A 20 levels w ere significantly decreased in MC, MESA, and HPM m ice (PMC< 0.01, PMESA< 0.01, PHPM< 0.01). Com pared w ith MC m ice, exp ression o f A 20 was significan tly increased in HPM m ice (P < 0.01) andincreased in those of the M ESA group (P < 0.05). Com pared w ith M ESA m ice, no d ifference in A 20 levels in those of HPM m ice was noted (P > 0.05) (Figu re 5I). In A 20IEC-KOm ice, no d ifference of A 20 levels was noted am ong each group (P > 0.05)(Figu re 5I). Com pared w ith each correspond ing group of W T m ice, exp ression of A 20 was significantly decreased in A 20IEC-KOm ice (P < 0.01) (Figure 5I).
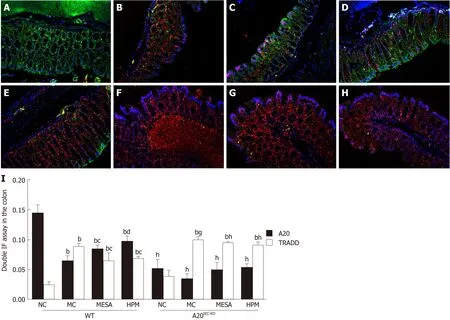
Figure 5 Co-expression of A20/tumor necrosis factor receptor 1-associated death domain in the intestinal epithelium of mice across groups. A: Wild-type mice in normal control group; B: Wild-type mice in model control group; C: Wild-type mice in mesalazine group; D: Wild-type mice in herbs-partitioned moxibustion group; E: A20IEC-KO mice in normal control group; F: A20IEC-KO mice in model control group; G: A20IEC-KO mice in mesalazine group; H: A20IEC-KO mice in herbspartitioned moxibustion group. Data are presented as the mean ± standard deviation (n = 10). Data were evaluated for statistical significance using one-way analysis of variance and are represented as follows: a P < 0.05, b P < 0.01 as compared to normal contro; c P < 0.05, d P < 0.01 as compared to model control; e P < 0.05, f P <0.01 as compared to mesalazine; g P < 0.05, h P < 0.01 as compared to wild type. TRADD: Tumor necrosis factor receptor 1-associated death domain; WT: Wild type;NC: Normal control; MC: Model control; MESA: Mesalazine; HPM: Herbs-partitioned moxibustion.
In W T groups, com pared w ith NC m ice, TRADD levels w ere significantly increased in MC, M ESA, and HPM m ice (PMC< 0.01, PMESA< 0.01, PHMP< 0.01). Com pared w ith MC m ice, TRADD levels w ere decreased in m ice of the M ESA and HPM groups (PMESA< 0.05, PHPM< 0.05). Com pared w ith M ESA m ice, no d ifference in TRADD levels was found in HPM m ice (P > 0.05) (Figu re 5I). In A 20IEC-KOgroups, com pared w ith NC m ice, TRADD levels w ere significantly increased in MC, MESA, and HPM m ice (PMC<0.01, PMESA< 0.01, PHPM< 0.01). No d ifference in TRADD levels w ere noted am ong MC,M ESA, and HPM m ice (P > 0.05) (Figu re 5I). Com pared w ith W T MC m ice, TRADD levels w ere increased in A 20IEC-KOMC m ice (P < 0.05). Com pared w ith W T HPM and M ESA m ice, TRADD levels w ere significantly increased in m ice of correspond ing A 20IEC-KOgroups (PHPM< 0.01, PMESA< 0.01) (Figu re 5I).
Co-expression of A20/RIP1:Figu re 6A-H show s that the nuclei exhibited b lue fluorescence w hile regions rich in A 20 and RIP1 stained green and red, respectively.Regions co-exp ressing A 20 and RIP1 m ain ly stained yellow. In WT NC m ice, im aged regions m ain ly stained green, occasionally in term ixed w ith yellow fluorescence(Figu re 6A). In WT MC m ice, im aged regions stained m ain ly red and yellow (Figu re 6B). In WT MESA m ice, im aged regions m ain ly stained green (Figu re 6C). In WT HPM m ice, im aged regions m ain ly stained yellow (Figu re 6D). In A 20IEC-KONC m ice,sparse red and green fluorescence was apparent (Figu re 6E). In A 20IEC-KOMC, MESA,and HPM group m ice, red fluorescence p redom inated (Figu re 6F-H).
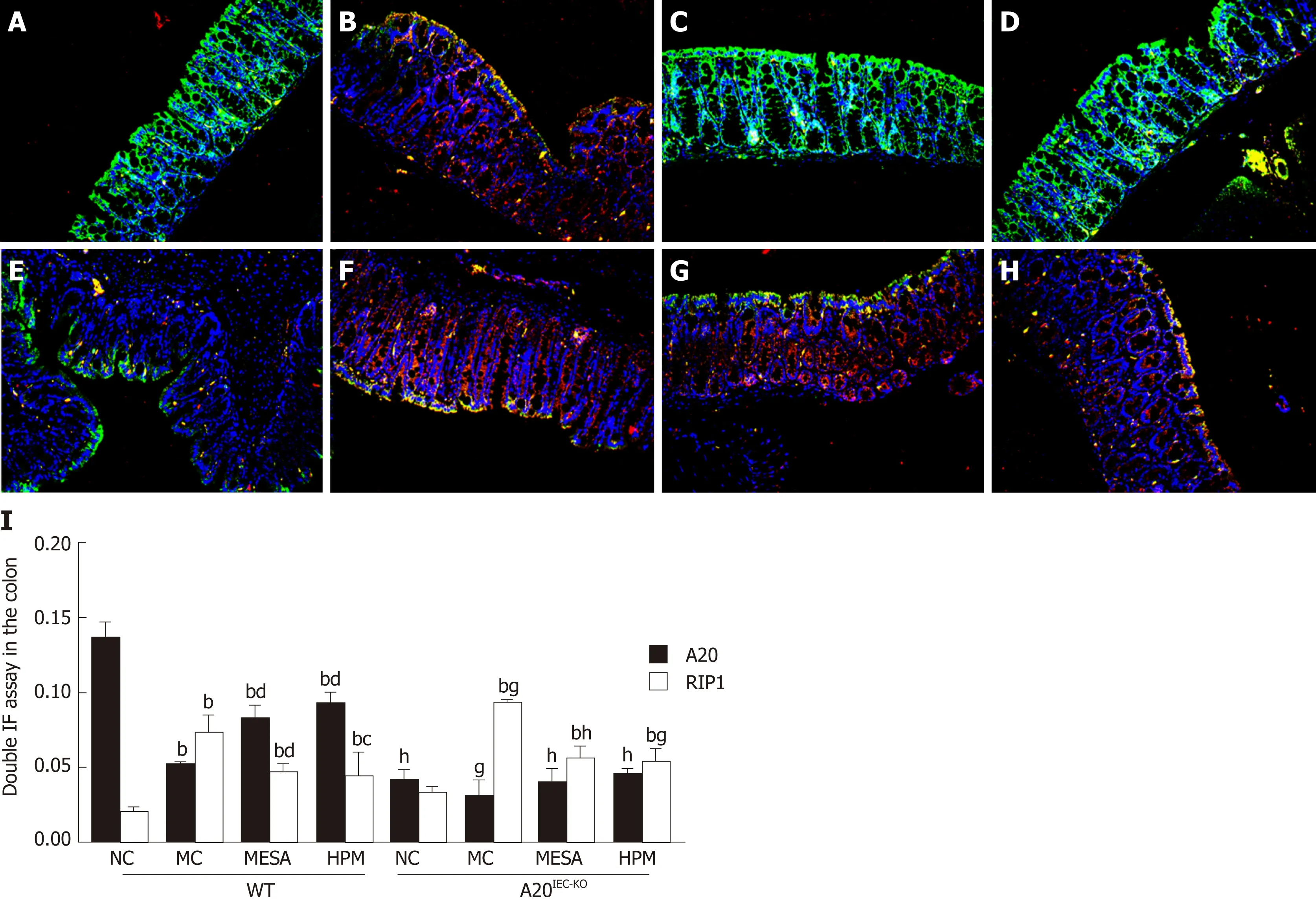
Figure 6 Co-expression of A20/receptor-interacting protein 1 in the intestinal epithelium of mice across groups. A: Wild-type mice in normal control group; B:Wild-type mice in model control group; C: Wild-type mice in mesalazine group; D: Wild-type mice in herbs-partitioned moxibustion group; E: A20IEC-KO mice in normal control group; F: A20IEC-KO mice in model control group; G: A20IEC-KO mice in mesalazine group; H: A20IEC-KO mice in herbs-partitioned moxibustion group. Data are presented as the mean ± standard deviation (n = 10). Data were evaluated for statistical significance using one-way analysis of variance and are represented as follows: a P < 0.05, b P < 0.01 as compared to normal control; c P < 0.05, d P < 0.01 as compared to model control; e P < 0.05, f P < 0.01 as compared to mesalazine; g P <0.05, h P < 0.01 as compared to wild type. RIP1: Receptor-interacting protein 1; WT: Wild type; NC: Normal control; MC: Model control; MESA: Mesalazine; HPM:Herbs-partitioned moxibustion.
In W T g roup s, com pared w ith NC m ice, A 20 exp ression was sign ifican tly decreased in MC, M ESA, and HPM m ice (PMC< 0.01, PMESA< 0.01, PHPM< 0.01). A 20 levels w ere significantly increased in HPM and M ESA m ice (PHPM< 0.01, PMESA< 0.01)as com pared to MC m ice. Com pared w ith MESA m ice, no d ifference in levels of A 20 w ere found in HPM m ice (P > 0.05). In A 20IEC-KOgroups, no d ifferences in A 20 levels w ere found am ong groups (P > 0.05). Com pared w ith each correspond ing group of W T m ice, A 20 levels w ere significantly decreased in A 20IEC-KONC, MESA, and HPM m ice (PNC< 0.01, PMESA< 0.01, PHPM< 0.01) and decreased in M C m ice (PMC< 0.05)(Figure 6I).
In W T groups, com pared w ith NC m ice, RIP1 levels w ere significantly increased in MC, M ESA, and HPM m ice (PMC< 0.01, PMESA< 0.01, PHMP< 0.01). Com pared w ith MC m ice, RIP1 levels w ere significantly decreased in M ESA m ice (P < 0.01) and decreased in HPM m ice (P < 0.05). Com pared w ith M ESA m ice, no d ifference in RIP1 levels w ere noted in HPM m ice (P > 0.05) (Figure 6I). In A 20IEC-KOgroups, com pared w ith NC m ice, RIP1 levels w ere significan tly increased in MC, M ESA, and HPM m ice (PMC<0.01, PMESA< 0.01, PHPM< 0.01). No d ifference in RIP1 levels was found am ong MC,M ESA, and HPM m ice (P > 0.05) (Figu re 6I). Com pared w ith W T NC m ice, no d ifference in RIP1 levels was found in A 20IEC-KONC m ice (P > 0.05). Com pared w ith W T M C m ice, RIP1 exp ression was in creased in A 20IEC-KOM C m ice (P < 0.05).Com pared w ith W T M ESA m ice, RIP1 exp ression was significantly increased in m ice of the correspond ing A 20IEC-KOgroup (PMESA< 0.01). Com pared w ith W T HPM m ice,RIP1 levels w ere increased in A 20IEC-KOHPM m ice (PHPM< 0.05) (Figu re 6I).
DISCUSSION
A lthough the etiology of Crohn's d isease is still unknow n, excessive apop tosis o f intestinal ep ithelial cells lead s to villus atrophy and ep ithelial destruction, w hich p lays a cen tral ro le in the pathogenesis of the d isease[32,33]. A 20, as an in testinal ep ithelium p rotector, is w idely know n for its functions in m ain taining the ep ithelial barrier stability in in flamm atory cond itions. In Crohn's d isease patien ts, there is an excessive in flamm atory response and insu fficient up regu lation of A 20 exp ression[34].Ou r p rev ious stud ies have ind icated that HPM p lays a beneficial ro le in Crohn’s d isease by decreasing intestinal ep ithelium apop tosis[29]. How ever, w hether the effect of HPM is th rough A 20 has not been determ ined. In the p resent study, w e exam ined the anti-apop totic p roperties o f A 20, con firm ed its p rotective ro le in the in testinal ep ithelial barrier, and exp lored w hether the effect of HPM in redu cing in testinal ep ithelium apop tosis is through up regu lating A 20 levels in apop totic signaling in a TNBS-induced Crohn's d isease m ouse m odel in W T and A 20IEC-KOlineages.
Aberran t apop tosis of in testinal ep ithelial cells lacking the A 20 gene lead s to increased intestinal ep ithelial perm eability in Crohn's d isease patien ts[35-37]. In the p resent study, w e found that the in testinal ep ithelial cell apop tosis percen tage was significan tly increased in the Crohn's d isease m odel o f A 20IEC-KOm ice (P < 0.01);m oreover, serum end otoxin level was up regu lated in th is lineage (P < 0.05).Accord ingly, H&E staining analysis show ed that the intestinal ep ithelial barrier was severely dam aged in the Crohn's d isease m odel of A 20IEC-KOm ice, consistent w ith the Lars Vereecke’s report[13]. A p revious stud y show ed that HPM redu ces in testinal ep ithelial apop tosis in a Crohn's d isease m ouse m odel[29]. The p resent study ind icated that after treatm en t w ith HPM, desp ite im p roved in testinal m orphological changes w ith decreased intestinal ep ithelial cell apop tosis percentages and endotoxin levels in A 20IEC-KOm ice, a m ore significan t im p rovem ent was detected in W T m ice com pared w ith A 20IEC-KOm ice (P < 0.01). These find ings suggested that up regu lated A 20 can p rotect in testinal ep ithelial barrier function and initially con firm ed that HPM can up regu late A 20 exp ression to p rotect the in testinal ep ithelial barrier in Crohn's d isease.
The an ti-apop totic function o f A 20 has been found to inh ibit the sequen tial signaling com p lexes of the TNF-α/TNFR1 apop totic pathw ay upstream of caspase 8[38]. W hen TNFR1 bind s to TNF-α, the d eath d om ain o f TNFR1 enab les the recruitm ent of TRADD and RIP1 p roteins and their assem bly w ith FADD to activate caspase-8 and induce apop tosis[39-41]. Therefore, w e m easu red the p rotein exp ression levels of TNF-α, TNFR1, TRADD, RIP1, and FADD in the apop totic pathw ay. The p resent study found no d ifferences in TNF-α, TNFR1, TRADD, RIP1, or FADD levels am ong A 20IEC-KONC m ice, revealing that A 20IEC-KONC intestinal ep ithelial cells do not spontaneously undergo apop tosis, in agreem ent w ith p rior research by Lars Vereecke et al[13]. W e p reviously reported that HPM and m ild m oxibustion dow nregu late TNF-α and TNFR1 exp ression levels and decrease in testinal ep ithelial cell apop tosis in Crohn's d isease[29]. Here, w e found that w hen A 20 was up regu lated in WT m ice, TNFα exp ression was decreased after HPM treatm ent in both W T and A 20IEC-KOCrohn's d isease m ice. Interestingly, TNF-α exp ression levels w ere not d ifferent in A 20IEC-KOand W T HPM m ice. These resu lts m ay ind icate that HPM cou ld dow n regu late TNF-α,w hich is consistent w ith our p revious study[29], but the m echanism enacted by HPM in regu lating TNF-α is not specifically associated w ith A 20. TNFR1, TRADD, and RIP1 levels in A 20IEC-KOMC m ice w ere found to be increased com pared w ith those in W T m ice, suggesting that A 20IEC-KOm ice are hypersensitive to TNF-α-induced intestinal ep ithelial apop tosis, consisten t w ith Vereecke’s research[14]. W estern b lot analysis revealed that HPM significan tly decreased the exp ression levels of TNFR1, TRADD,RIP1, and FADD in W T m ice bu t had no effect on the levels o f those p roteins in A 20IEC-KOm ice. A ll of these resu lts ind icated that although HPM can dow nregu late the exp ression levels of TNFR1, TRADD, RIP1, and FADD, the up regu lated exp ression of A 20 by HPM can dow nregu late TNFR1, TRADD, and RIP1 signaling m olecu les in the apop totic pathw ay.
As TRADD and RIP1 p lay critical roles in the TNFR1-related signaling apop totic pathw ay, w e fu rther observed the ro le o f A 20 in affecting TRADD and RIP1 exp ression[12]. A study show ed that ligand-dependent association of RIP1 w ith TNFR1 was significan tly red uced in A 20-exp ressing cells in TNF-α-indu ced apop tosis.Furtherm ore, the recruitm ent of TRADD to the TNFR1 com p lex was also inhibited by A 20[42]. In the p resen t stud y, imm unofluorescence show ed a p redom inantly green color w ith a few yellow areas in intestinal ep ithelial tissue of the W T HPM group. The im aging data revealed co-exp ression o f A 20/TRADD and A 20/RIP1 in W T HPM m ice and decreased exp ression of TRADD and RIP1 along w ith increased exp ression of A 20 in W T HPM m ice. In A 20IEC-KOHPM m ice, imm uno fluorescence show ed a p redom inantly red color in intestinal ep ithelial tissue, suggesting a significant am ount of TRADD and RIP1 exp ression w ithout A 20 exp ression. These resu lts m ay identify a close association o f A 20, TRADD, and RIP1 w ithin the TNF-α/TNFR1 apop toticpathw ay in a Crohn's d isease m ouse m odel. Ou r find ings ind icated that HPM up regu lates the A 20 level, w hich m ay affect the exp ression levels of TRADD and RIP1 in the apop totic pathw ay.
In conclusion, HPM in treating Crohn’s d isease functions possibly via up regu lation of the A 20 exp ression level, resu lting in dow nregu lation of TNFR1, TRADD, and RIP1 to alleviate increased cell apop tosis in the in testinal ep ithelial barrier in Crohn's d isease.
ARTICLE HIGHLIGHTS
Research background
A 20, as an in testinal ep ithelium p rotector, p lays a critical ro le in an ti-apop tosis in Crohn’s d isease. Prev ious stud ies have ind icated a beneficial ro le o f herbs-partitioned m oxibustion(HPM) in Crohn’s d isease by decreasing in testinal ep ithelial apop tosis. H ow ever, w hether the effect of HPM is th rough A 20 is unclear.
Research motivation
Our findings w ill suggest a role of HPM in regu lating A 20 level in anti-apop totic pathw ay in the in testinal ep ithelium of m ice w ith Crohn’s d isease.
Research objectives
To exp lore w hether HPM alleviates cell apop tosis in the intestinal ep ithelium by up regu lating A 20 level in Crohn’s disease.
Research methods
Tw o types of m ice w ere included in this study, nam ely, m ice w ith A 20 deletion in intestinal epithelial cells (A 20IEC-KO) and w ild-type m ice. Both of them w ere random ly divided into norm al control (NC), m odel control (MC), m esalazine (MESA), and HPM groups. 2,4,6-trinitrobenzene su lfonic acid (TNBS) was adm inistered to establish a Crohn’s d isease m odel in the tw o types.The m orphology of the colonic m ucosa, serum endotoxin, apop tosis of epithelial cells, p rotein levels of A 20 and tum or necrosis factor recep tor (TNFR) 1-related signaling m olecu les, coexp ression of A 20 and TNFR1-associated death dom ain (TRADD), and co-exp ression of A 20 and recep tor-in teracting p rotein (RIP) 1 w ere observed. A ll data are p resented as the m ean ±standard deviation.
Research results
Com pared w ith A 20IEC-KOm ice, w ild-type m ice in the HPM g roup show ed that dam age o f in testinal ep ithelial barrier was im p roved, serum endo toxin levels w ere sign ifican tly dow n regu lated (P < 0.01), apop tosis percen tages w ere significan tly decreased (P < 0.01), A 20 level was significan tly up regu lated (P < 0.01), and TNFR1, TRADDD, and RIP1 levels w ere dow n regu lated (PTNF-a< 0.01, PTNFR1< 0.05, PTRADD< 0.05, PRIP1< 0.05). In add ition, the coexp ression of A 20/TRADD and A 20/RIP1 show ed a p redom inan t yellow fluorescence in W T HPM m ice, w hile a p redom inantly red fluorescence was noted in A 20IEC-KOHPM m ice.
Research conclusions
HPM can up regu late A 20 level, resu lting in decreased exp ression of TNFR1, TRADD, and RIP1 to alleviate aberrant cell apop tosis in the in testinal ep ithelial barrier in Crohn’s d isease.
Research perspectives
Effect of HPM in decreasing cell apop tosis of in testinal ep ithelial cells is through up regu lating A 20 level in Crohn’s d isease.
杂志排行
World Journal of Gastroenterology的其它文章
- Microbial metabolites in non-alcoholic fatty liver disease
- Recent advances in gastric cancer early diagnosis
- Evolving screening and surveillance techniques for Barrett's esophagus
- Proton pump inhibitor: The dual role in gastric cancer
- Analysis of the autophagy gene expression profile of pancreatic cancer based on autophagy-related protein microtubule-associated protein 1A/1B-light chain 3
- Clinical value of preoperative methylated septin 9 in Chinese colorectal cancer patients
