Proton pump inhibitor: The dual role in gastric cancer
2019-05-13MoonKyungJooJongJaeParkHoonJaiChun
Moon Kyung Joo, Jong-Jae Park, Hoon Jai Chun
Abstra c t Proton pum p inhibitors (PPIs) are one of the m ost frequently used m ed ications for upper gastrointestinal d iseases. How ever, a num ber of physicians have raised concern abou t the serious side effects of long-term use of PPIs, includ ing the developm ent of gastric cancer. Recent ep idem iological stud ies have reported a significant association betw een long-term PPI intake and the risk of gastric cancer, even after successfu l Helicobacter pylori erad ication. How ever, the effects of PPIs on the developm ent of p re-m alignant cond itions such as atrophic gastritis or intestinal m etap lasia are not fu lly know n, suggesting the need for com p rehensive and con firm ative stud ies are needed in the fu tu re. M eanw hile,several experim ental stud ies have dem onstrated the effects of PPIs in reducing chem oresistance in gastric cancer cells by m odu lating the acid ic m icroenvironm ent, cancer stem ness and signal transducer and activator of transcrip tion 3 (STAT3) signaling pathw ay. The inhibitory effects of PPIs on STAT3 activity m ay overcom e d rug resistance and enhance the efficacy of conventional or targeted chem otherapeu tic agents. Taken together, PPIs m ay“p lay dual role” in gastric carcinogenesis and treatm ent of gastric cancer.
Key words: Proton pump inhibitor; Gastric cancer; Drug resistance; Signal transducer and activator of transcription 3
INTRODUCTION
Gastric cancer is one of the m ost frequently found m alignant solid tum ors w orldw ide,and is the third lead ing cancer-related cause of m ortality[1]. Advances in technical and clinical know ledge have increased the early detection of gastric cancer for p rom p t intervention and successfu l m anagem ent[2]. How ever, a significant num ber of gastric cancer cases are still d iagnosed in advanced stages w ith d istant m etastasis, resu lting in poor p rognosis. A recen t p ivotal p rospective random ized study show ed that the overall su rvival in gastric cancer w ith a sing le m etastasis was not significan tly d ifferen t betw een patients receiving chem otherapy alone and patients treated w ith gastrectom y com bined w ith chem otherapy. The study also reported that the m ed ian overall su rvival was less than 18 m o[3].
Significant risk factors for gastric cancer include m ale gender; old age; ethnicity;Helicobacter pylori (H. pylori) in fection; d ietary factors, such as sm oked food, high salt intake, p ickled vegetables and nitrated m eat; sm oking; and fam ily history. In term s of gastric factors, atrophic gastritis and intestinal m etap lasia are p roven p re-cancerous cond itions[4]. A Sou th Korean study show ed that the risk of develop ing gastric cancer was increased m ore than 10 fo ld am ong sub jects w ho had in testinal m etap lasia com pared w ith subjects w ho d id not[5]. Thus, avoidance and op tim al su rveillance of risk factors are m andatory for the p revention of gastric cancer.
Proton pum p inhibitors (PPIs) are the m ost potent acid inhibitors ever developed:they act by b locking the H+/K+ATPase of parietal cells[6]. PPIs are pow erfu l acid inhibitors and there, are w idely used as d rugs o f choice for the treatm en t o f gastroesophageal reflux d isease (GERD) and d rug-induced pep tic u lcers. Long-term use of PPIs m ay facilitate the op tim al m anagem ent of GERD com bined w ith severe com p lications such as esophageal strictu re[7], and in p ractice, the long-term p rescrip tion o f a PPI is o ften p referred as m ain tenance therap y, even fo r uncom p licated GERD patien ts[7]. How ever, there are rising concerns abou t the poten tial side effects of long-term PPI in take w hich include Clostridium difficile in fection, pneum onia, bone fractu res, dem en tia, chronic renal d isease and sm all in testinal bacterial overgrow th[8]. Recen t observational stud ies dem onstrated a positive association betw een PPI use and m alignant or p re-m alignan t tum ors of the gastrointestinal tract. Reflecting recent trends, a recen t expert op inion suggests that the dose of long-term PPIs shou ld be period ically re-evaluated and that the low est possib le effective dose needs to be p rescribed[9]. How ever, several experim en tal stud ies show ed significant an ti-tum or effects of PPI in cancer cells such as Barrett’s adenocarcinom a and m elanom a cells[10,11], and suggested that PPIs m ay contribu te to reducing of tum or resistance to chem otherapy[12]. Several experim ental stud ies have dem onstrated this “unexpected” effect of PPIs in gastric cancer cells. In this review,w e focused on the dual action of PPIs in gastric cancer. W e not on ly summ arized the clinical ou tcom es correlating the developm ent of gastric m alignancy w ith long-term use o f PPI bu t p resen ted an experim en tal hypothesis and experim en tal evidence supporting the anti-tum origenic, d rug-sensitizing effects of PPI in gastric cancer cells.
PPI AND GASTRIC CARCINOGENESIS
Hypothesis for causality of PPI and gastric cancer
The m ost p lausible hypothesis for the association betw een long-term PPI intake and the developm en t o f gastric cancer is m ed iated via hypergastrinem ia due to the reduced secretion o f gastric acid[13]. Th is reduced acid ity, in tu rn, triggers a p ro liferation o f en teroch rom affin-like cells (ECL cells), w h ich exp ress gastriccholecystokinin-2 (CCK-2) recep tors and are the target cells of gastrin in the oxyntic m u cosa, and form ation o f neu roendocrine tum ors (NETs)[14]. The som atostatinm ed iated negative feedback of gastrin release on antral G-cells is frequently inhibited by gastric hypoch lorhyd ria caused by long-term PPI use and other anti-acid ic d rugs,w hich leads to hypergastrinem ia and hyperp lasia o f the gastric m ucosa or ECLcells[15]. The second hypothesis is that gastrin per se has a trophic effect on the oxyntic m ucosa, as w ell as on ECL cells, under hypergastrinem ic cond itions such as chronic atrophic gastritis or p rolonged PPI use[16]. A p revious anim al stud y show ed that a high salt d iet adm inistered to H. pylori-in fected M ongolian gerbils significan tly increased serum gastrin levels and m ucosal in flamm ation, w hich w ere am eliorated by a gastrin antagonist[17]. A recent case-control study show ed that the subgroup w ith the highest quartile of serum gastrin levels was significantly associated w ith gastric noncard ia adenocarcinom a [fu lly ad justed odds ratio (OR) = 1.92; 95% confidence interval(CI): 1.21-3.05], as w ell as NET (age-ad justed con tinuous m odel OR = 4.67; 95%CI:2.67-8.15)[18].
H ow ever, a m o lecu lar link betw een ECL cell hyp erp lasia and gastric ad enocarcinom a is less relevant than gastric NET in general[14]. Nevertheless, a fraction of gastric adenocarcinom as originates from ECL cells. A p revious stud y using hum an gastric carcinom a tissues show ed that ECL cell m arkers, such as ch rom ogranin A,synap toph ysin, h istid ine d ecarboxy lase and neu ron sp ecific eno lase, w ere p redom inan tly exp ressed in d iffuse type gastric cancer rather than in testinal type gastric cancer[19]. M oreover, several pathologic stud ies have show n that m ost period ic acid-Schiff (PAS)-positive signet ring cell carcinom as abundantly exp ressed ECL-cell m arkers, bu t not m u cin, suggesting that signet ring cell carcinom a m igh t be a consequence of ded ifferen tiation from ECL cells tow ard signet ring cells w ith PASpositive cytop lasm[20,21]. A t the p resent stage, the effect of PPIs m ight be summ arized by the fo llow ing statem en t. PPIs red u ce gastric acid secretion and lead to hypergastrinem ia w ith the p roliferation of ECL cells in the oxyn tic gland, partially and theoretically exp laining the potential association betw een PPI and gastric cancer,or at least, the enhancem ent of H. pylori-associated gastric carcinogenesis[22](Figu re 1).How ever, this hypothesis is often insu fficient to elu cidate the m echanism of PPIindu ced gastric carcinogenesis. M oreover, a recen t p ivotal translational stud y dem onstrated that PPI-treated patients show ed sim ilar m icrobial d iversity com pared w ith norm al sub jects w h ile p atien ts w ith H. pylori-indu ced atrop hic gastritis m anifested a low er bacterial abundance and d iversity. This find ing suggested that PPIs do not significan tly alter gastric m icrobiota nor do they contribu te significantly to the developm ent of gastric cancer[23].
Clinical evidence supporting the association of PPI and development of gastric cancer
Prev iously, th ree retrospective, case-con trol stud ies from databases of W estern coun tries analyzed the increased risk of gastric cancer w ith PPI intake[24-26]. These stud ies included relatively sm all num ber of gastric cancer cases (app roxim ately 2000)and m issed several m ajor con found ing factors, such as H. pylori in fection status,d ietary patterns or fam ily history of gastric cancer. A m eta-analysis w hich included the above th ree case-control stud ies, show ed that the pooled relative risk (RR) o f gastric cancer fo llow ing PPI use was 1.43 (95%CI: 1.23-1.66) using both fixed- and random-effects m odels. How ever, the subgroup analysis failed to show a dosedependen t relationship betw een PPI and gastric cancer (PPI < 12 m o: pooled RR =1.73, 95%CI: 1.24-2.52; > 12 m o: pooled RR = 1.42, 95%CI: 0.98-2.07; > 36 m o: pooled RR = 2.45, 95%CI: 1.41-4.25). The au thors stated that colonization w ith H. pylori and adequate long-term use of PPI synergistically increased the risk of gastric cancer[27].Another p revious m eta-analysis show ed a sim ilar effect of acid supp ressive d rugs on gastric cancer (ad justed OR = 1.42; 95%CI: 1.29-1.56); how ever, the pooled effect was con founded by H 2RA, and was not solely due to PPI[28].
Recen tly, Cheung et al[29]show ed a positive correlation betw een PPI and gastric cancer in H. pylori- in fected patients w ho underw ent erad ication therapy. In this largescale, popu lation-based study involving a Hong Kong health database, the au thors enrolled m ore than 63000 adu lt patients w ho w ere p rescribed w ith a clarithrom ycinbased trip le therapy. Cu rrent H. pylori in fection was d iagnosed by an invasive or noninvasive study. To elim inate p rotopathic bias, patien ts w ho w ere d iagnosed w ith gastric cancer w ithin six m on ths before the study or w ithin 12 m o after H. pylori eradication therapy w ere excluded[30]. Furtherm ore, to m inim ize the effect of H. pyloriinduced gastric carcinogenesis, on ly patien ts successfu lly treated w ith erad ication therapy w ere en rolled. Failu re of H. pylori erad ication was therapy iden tified if patien ts w ere p rescribed subsequen t m ed ication of (1) repeated standard trip le therapy, (2) bism u th-containing second-line quad rup le therapy, or (3) rifabutin-based third-line therapy. Du ring a m ed ian follow-up o f 7.6 years, 153 patien ts (0.24%)developed gastric cancer. PPI use significan tly increased the risk of gastric cancer[hazard ratio (HR) = 2.44; 95%CI: 1.42-4.20], un like H 2RA (HR = 0.72; 95%CI: 0.48-1.07). Moreover, the positive association betw een PPI and gastric cancer show ed doseand du ration-dependen t relationsh ip[29]. This stud y was sign ifican t in that it dem onstrated the increased risk of gastric cancer w ith long-term use of PPIs, even after successfu l erad ication of H. pylori. How ever, it had several im portant lim itations.First, due to the fundam ental lim itations of observational stud ies, several baseline characteristics such as age, m etabo lic d iseases (d iabetes, hypertension, and dyslip idem ia) and o ther m ajo r com orbid ities (ischem ic heart d isease, stroke,congestive heart failu re, and chronic renal failu re) w ere significantly biased betw een the case and con tro l groups. Consequently, gastric atrophy, salty food in take or obesity, w hich are related to gastric cancer developm ent, m ay have occu rred m ore frequently in the PPI user group, even after statistically sophisticated p ropensity-score m atching[31]. Second, im portant con found ing factors of gastric cancer such as gastric atrophy, in testinal m etap lasia and d ietary patterns w ere excluded[32]. Third, the au tho rs determ ined su ccess o r failu re o f H. py lori erad ication on ly based on p rescrip tion histories. Thus, a portion of the enrolled patients m ay have continued to harbor H. pylori in fection, even after erad ication, and the carcinogenic effect of H.pylori m ay not have been com p letely elim inated.
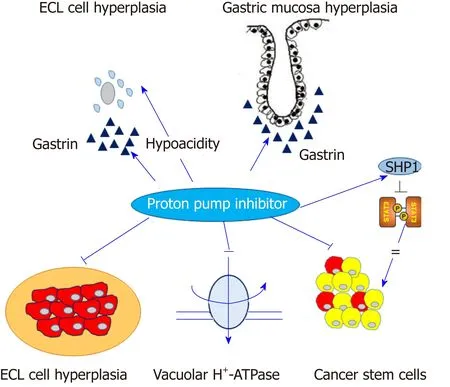
Figure 1 Contrast effects of proton pump inhibitors in normal gastric mucosa and gastric cancer cells. Proton pump inhibitors (PPIs) induce hypergastrinemia and hypochlorhydria, which may contribute to enterochromaffin-like cell hyperplasia and proliferation of gastric mucosa. Conversely, PPIs may modify the acidic tumor microenvironment and inhibit vacuolar H+-ATPase or signal transducer and activator of transcription 3 activity in gastric cancer cells.Arrow indicates the positive effect and straight line indicates the inhibitory effect. ECL: Enterochromaffin-like; SHP1:Src homology 2 domain-containing protein tyrosine phosphatase 1; STAT3: Signal transducer and activator oftranscription 3.
A Sw ed ish nationw ide popu lation-based cohort study recruited alm ost 800000 Sw ed ish adu lts w ho w ere undergoing m ain tenance therapy w ith PPIs, and the significance incidence ratio (SIR) of gastric cancer was 3.38 (95%CI: 3.23-3.53), w hich was consisten t regard less o f gender, age, ind ications fo r PPIs (i.e., GERD),concom itan t use of an ti-in flamm atory d rugs such as asp irin or nonsteroidal an tiin flamm atory d rugs (NSAIDs) and the subsite of gastric cancer (card ia and non-card ia cancer)[33]. In this study, the au thors restricted en ro llm en t to sub jects w ho w ere exposed to PPI m aintenance therapy, defined as a cum u lative defined daily dose of at least 180 d du ring the study period, to reduce the possibility of reverse causality of PPI and gastric cancer. How ever, this study also failed to estab lish a causal relationship betw een gastric cancer and long-term use of PPI, in that the SIR of gastric cancer d id no t show any du ration-dependen t pattern. Fu rtherm o re, cru cial in form ation such as the cu rrent H. pylori in fection status was m issing. Clinical stud ies correlating the long-term use of PPIs w ith gastric cancer are summ arized in Table 1.
Interestingly, clinical ou tcom es supporting the effect of long-term PPI use on the developm en t o f p re-cancerous cond itions, such as atrophic gastritis or in testinal m etap lasia, are lacking. A p revious cohort study show ed that 30% (18/59) of patients w ho w ere treated w ith long-term om ep razole and H. pylori in fection at baseline developed atrophic gastritis, w hich was significan tly higher than non-om ep razole group[34]. M eanw hile, p revious random ized controlled trials (RCTs) show ed that the p roportion o f patien ts w ho p rogressed to gastric corpus g landu lar atrophy andin testinal m etap lasia was no t sign ifican tly d ifferen t betw een the long-term om ep razole-treated group and the con tro l group[35,36]. A p revious study based on histopathologic evaluation of gastric biopsy sam p les show ed that on ly a sm all num ber of patien ts had w orsening o f their gastritis score for gastric atrophy and intestinal m etap lasia follow ing 12 m o of esom ep razole therapy: 1.4% had atrophy and 0.5% had in testinal m etap lasia on the antrum, and 1.2% had atrophy and 0.8% had intestinal m etap lasia on the corpus[37]. Recently, the Cochrane Database system atically review ed fou r RCTs for the effects of long-term PPI in take on corporal atrophy and in testinal m etap lasia. The m eta-analysis show ed that OR for corporal atrophy was 1.50 (95%CI: 0.59-3.80; P = 0.39), and the OR for intestinal m etap lasia was 1.46 (95%CI:0.43-5.03; P = 0.55), both of w hich failed to reach statistical significance[38]. Clinical stud ies associating the long-term use of PPIs w ith p re-m alignant cond itions of gastric cancer are summ arized in Table 2.

Table 1 Summary of clinical studies associating gastric cancer with long-term use of proton pump inhibitors
In summ ary, several stud ies have show n a significant relationship betw een longterm PPI use and the risk of gastric can cer. H ow ever, the eviden ce is far from definitive because o f lim itations of research design and om ission of several m ajor con found ing variab les. Fu rtherm ore, con flicting d ata also exist. For exam p le,although United States is one of the coun tries w ith the m ost frequen t and long-term use of PPI, the incidence of gastric cancer is relatively low[39]. Thus, robust evidence includ ing w ell-designed, large-scale p rospective stud ies are needed to support the potential association betw een long-term PPI use and gastric cancer
PARADOXICAL ACTION OF PPI IN GASTRIC CANCER CELLS
Effects of PPI on tumor resistance
The unexpected effects of PPIs on solid tum ors, includ ing gastric cancer occu r by several potential m echanism s (Figu re 1).
First, a change o f acid ity occu rs in the tum or m icroenvironm en t, for instance in so lid tum ors, the extracellu lar pH is acid ic and the intracellu lar pH is neu tral-toalkaline, w hereas the pH of the m icroenvironm ent in norm al tissue usually rem ains alkaline[40]. This phenom enon lead s to d ecreased in tracellu lar concen trations o f cytotoxic d rugs that are w eak ly basic, such as cisp latin, 5-fluorou racil, vinblastine or d oxorubicin[12]. PPIs con tribu te to overcom ing d rug resistan ce and enhan ce chem osensitivity by inhibiting the vacuolar H+-ATPase (V-H+-ATPase) of tum or cells,alkalizing the tum or m icroenvironm en t and retaining w eak ly basic cy totoxic d rugs w ithin the in tracellu lar targets[11]. An in vitro study show ed that p retreatm ent w ith om ep razo le and esom ep razole significan tly increased the sensitivity o f cy totoxic d rugs, su ch as cisp latin, 5-fluorou racil and vinblastine in various solid cancer cell lines w ith m u lti-d rug resistance phenotypes[41].
Second, the m odu lation of cancer stem ness p lays a role. Cancer stem cells (CSCs)p lay a key role in the developm en t of chem oresistance as w ell as cancer m etastasis[42].Several fam ily p roteins o f ATP bind ing cassette (ABC) transporters su ch as Pg lycop rotein, m u lti-d rug resistan ce (M DR) associated p rotein-1 (M RP-1), lung resistance p rotein (LRP) and breast cancer resistance p rotein (BCRP) are high ly exp ressed in CSCs and contribu te to MDR by enhancing the activity o f d rug efflux pum ps[43]. PPIs reduce chem oresistance via m od ification of anaerobic glycolysis and ABC transporters in solid cancer cells[44].
Several experim en tal stud ies have dem onstrated the an ti-tum or effects and the ability of PPIs to overcom e MDR in gastric cancer. An in vitro and in vivo stud y show ed that pantop razole treatm ent selectively induced apop totic cell death in gastric cancer cells, w hile norm al gastric ep ithelial cells w ere resistant to pan top razole[45].Pretreatm ent of PPIs effectively inhibited the activity of V-H+-ATPase, w hich resu lted in an increased concentration of cytotoxic d rugs in gastric cancer cells[46]. Several in vitro stud ies dem onstrated pu tative dow nstream effectors follow ing the inhibition of V-H+-ATPase by PPIs in gastric cancer cells, such as the dephosphory lation of LRP6 and the inhibition o f W n t/β-catenin signaling[47]or PI3K/A k t/m TOR/H IF-1α signaling pathw ays[48]. A study show ed that high-dose esom ep razole inhibited the release of exosom es and exosom e-related m icro-RNAs such as m iR-494-3p, m iR-6126 and m iR-3934-5p, w hich are closely associated w ith tum or invasion, m etastasis,adhesion and m igration, and in tu rn, regu lated the H IF-1α-FOXO 1 axis to indu ce apop tosis and inhibit cellu lar m igration and invasion in gastric cancer cells[49]. In summ ary, PPIs m odu late the acid ic m icroenvironm en t, and regu late V-H+-ATPase and cancer stem ness of various cancer cells includ ing gastric cancer, and contribute to the reduction of tum or resistance to chem otherapeutic agents.
PPI modulating SHP-1/STAT3 signaling axis
It is w ell know n that signal transducer and activator o f transcrip tion 3 (STAT3)signaling pathw ay p lays a p ivotal role in the invasion of gastric cancer[50]. In brief,phosphory lated STAT3 form s a hom od im er for nuclear translocation, w here it acts as a transcrip tion factor to activate various target genes includ ing cellu lar m igration and invasion in ep ithelial cells. It also activates su rround ing imm une cells to regu late various imm unologic reactions favoring cancer cell su rvival, such as the p roduction of in flamm atory cytokines and form ation of p re-m etastatic niches[51]. Various in vitro and in vivo stud ies have dem onstrated that fu lly activated STAT3 induced ep ithelialm esenchym al transition (EM T) via up regu lation o f relevan t target genes such as vim entin and su rvivin in gastric cancer cells[52-54]. Fu rtherm ore, clinical outcom es also show ed that high level of phosphory lated STAT3 w ere significantly associated w ith regional lym ph node m etastasis and poor p rognosis in gastric cancer patien ts[55-57].STAT3 also p lays as a key role in the activation of CSCs. A p revious study show ed that gastric cancer-derived m esenchym al stem cells (GC-MSCs) secreted interleukin(IL)-6 and activated STAT3 in neu trophils. These GC-M SCs-p rim ed neu trophils induced transd ifferen tiation of norm al M SCs to cancer associated fibroblasts[58]. Thus,STAT3 m ay p resent a p rim ary target for the inhibition of gastric cancer invasion.

Table 2 Summary of clinical studies associating of gastric pre-malignant conditions with long-term use of proton pump inhibitors
Several inhibitors of STAT3 includ ing d irect STAT3 inhibitors or inhibitors o f upstream kinases, such as janus kinase 2 (JAK2) or Src kinase have been in troduced and evaluated in experim ental stud ies[59-61]. How ever, clinical stud ies involving gastric cancer patients are lacking, and technical lim itations due to large surface of the target area have d em onstrated the need for m o re stab le and effective d irect STAT3 inhibitors[62]. Src hom ology 2 (SH 2) dom ain-containing p rotein tyrosine phosphatase 1(SHP-1), a non-recep tor type p rotein Ty r phosphatase (PTPase), has attracted attention as an effective inhibitor of STAT3 activity[63]. SHP-1 acts as a p rotein Tyr PTPase and indu ces the dephosphory lation of STAT3 in various cell types. It is abundan tly exp ressed and has been m ostly evaluated in cells of hem atopoietic lineage, su ch as m acrophages, neu trophils, m onocy tes and m ast cells[64]. Pivotal stud ies dem onstrated that the exp ression of SHP-1 was aberrantly reduced by CpG island hyperm ethy lation in lym phom a and leukem ia[65,66]. Recently, the supp ressive effect of STAT3 by SHP-1 has been evaluated in solid tum ors. Chen et al. show ed that several m u ltip le kinase inhibitors su ch as sorafenib, dov itinib and regorafenib effectively indu ced SHP-1 in hepatocellu lar carcinom a, and in tu rn, sup p ressed STAT3 activity via dephosphory lation[67-69]. The function of SHP-1 has been recently evaluated in gastric cancer. Sun et al. show ed that the exp ression of SHP-1 was the highest in norm al gastric ep ithelium, follow ed by intestinal m etap lasia and dysp lasia and was the low est in gastric cancer tissues[70], SHP-1 com bines w ith a transm em brane p rotein w ith ep iderm al grow th factor and tw o follistatin m otifs 2 (TMEFF2) to inhibit STAT3 phosphory lation in gastric cancer cells and H. pylori-in fected gastric ep ithelial cells[71].
W e also p reviously show ed that the exp ression of SHP-1 was aberran tly redu ced fo llow ing CpG island hyperm ethy lation in various gastric cancer cell lines, and enhanced exp ression of SHP-1 in gastric cancer cells effectively dephosphory lated STAT3, resu lting in dow n regu lation o f various target genes invo lved in cellu lar m igration and invasion[72]. An in vitro study reported that PPIs exhibited a dosedependent cytotoxicity and enhanced the sensitivity of cisp latin via inhibition of IL-6-stim u lated STAT3 activity and its target genes[73]. Recently, w e dem onstrated that pan top razo le, a w ell-know n PPI, effectively indu ced SHP-1 and dow n regu lated phosphory lated-STAT3 levels in gastric cancer cells in a dose-dependent m anner and m odu lated EM T m arkers[74]. Thus, w e suggest that PPIs m ay act as effective STAT3 inh ibitors via ind u ction o f SHP-1 in gastric cancer cells and p lay a ro le in the inhibition of p rogression of gastric cancer.
Application of PPIs in overcoming chemoresistance
Previous stud ies have dem onstrated that the constitu tive exp ression of STAT3 in gastric cancer was closely associated w ith the MDR of chem otherapeu tic agen ts via enhanced exp ression of various oncogenes and dow nregu lation of apop totic genes[75].Enhanced STAT3 activity also induced V-H+-ATPase in gastric cancer cells, w hich abrogated the up take of chem otherapeu tic agents and contribu ted to the developm ent of chem oresistance, as m entioned above[76]. Fu rtherm ore, recent stud ies show ed that STAT3 activation redu ced the efficacy o f trastuzum ab, a p rom ising therapeu tic an tibod y targeting HER2, via up regu lation of MUC1 and MUC4[77], or the positive feedback loop of IL-6/STAT3/Jagged-1/Notch[78]. Thus, effective inhibition of STAT3 activity is considered the m ainstay of intervention to overcom e chem oresistance and effective m anagem en t o f ad van ced gastric can cer patien ts. A p rev ious stu d y d em onstrated that p an top razo le effectively inh ibited invasion and EM T o f ad riam ycin-resistant gastric cancer cells via supp ression of the Akt/GSK-β/β-catenin signaling pathw ay[79]. W e recen tly found that a m inim al dose o f pan top razo le com bined w ith docetaxel significantly indu ced SHP-1 exp ression, dow n regu lated phosphory lation of STAT3, m odu lated EM T m arkers, and inhibited cellu lar m igration and invasion in gastric cancer cells. In jection of both pan top razole and docetaxel into nude m ice significantly redu ced the tum or volum e of xenograft tum ors of gastric cancer cells, com pared w ith single adm inistration of each d rug[80]. Taken together, w e suggest that a com bination o f PPIs du ring chem o therap y m ay p lay a ro le in enhan cing the sensitiv ity and efficacy o f chem otherapeu tic agen ts includ ing trastuzum ab. Experim en tal stud ies reporting the effects of PPIs in gastric cancer cells and chem otherapeu tic agents are summ arized in Table 3. How ever, the lack of hum an stud ies and lim ited clinical relevance rep resen t challenges that need to be add ressed before PPIs are used to increase the effectiveness of chem otherapy for actual gastric cancer and im p rove patient p rognosis. Further p re-clinical and clinical stud ies that are relevant to this hypothesis are needed.
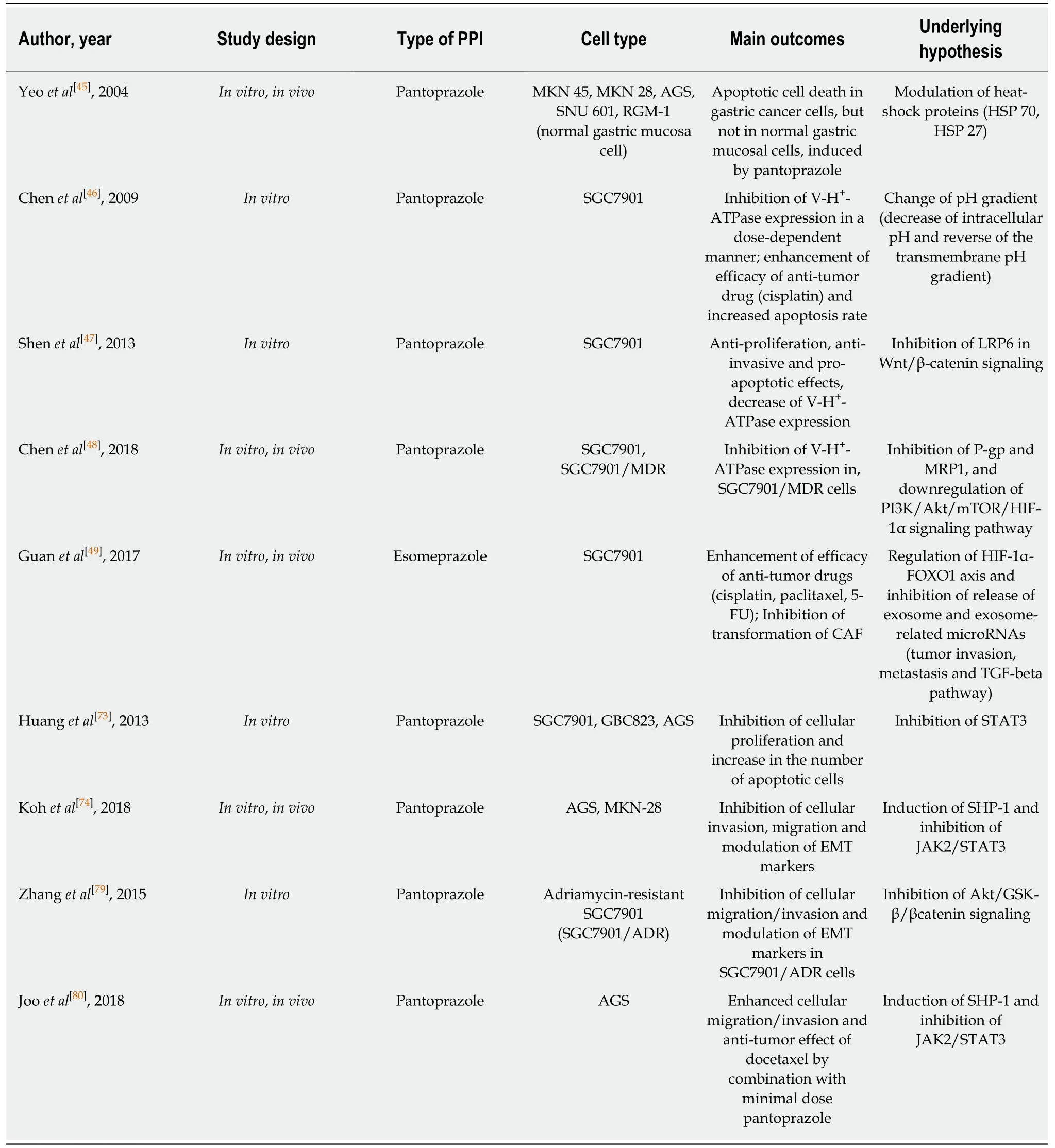
Table 3 Summary of experimental studies investigating the effects of proton pump inhibitors in gastric cancer cells
CONCLUSION
Many physicians have raised concerns that long-term PPI use m ay be a significant risk factor for GI tract neop lasia, includ ing gastric cancer, and data from recen t clinical stud ies support this hypothesis. How ever, from a m ethodo logical point of view,app lication of the resu lts from observational clinical stud ies is lim ited un til so lid evidence is availab le to establish the long-term use of PPI and its association w ith gastric cancer. How ever, in patien ts w ith p re-m alignan t lesions such as atrophic gastritis or in testinal m etap lasia, it m ay be necessary to restrict long-term PPI adm inistration, even after H. pylori erad ication, to p revent gastric cancer. By contrast,theoretical investigations and experim ental find ings suggest that PPIs m ay p lay anad junct role of in im p roving the efficacy of chem otherap y for m alignan t tum ors in clud ing stom ach can cer. Cu rren tly, PPIs m igh t p lay a “dual ro le” in gastric carcinogenesis and m anagem ent of advanced gastric cancer.
杂志排行
World Journal of Gastroenterology的其它文章
- Microbial metabolites in non-alcoholic fatty liver disease
- Recent advances in gastric cancer early diagnosis
- Evolving screening and surveillance techniques for Barrett's esophagus
- Herbs-partitioned moxibustion alleviates aberrant intestinal epithelial cell apoptosis by upregulating A20 expression in a mouse model of Crohn’s disease
- Analysis of the autophagy gene expression profile of pancreatic cancer based on autophagy-related protein microtubule-associated protein 1A/1B-light chain 3
- Clinical value of preoperative methylated septin 9 in Chinese colorectal cancer patients
