Hydrothermal Synthesis of Clinoptilolite and Its Ion Exchange Performance for CH4/N2 Separation
2019-03-22PENGShenlaiRAZAUllahBAIShiyangSUNJihongWUXia
PENG Shenlai, RAZA Ullah, BAI Shiyang, SUN Jihong, WU Xia
(Beijing Key Laboratory for Green Catalysis and Separation, College of Environmental and Energy Engineering,Beijing University of Technology, Beijing 100124, China)
Abstract: Clinoptilolite was synthesized via hydrothermal method using aluminum hydroxide as aluminum source and silica sol (Ludox) as silicon source. The influences of synthesized parameters (i.e., molar ratio of n(OH-)/n(Si), crystallization time and crystallization temperature, etc.) on the structure of prepared clinoptilolite and its CH4/N2 separation performance were investigated by various characterization techniques, including XRD, TEM, BET, FT-IR, TG, 29Si-NMR. Particularly, the kinetic behaviors of crystallization rate were evaluated, and the apparent activation energies for induction and growth period according to Arrhenius equation are 91.5 and 7.2 kJ/mol, respectively. Meanwhile, Ca2+ and K+ clinoptilolites were prepared through water solution ion exchange method. Moreover, the CH4/N2 separation performance was also evaluated for clinoptilolites with/without ion exchange. Experimental results suggest that equilibrium selectivity of CH4/N2 is strongly depended on the type of exchanged cationic charge, its quantity and distribution in the skeleton. K-clinoptilolite exhibited a good separation performance, and could be a promising absorbent for coal bed methane recovery.
Key words: clinoptilolite; hydrothermal synthesis; ion exchange; CH4/ N2 separation
Recently, the utilization of coal bed methane is very important in the application of energy and environmental fields[1]. However, separation of its major component (CH4/N2) is a big challenge due to the similar physicochemical properties of CH4and N2[2]. In the past, a number of techniques have been applied for gaseous separation, i.e. cryogenic distillation[3], membrane filtration[4]etc. which are either too expensive or less efficient. An alternative technology is the Pressure Swing Adsorption (PSA) used for separation of nitrogen from methane[5-6], which is one of the most established industrial processes employed for separation of gases, due to its low energy, low cost, safe and simple operation. The PSA, in which adsorbent can selectively adsorb a particular gas from a gaseous mixture, is a crucial technique. Therefore, selection or development of appropriate absorbent for PSA method has been attracting attention all over the world. Although activated carbon has been used in the past for CH4/N2separation, the adsorption capacity and selectivity are too low[7-8]. Recently, a number of porous materials, such as zeolite 4A[9], zeolite 13X[10], and carbon molecular sieve[11]have been developed. However, most of these expensive absorbents are restrained in industrial application because of their cost.
As a member of heulandite group of zeolites, clinoptilolite consists of three types of channels. Among which channel A with 10-membered rings (0.72 nm × 0.44 nm) are parallel to channel B (8-membered rings, 0.47 nm × 0.41 nm), while the third channel C (0.55 nm × 0.40 nm) intersects both channel A and B[12]. Different kinds of cations, such as Mg2+, Na+, K+, and Ca2+, are located in these channels, giving them a unique pore structure and thus allowing smaller molecules such as N2(kinetic diameter 0.36 nm) to diffuse quickly while hindering the diffusion of slightly larger molecules such as CH4(kinetic diameter 0.38 nm).
In last two decades, many studies regarding the equilibrium and diffusion properties of the ion-exchanged clinoptilolites were focused on the thermodynamic properties and tailored performance through cations exchange, in order to improve the selectivity of N2/CH4or CH4/N2adsorption based on PSA processes[13]. From the comments of the researches in recent years, the kinetic separation ability of clinoptilolite strongly depends on the composition and the type of charge balancing cations[14]. Although clinoptilolite is a promising adsorbent for kinetic separation of N2from CH4, there was no artificial synthesized clinoptilolite-like zeolite until 1963[15]. Since then different researchers have reported the successful synthesis of clinoptilolite under various conditions. For example, Na/K-clinoptilolite was synthesized through hydrothermal method in a weak basic solution of pH value of 7.9 at 473 K for 600 h[16], but the overlong synthesis period was one of the main drawbacks for the development of artificial synthesized clinoptilolite. Fortunately, Chi et al.[17]have successfully shortened the crystallization time to 15 h at 448 K with mass fraction of 10% seeds, however, the synthesized clinoptilolite was not pure enough and contains some other zeolites. Yuan et al.[18]improved this preparation method and obtained the clinoptilolite with much higher purity, but 144 h was needed and mass fraction of 10% seeds were also necessary. Although synthesis of clinoptilolite without using of seeds was reported by Satokawa et al.[19], the data regarding the synthesized clinoptilolite and its application for gas separation has been rarely reported.
In this paper, clinoptilolite was synthesized via hydrothermal method. The influence of various parameters, such asn(OH-)/n(Si), crystallization time and synthesis temperature were investigated in detail. Their structural properties and textural parameters before and after K+and Ca2+exchanged were characterized by various methods, including X-ray diffraction (XRD), scanning electron microscope (SEM), transmission electron microscope (TEM), N2sorption isotherms, Fourier transform infrared (FT-IR), thermal gravimetric and differential thermal gravimetric (TG-DTG),29Si magic angle spinning nuclear magnetic resonance(NMR). Meanwhile, the equilibrium selectivity of CH4/N2separation was preliminary explored. Experimental results suggested that K-clinoptilolite could improve the separation capability for CH4/N2and showed a potential candidate in the coal bed methane recovery.
1 Experimental
1.1 Materials
Silica source was colloidal silica (Ludox JN-30, mass fraction of 30%, Qingdao Haiyang Chemical Co. Ltd.) with average particle size of 10-20 nm and density of 1.2 g/cm3. Alumina source was Al(OH)3(mass fraction of 99.5%, Tianjin Fuchen Chemical Reagents Factory, A.R. grade), KOH (mass fraction of 82.0%), NaOH (mass fraction of 96.0%), KCl (mass fraction of 99.5%). CaCl2(mass fraction of 96.0%) was purchased from Beijing Chemical Works. All chemicals were A.R. grade, and deionized water with resistivity of 18.25 MΩ·cm at 298 K was used.
1.2 Synthesis of clinoptilolite and its ion exchange
The mixture was firstly prepared by mixing the calculated amount of NaOH, KOH and Al(OH)3with 10 mL deionized water in a 50 mL Teflon lined beaker at 393 K, and kept for stirring until it changed to a clear solution. Then, a certain amount of deionized water was added and Ludox was followed dropwisely to the above solution to make sure thatn(H2O)/n(SiO2) was 25 andn(OH-)/n(Si) was between 0.40 and 0.58, while stirring for 2 h. After that, the gel was placed in a stainless-steel autoclave with Teflon liners at a certain temperature (413-443 K) for a certain time (96-192 h). Finally, the resultant solid product was recovered by filtration and washed with enormous amount of deionized water, and dried in air at 353 K overnight, named as Na/K-clinoptilolite.
The above synthetic Na/K-clinoptilolite was exchanged with 1.0 mol/L KCl or CaCl2aqueous solution with a ratio of 1 g zeolite to 100 mL salt solution at 353 K for 2 h, followed by washing thoroughly with deionized water to ensure the removal of all the remaining ions. The ion exchange procedure was repeated four times, and then the K or Ca-clinoptilolite was obtained.
1.3 CH4/N2 adsorption
Firstly, 0.15 g of clinoptilolite was put into a sample tube, then the sample tube was fixed in the JWGB JW-BK300 machine and pre-heated to 393 K for 6 h under high vacuum (lower than 5 Pa) condition. After heating, N2or CH4adsorption process started at a constant temperature, until the adsorbed gas reached its maximum value, i.e., when pressure became equal to the saturated vapour pressure (p0) of N2or CH4.
1.4 Characterizations
XRD patterns were collected on XD-6 X-ray diffractometer (Beijing Purkinje General Instrument Co. Ltd) using CuKαradiation (λ=0.154056 nm) in the range of scattering angle 2θof 5°-50° and the accelerating voltage was set at 36 kV with a current of 20 mA.29Si NMR experiments were performed on Bruker AVANCE III 600 spectrometer at a resonance frequency of 119.2 MHz. The chemical shift of29Si was referenced to TMS. For CH4and N2sorption, JWGBJW-BK300 from Beijing Sci.&Tech. Co.Ltd was used. TG-DTG analysis were carried out on a Perkin-Elmer Pyris I at a heating rate of 10 K/min under the air atmosphere with a flow rate of 20 mL/min. JEOLJEM-220 SEM was used for studying surface morphology under the condition of 25 kV. TEM images were obtained using JEOL-2010, operating at 200 kV. FT-IR spectrum in wave number range 400-4000 cm-1was obtained using IR Prestinge-21. N2adsorption-desorption isotherms were performed at 77 K using JWGB JW-BK300 from Beijing Sci. & Tech. Co. Ltd. Prior to each measurement, all of samples were degassed for 6 h at 393 K under high vacuum. Barret-Joyler-Helanda (BJH) is used to calculate mesopore size distribution on the basis of isothermal desorption branch, and Horvath-Kawazoe(HK) model is used for determining micropore size distribution[20].
2 Results and discussion
Fig.1(1)-(5) shows the XRD patterns of Na/K-clinoptilolites prepared with different molar ratios of OH-/Si. As indicated in Fig.1(5), only amorphous structures could be observed whenn(OH-)/n(Si) was 0.40. Whenn(OH-)/n(Si) was increased to 0.50-0.55, all the diffraction peaks, i.e. (020), (200), and (400), can be indexed as clinoptilolite type[21]. However, whenn(OH-)/n(Si) was above 0.58 (Fig.1 (1) and (2)), additional phillipsite phase, which usually coexists with clinoptilolite, could be observed[22]. Crystallization time needed in the synthesized process was also investigated in detail as shown in Fig.1(6)-(10). According to the results, 144 h was the best crystallization period. When the crystallization period was decreased to 96 h or 120 h, as shown in Fig.1(6), and (7), clinoptilolite structures was unobvious with the small irregular particles as shown in Fig.2(a),which may be ascribed to the amorphous aluminosilicate. However, when the crystallization time was prolonged beyond 144 h, extra diffraction peaks appeared at 2θ=6.46°, which could be assigned to mordenite phase[19], as shown in Fig.1(9) and (10).
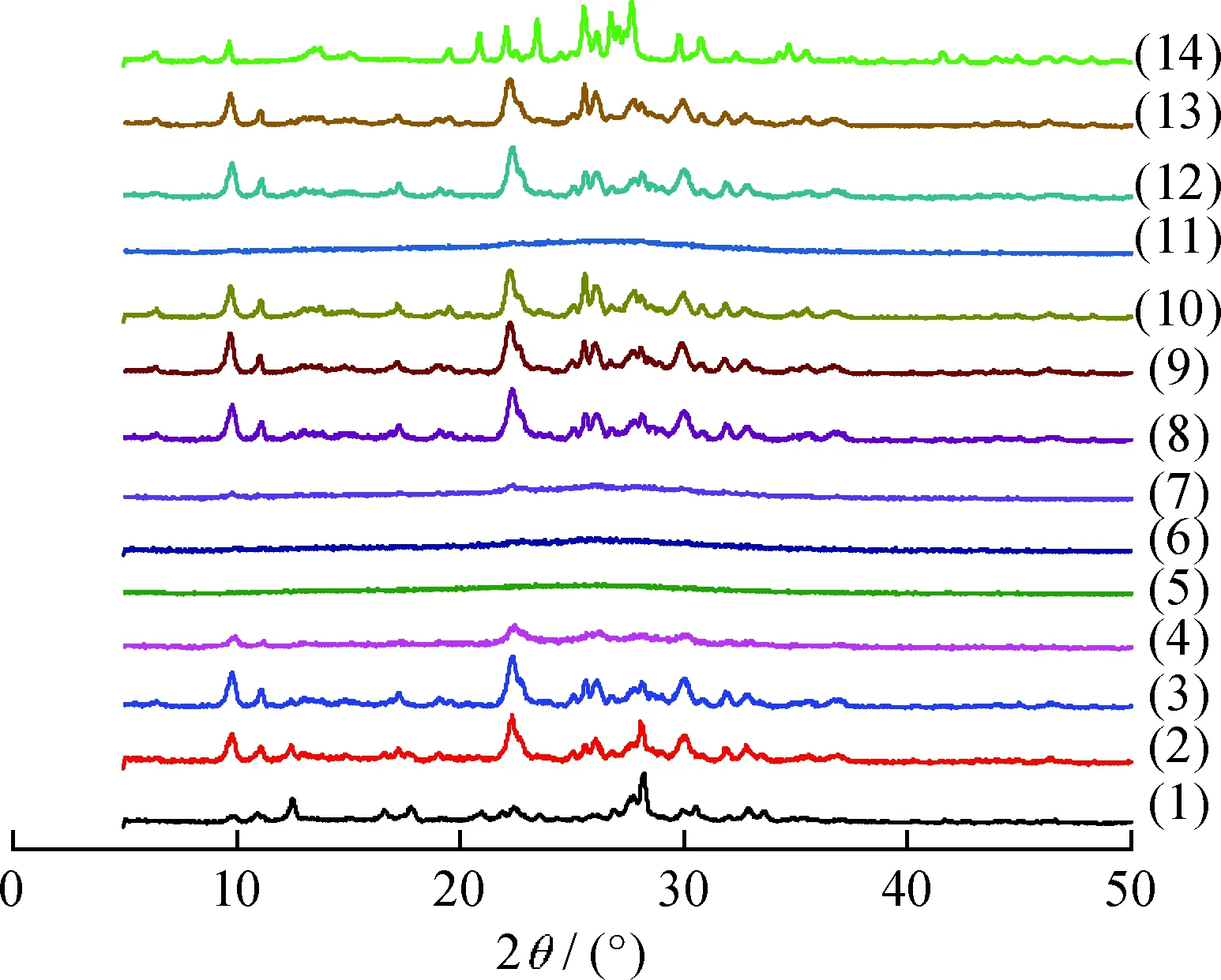
Fig.1 XRD patterns of Na/K-clinoptiloliten(OH-)/n(Si): (1) 0.60; (2) 0.58; (3) 0.55; (4) 0.50; (5) 0.40;Crystallization time/d: (6) 4; (7) 5; (8) 6; (9) 7; (10) 8;Crystallization temperature/K: (11) 413; (12) 423;(13) 433; (14) 443
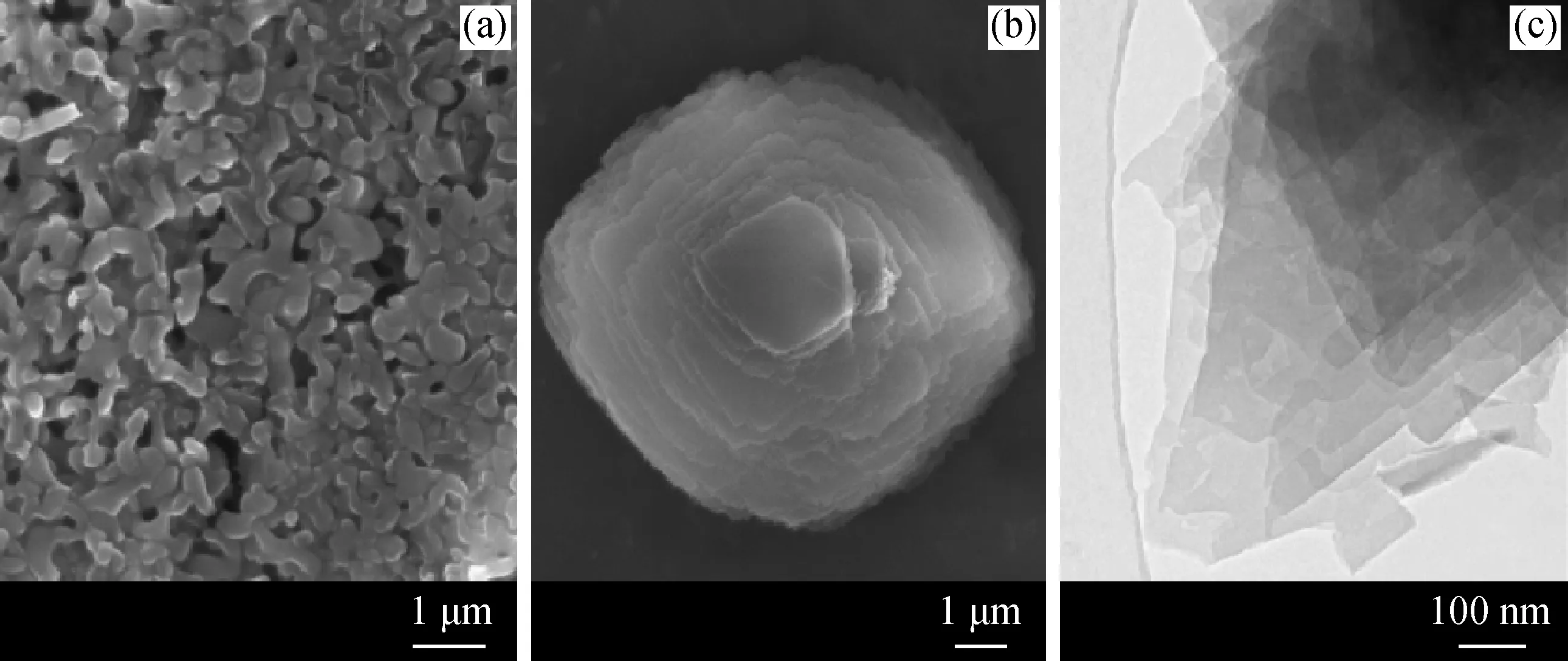
Fig.2 SEM images of Na/K-clinoptilolite with different crystallization time and corresponding TEM image(a) 4 d SEM; (b) 6 d SEM; (c) 6 d TEM
Crystallization temperature is also a key factor for the crystallinity of clinoptilolite. The effect of temperature on the crystallinity is illustrated in Fig.1(11)-(14). It can be observed that, when the crystallization temperature was 423 K asn(OH-)/n(Si)=0.55 and crystallization time of 144 h, pure clinoptilolite can be synthesized as shown in Fig.1(12). While amorphous phase was observed at 413 K (Fig.1(11)) and coexisted mordenite structure appeared at 433 K (Fig.1(13)), the clinoptilolite structure disappeared, and completely transformed to the mordenite phase at 443 K (Fig.1(14)).
As discussed above, the optimum synthesized parameters can be selected asn(OH-)/n(Si)=0.55, hydrothermal crystallization time of 144 h, and temperature at 423 K. This result was further investigated by observing their SEM and TEM images, as shown in Fig.2(b) and (c), which depicted the plate-like particles in size of around 10 μm, corresponding to the morphology of normal clinoptilolite.
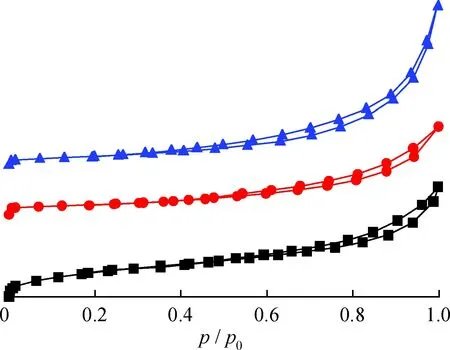
Fig.3 N2 adsorption-desorption isotherms at 77 K Na/K-clinoptilolite; K-clinoptilolite; Ca-clinoptilolite
The N2adsorption-desorption isotherms of Na/K-clinoptilolite, K-clinoptilolite and Ca-clinoptilolite are provided in Fig.3, and their corresponding physical parameters are listed in Table 1. All curves exhibit a hysteresis loop of type H3, according to IUPAC classification, in thep/p0range of 0.5-1.0. An abrupt increase of nitrogen adsorption at very low relative pressures (p/p0<0.1) is observed for all samples, suggesting the abounding microporosity. The calculated BET surface areas are 71.6, 30.2 and 25.3 m2/g for Na/K-clinoptilolite, K-clinoptilolite and Ca-clinoptilolite, respectively. Thus, for the ion-exchanged samples, the BET surface areas from large to small are in order of Na/K-clinoptilolite, K-clinoptilolite and Ca-clinoptilolite. Interestingly, after the ion exchange, the pore volume of K-clinoptilolite and Ca-clinoptilolite were increased to 0.12 and 0.21 cm3/g respectively, compared with Na/K-clinoptilolite (0.15 cm3/g).

Table 1 Textural properties of synthesis andion exchange clinoptilolite
1) Estimated from the amounts adsorbed at a relative pressurep/p0=0.99
As indicated in Fig.4, the same FT-IR spectra profiles can be noticed for clinoptilolites before and after ion-exchanged. The broad bands from 3000 to 4000 cm-1are attributed to the characteristic —OH bond (typically at 3434 cm-1), the bands at 1639 and 3635 cm-1are corresponded to the isolated OH stretching vibration and bending vibration of H2O. Meanwhile, the band near 1032 cm-1can be assigned to the asymmetric stretching vibration modes of internal T—O bonds in TO4tetrahedra where T is Si or Al. The peaks at 793 and 465 cm-1represent the stretching vibration modes of O—T—O groups and the bending vibration modes of T—O bonds, respectively[23].
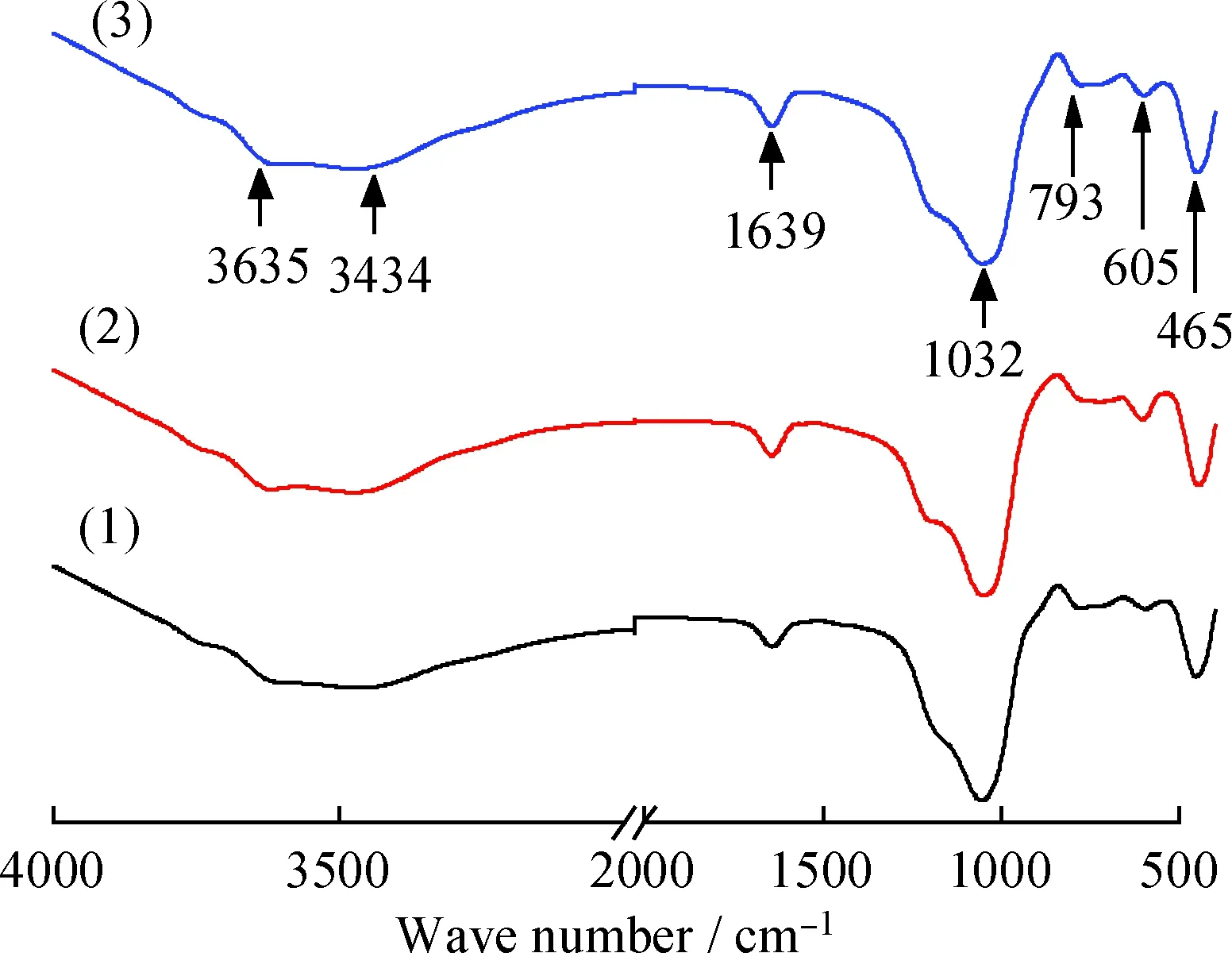
Fig.4 FT-IR spectra of before and afterion exchanged clinoptilolite(1) Na/K-clinoptilolite; (2) K-clinoptilolite; (3) Ca-clinoptilolite
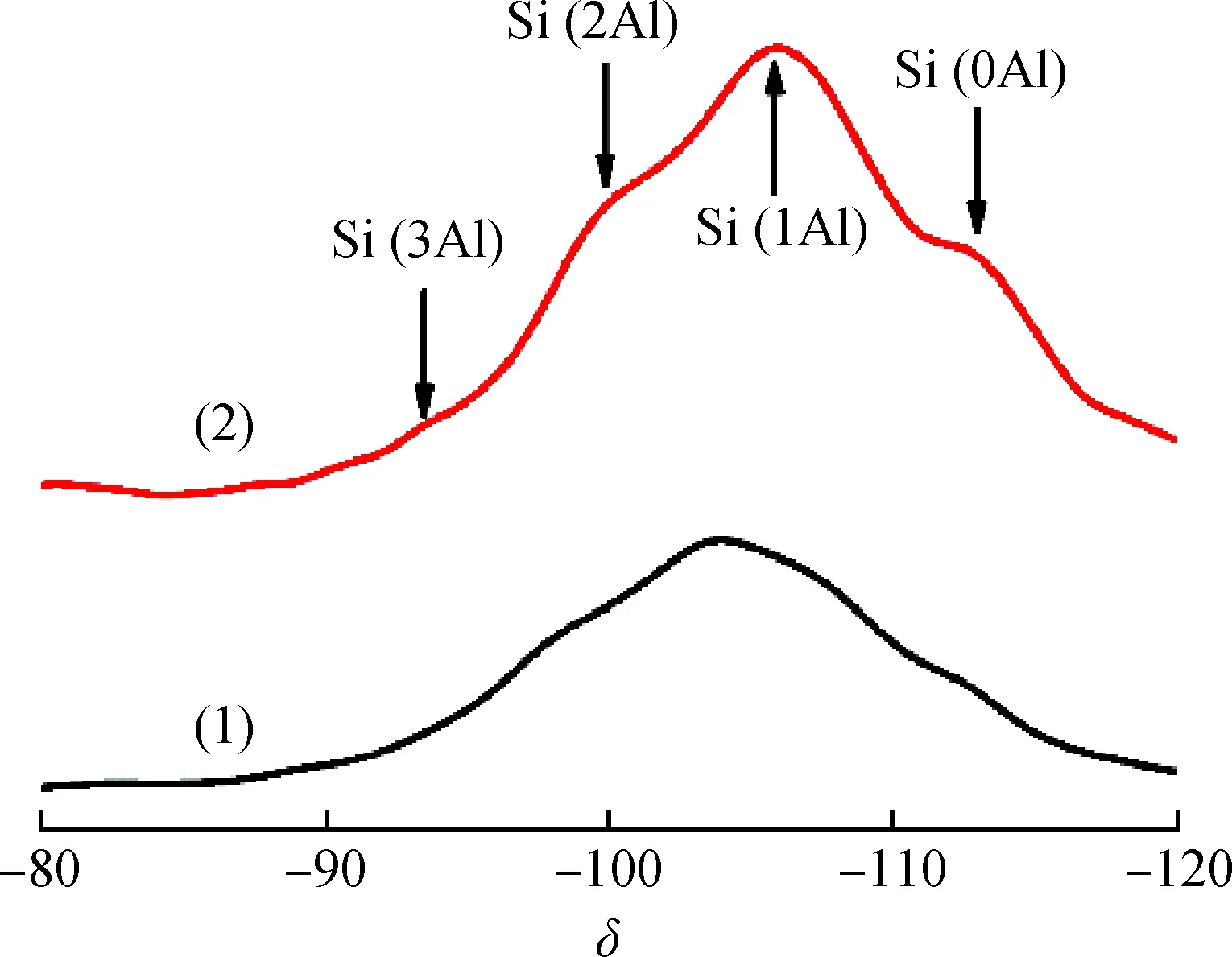
Fig.5 29Si NMR spectra of before and afterion exchanged clinoptilolite(1) Na/K-clinoptilolite; (2) Ca-clinoptilolite
Fig.5 shows29Si NMR spectra of Na/K-clinoptilolite and Ca-clinoptilolite. There are four resonance signals, corresponding to Si in between one and four aluminum neighbors with following configurations:δat -95, -99, -105, and -112 belong to Si(3Al), Si(1Al), Si(2Al), and Si(0Al), respectively[24]. According to29Si NMR profiles, then(Si)/n(Al) ratio of the clinoptilolite skeleton can be calculated as 4.1 and 4.5 for Na/K-clinoptilolite and Ca-clinoptilolite, respectively.
Fig.6 is the TG-DTG profiles of Na/K-clinoptilolite and Ca-exchanged clinoptilolite. As can be seen, the total mass loss determined by TG analysis are around 13.9% and 11.2% for Na/K-clinoptilolite and Ca-clinoptilolite, respectively. Correspondingly, the first distinct peak from DTG curves in the temperature range of 303 to 473 K is assigned to the desorption of physisorbed water, and the mass loss are about 5.5% and 3.1% for Na/K-clinoptilolite and Ca-clinoptilolite, respectively. Subsequently, the mass loss are 5.9% and 5.6% for Na/K-clinoptilolite and Ca-clinoptilolite at temperature range of 473 to 773 K, corresponding to strong chemisorbed water[25]. Finally, the mass loss of 2.5% for Na/K-clinoptilolite and Ca-clinoptilolite during the temperature period of 773 to 873 K can be assigned to the crystalline water.
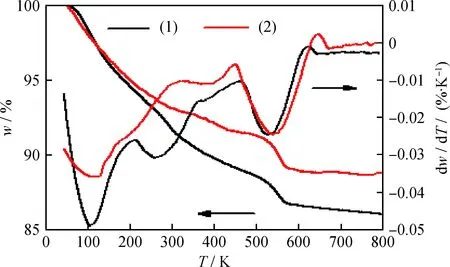
Fig.6 TG-DTG curves of before and afterion exchanged clinoptilolite(1) Na/K-clinoptilolite; (2) Ca-clinoptilolite
According to ICP analysis (Table 2) and29Si NMR spectra, the calculated chemical formula could be K11.2Na3.2Si29.8Al7.2O77.6·26.5H2O for the Na/K-clinoptilolite, and K0.31Na3.61Ca3.06Si29.42Al6.58O73.73·25.2H2O for Ca-clinoptilolite. These results are in good agreement with that of the EDX data (Table 2) originating from mapping images.
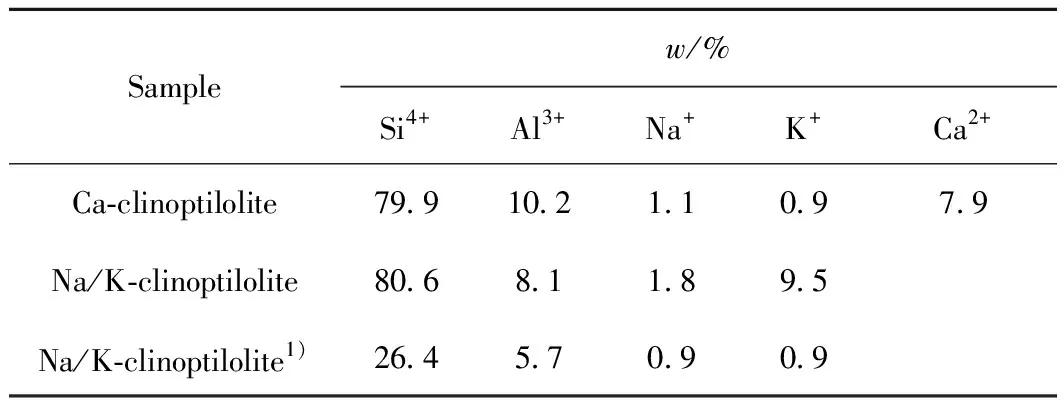
Table 2 ICP analysis of synthesized clinoptilolitesand Ca2+ exchange
1) Originating from EDX data
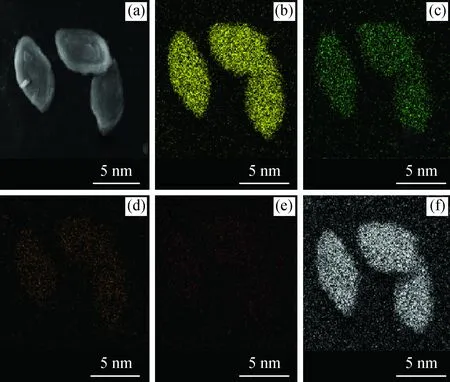
Fig.7 Mapping images of Na/K-clinoptilolite(a) Na/K-clinoptilolite;(b) Si; (c) Al; (d) K; (e) Na; (f) O
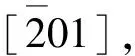
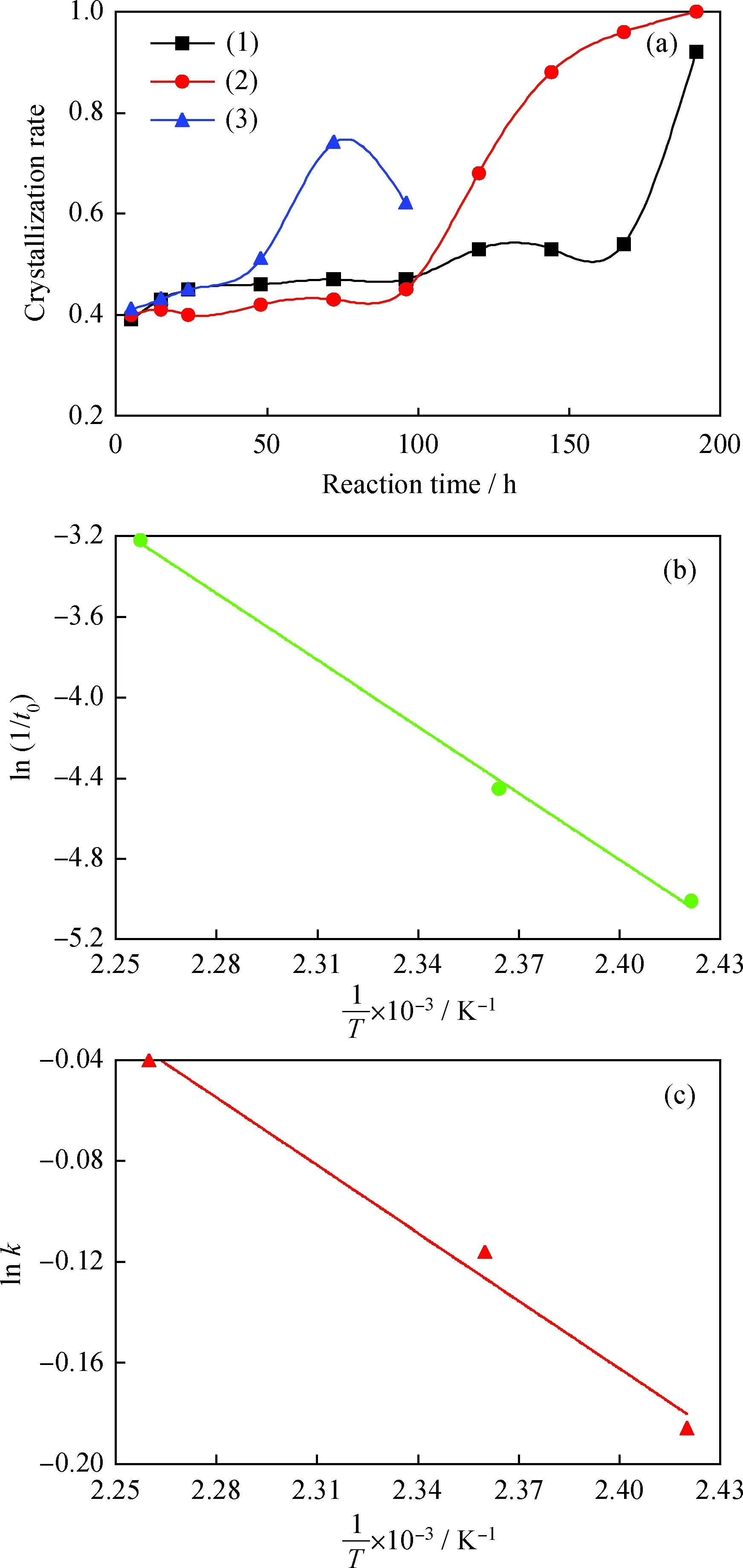
Fig.8 Crystallization curves and Arrhenius plots forNa/K-clinoptilolite at different temperatures(a) Crystallization curves for Na/K-clinoptilolite;(b) Arrhenius plots of induction period;(c) Arrhenius plots of growth process(1) 413 K; (2) 423 K; (3) 443 K
(1)
Wheret0is the induction time (h),Anis frequency factor,Enis activation energy (kJ/mol),Tis crystallization temperature (K), andRis Avogadro constant. The logarithmic plot of the nucleation rate versus the reciprocal of the temperature is shown in Fig.8(b), which can be expressed asy=-11010x+21.618 with high coefficient (R2=0.996), and Pearson correlation coefficient -0.999. Thus, theEnvalue for the induction period obtained from above mentioned method is 91.5 kJ/mol.
After induction period, the rapid growth of crystallites led to an abrupt change in the slope of crystallization curves, as shown in Fig.8(a)-(2). The sigmoidal shape of the crystallization curves implies that the rate of crystal growth follows an Avrami-Erofeev expression, which is also known as the KJMA equation[27]. The rate of crystal growth (k) can be obtained by the slope of the rapid growth of crystallites. The activation energy (Eg) for the growth period can be obtained according to the following equation:
(2)
Wherekis the rate of crystal growth,Agis frequency factor,Egis activation energy (kJ/mol),Tis crystallization temperature (K), andRis Avogadro constant. The logarithmic plot of growth rate versus the reciprocal of the temperature is shown in Fig.8(c), which can be expressed asy=-864.72x+1.9154 with coefficientR2of 0.971, and Pearson correlation coefficient of -0.993. Thus, theEgvalue obtained from the above method is about 7.2 kJ/mol for the growth period. Obviously, theEnvalue in induction period for Na/K-clinoptilolite is much higher than that (Eg) in growth period, which suggests that induction period belongs to kinetic-controlled behavior, but growth period is attributed to thermodynamics dominance. The activation energys of induction and growth period for zeolite ZSM-5 are 25 and 29 kJ/mol[28], and zeolite A are 75 and 71 kJ/mol[29]. The reasons of the big difference between the two stages in the synthesis process are needed to be further understood and this work is currently on-going.
Fig.9 CH4/N2 adsorption isotherms and selectivity curvesAmount adsorbed: (a) 273 K; (b) 298 K(1) N2; Na/K-clinoptilolite; (2) N2; K-clinoptilolite; (3) N2; Ca-clinoptilolite;(4) CH4; Na/K-clinoptilolite; (5) CH4; Na/K-clinoptilolite; (6) CH4; Ca-clinoptiloliten(CH4)/n(N2): (c) 273 K; (d) 298 K(1) Na/K-clinoptilolite; (2) K-clinoptilolite; (3) Ca-clinoptilolite; (4) tangent of (2)
The equilibrium adsorption isotherms of N2and CH4on Na/K-clinoptilolite, Ca-clinoptilolite and K-clinoptilolite at 273 K and 298 K are given in Fig.9(a) and (b), respectively. It can be seen that K-clinoptilolite has the maximum adsorption capacity of 1.09 mmol/g for CH4and 0.33 mmol/g for N2at 273 K, 0.76 mmol/g for CH4and 0.41 mmol/g for N2at 298 K, while Ca-clinoptilolite has the minimum adsorption capacity of 0.17 mmol/g for CH4and 0.15 mmol/g for N2at 273 K, 0.11 mmol/g for CH4and 0.18 mmol/g for N2at 298 K. Based on the literature, the type and location of cations presented in the porous network are the main factors that contribute to the adsorption and diffusion properties of the clinoptilolites[30]. Gu et al.[31]reported the use of granular activated carbons for the adsorption of CH4and N2. The adsorbed amount of CH4and N2were 1.5 mmol/g and 1.2 mmol/g, respectively, at 2 MPa and 298 K. As evaluated by Silva et al.[32], the amounts of CH4and N2adsorbed on zeolite 5A were 1.5 mmol/g and 0.9 mmol/g at 3 kPa and 305 K, respectively. Although the adsorption capacity of K-clinoptilolite is slightly lower than the above adsorbents, the selectivity of K-clinoptilolite is better than them.
Different ions on the channel congestion have significant impact on the diffusion rate of N2and CH4, especially the one with pore size range between 0.15-0.55 nm. The most effective channel jam is located on the channel intersections of Na+and Ca2+[33]. Na+and Ca2+occupy both M(1) and M(2) sites and these sites are located on the channel intersections. Thus, full occupancy of sites M(1) and M(2) with Na+and Ca2+can result in effective blocking of all three channels, and lead to very slow diffusion rate through molecular sieving. Due to the pore blocking, the equilibrium capacity of the clinoptilolites can be also greatly reduced. However, for K-clinoptilolite, K+could cause very little impact on the intersections channel due to its complete occlusion channel C.
Meanwhile, the selectivity ratio defined here is the amount of adsorbed CH4per amount of N2adsorbed, as shown in Fig.9(c) and 9(d). It can be observed that, for all samples, the CH4/N2selectivity goes down when relative pressure increases. Furthermore, K-clinoptilolite has the best selectivity at 273 K, higher than that at 298 K, which can be attributed to the interaction between kinetics and thermodynamics[30]. Franckiewicz et al.[14]investigated the application of clinoptilolite for CH4/N2separation through PSA process, and suggested that the values of kinetic separation factor depend on the composition and the type of charge balancing cations. As reviewed by Ackley et al.[13], CH4adsorption should be kinetic control without thermodynamic restriction. However, high temperature is beneficial to molecular motion in the micropore channels and therefore, thermodynamics is prevailing, even kinetic hindrance is minimum. On the other hand, the molecular motion in the micropore channels will slow down at low temperature, which results in the kinetic behavior dominance in the whole process of adsorption. Therefore, the equilibrium (thermodynamic) diffusion of CH4molecules in the micropores is very limited at low temperatures.
The energy consumption of PSA system in industry is associated with switched pressure. From Fig.9(c), the best work adsorption pressure is at about 35 kPa, originating from intersection of two tangents. The maximum amount of adsorbed CH4per amount of adsorbed N2is around 4.5 at 273 K for K-clinoptilolite. These results indicate a higher adsorption capacity and selectivity factor, which suggest that K-clinoptilolite might be a promising candidate for CH4/N2separation
The measured isotherms follow with the Langmuir equation, which can be expressed as:
(3)
Whereqis molar adsorbed amount (mmol/g),qsis molar saturated amount adsorbed (mmol/g) andBis Langmuir constant. The fitted Langmuir parameters are listed in Table 3.
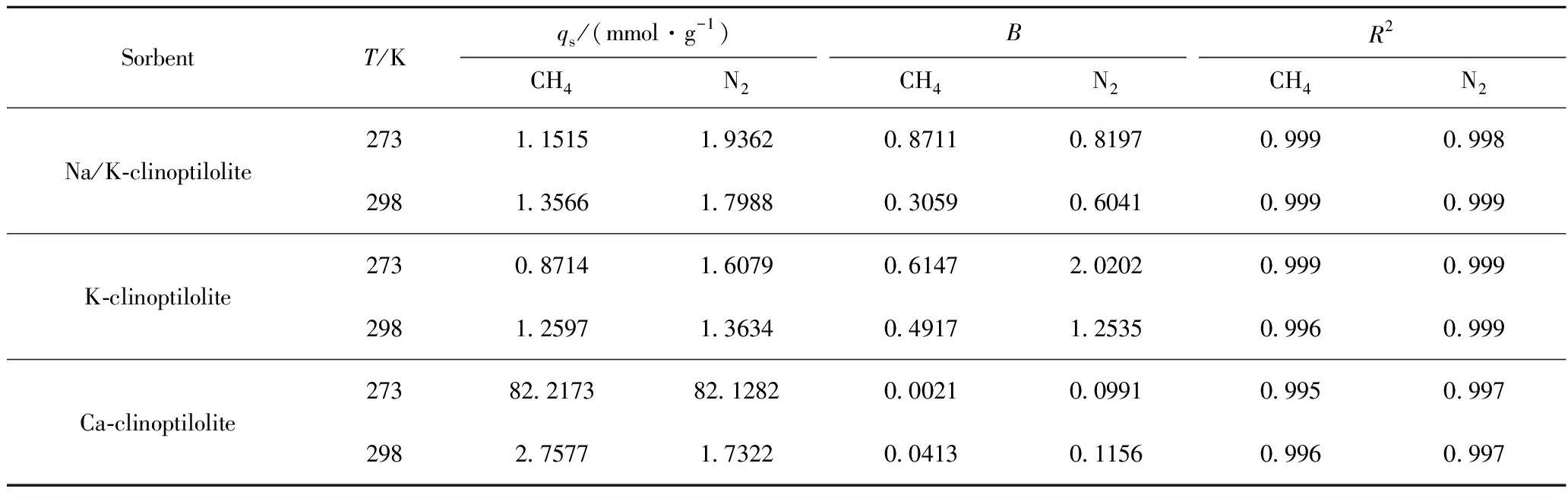
Table 3 Langmuir parameters for N2 and CH4 adsorption on clinoptilolites before and after ion-exchanged at 273 K and 298 K
3 Conclusions
Na/K-clinoptilolite was synthesized through hydrothermal route. The structural features and textural properties of the prepared clinoptilolites before and after ion-exchange (Ca2+or K+) were characterized with XRD, SEM, TEM, N2sorption isotherms, FT-IR, TG and29Si NMR methods. The optimum conditions of the synthesized parameters were determined as follows:n(OH-)/n(Si)=0.55, crystallization time was 6 days, and crystallization temperature was 423 K. Particularly, the crystallization kinetics profiles were evaluated at different temperatures. For the induced period, the activation energy of Na/K-clinoptilolite was 91.5 kJ/mol, and for the growth period, it was 7.2 kJ/mol. Meanwhile, the application of CH4/N2separation via ion-exchanged clinoptilolite was preliminary studied. Experimental results demonstrated that K-clinoptilolite exhibited maximum adsorption capacity of 1.09 mmol/g for CH4with CH4/N2selectivity of 4.5 at relative pressure of 0.35 at 273 K. The adsorption capacity and selectivity is strongly dependent on the type of exchanged cationic charges, quantity as well as their distribution within the skeleton.
