低植酸水稻种质资源筛选、遗传生理调控与环境生态适应性研究进展
2019-03-22苏达吴良泉renRasmussen周庐建程方民
苏达 吴良泉 Søren K Rasmussen 周庐建 程方民,*
低植酸水稻种质资源筛选、遗传生理调控与环境生态适应性研究进展
苏达1,2吴良泉2Søren K Rasmussen3周庐建4程方民4,*
(1福建农林大学 作物科学学院 作物遗传育种与综合利用教育部重点实验室, 福州 350002;2福建农林大学 国际镁营养研究所, 福州 350002;3哥本哈根大学 植物与环境科学系, 丹麦 哥本哈根;4浙江大学 农业与生物技术学院, 杭州 310058;*通讯联系人, E-mail: chengfm@zju.edu.cn)
提高或维持水稻产量的同时,提高稻米品质已成为目前水稻育种的首要目标之一。其中,通过降低籽粒中植酸等抗营养因子,增加锌、铁生物有效性以提升水稻营养品质,是目前水稻品质改良的一个重要方向。本文主要综述了水稻籽粒中植酸合成的代谢路径、低植酸水稻的筛选及相关功能基因的遗传特点、植酸生理代谢的调控网络、低植酸水稻农艺性状劣变和生态适应性降低的生理原因、籽粒植酸合成的环境调控效应等相关研究进展。可为低植酸水稻品质改良以及栽培调优提供借鉴。
植酸;水稻;籽粒品质;遗传调控;生态效应
植酸(C6H18O24P6,IP6,六磷酸肌醇)是作物籽粒中磷的最主要贮存形式,约占籽粒总磷含量的60%~90%[1]。植酸与籽粒其他内含物(如淀粉、蛋白质和脂类等)一起随灌浆充实逐渐积累。在此过程中,植酸可与籽粒中的矿质离子(Zn2+、Fe2+、Ca2+、Mg2+等)以及活性蛋白络合,形成难溶性的植酸盐(phytin),并以圆球状复合晶体(globoid crystal)的形式贮存于籽粒中[2]。由于人和单胃动物消化系统中缺乏分解植酸的内源植酸酶,因此,作物籽粒中植酸(盐)的存在大幅降低了有关矿质营养元素(尤其是锌和铁)的生物有效性以及人体对活性蛋白和氨基酸的有效吸收[3, 4]。因此,大量摄取植酸含量相对较高的谷物和豆类,被认为是发展中国家人群铁、锌缺乏的主要原因。同时,在畜牧业生产中,不能被单胃动物有效吸收的植酸磷,70%以上以粪便形式排泄到环境,也加剧了土壤污染和水体富营养化[1, 5]。
水稻是我国重要的粮食作物,在传统高产育种和优化栽培体系的基础上,进一步挖掘其品质潜力,是水稻品质育种的主要目标之一。其中,培育和筛选低植酸种质资源是目前解决水稻籽粒植酸等抗营养问题以及谷物营养品质改良的重要手段,也是保障锌、铁生物强化,磷资源可持续利用以及农业生态保护的有效措施[1, 6, 7]。为此,本文主要综述了植酸的合成代谢及分子遗传特点、植酸合成的生理调控网络、低植酸水稻的农艺性状和环境生态适应性表现等内容,相关研究进展可为低植酸水稻品质改良以及相应的栽培调控提供借鉴。
1 植酸合成的代谢路径及其关键酶
植酸的合成和代谢路径主要分为三个阶段:肌醇合成、多磷酸肌醇合成以及植酸合成后从胞质向液泡的转运(图1)。1)肌醇(-inositol)合成阶段。肌醇-3-磷酸合成酶(-inositol-3-phosphate synthase,MIPS)以NADH(Nicotinamide adenine dinu cleotide)为辅酶,将光合产物葡萄糖-6-磷酸(Glucose-6-phosphate,G-6-P)转化为肌醇-3-单磷酸[Ins(3)P1][8,9]。肌醇-3-磷酸随后在肌醇-3-磷酸水解酶[Ins(3)P1- monophosphatase,IMP]的催化下水解肌醇环上的磷酸基团,生成肌醇。2)多磷酸肌醇的合成阶段。可分为脂独立途径(磷酸肌醇顺序磷酸化路径,lipid-independent)和脂依赖途径(磷脂酰肌醇代谢路径,lipid-dependent)。两条代谢途径的最初产物均为肌醇,终产物均为1,3,4,5,6-五磷酸肌醇[Ins(1,3,4,5,6)P5],区别在于代谢分支路径中是否会有磷酸酯的出现。其中,脂独立途径中,肌醇在肌醇激酶(-inositol kinase,MIK)的催化下重新生成肌醇-3-磷酸,之后在磷酸甘油酸激酶(phosphoglycerate kinase,PGK)和多磷酸肌醇激酶(如多磷酸-肌醇5,6-激酶,Inositol 1,3,4-trisphosphate 5/6-kinase)催化下,逐渐顺序磷酸化并最终合成植酸。前人研究多表明,此途径是作物籽粒植酸合成的主要路径。而在脂依赖途径中,肌醇首先在磷脂酰肌醇合酶(phosphatidylinositol synthase)的催化下合成磷脂酰肌醇(phosphatidylinositol),随后由磷酸磷脂酶C(Phospholipase C, PLC)水解生成肌醇-1,4,5-三磷酸[-inositol-1,4,5-trisphosphate,Ins(1,4,5)P3],继而进一步磷酸化生成植酸。因此,脂依赖途径中有磷脂酰肌醇以及肌醇-1,4,5-三磷酸等第二信使参与。与脂独立途径相比,脂依赖途径对植物籽粒器官植酸含量的影响较小。上述两条代谢路径的共同终产物均为1,3,4,5,6-五磷酸肌醇,其后在1,3,4,5,6-5-肌醇-2-磷酸激酶[Ins(1,3,4,5,6) P5-2-kinase]的催化下生成植酸[10];3)植酸合成后的转运路径。胞质中合成的植酸,需经ABC跨膜转运蛋白(ATP-binding cassette)家族中MRP蛋白(multidrug resistance-associated protein,MRP)的运输作用,将胞质中合成的植酸最终转移至液泡中。此外,真核细胞植酸还可在六磷酸肌醇激酶的催化下继续磷酸化生成高阶磷酸肌醇盐(如7、8-磷酸肌醇),参与磷和ATP能量代谢等生理过程的调节[8]。同时,在籽粒萌发过程中,植酸可在内源植酸酶或磷酸化酶的催化下去磷酸化,重新降解为不同价位的磷酸肌醇(如IP1、IP2、IP3、IP4、IP5)和肌醇。总之,植酸合成主要有以下三个特点:1)代谢途径同时进行,不同代谢途径之间相互协调和补充[11-14];2)反应过程非线性,磷酸化和去磷酸化同时进行;3)植酸合成过程中的关键酶大多数具有多功能性特性,不局限于作用某一特定底物[15]。

G-6-P,葡萄糖-6-磷酸; Inositol,肌醇;Ins(3)P1, 肌醇-3-单磷酸;Ins(3,4)P2,肌醇-3,4-二磷酸;Ins(3,4,6)P3,肌醇-3,4-6-三磷酸;Ins(3,4,5,6)P4,肌醇-3,4-5-6-四磷酸;Ptd Ins,磷酯酰肌醇;PtdIns(4)P,磷脂酰肌醇-4-单磷酸;PtdIns(4,5)P2,磷脂酰肌醇4,5二磷酸;Ins(1,4,5)P3,肌醇1,4,5三磷酸;Ins(1,4,5,6)P4,肌醇1,4,5,6四磷酸;Ins(1,3,4,5,6)P5,肌醇1-3,4-5-6-五磷酸;Ins(1,2,3,4,5,6)P6,肌醇1-2-3-4-5-6-六磷酸(植酸)。
[1]-肌醇-3-磷酸合成酶;[2]-肌醇-3-磷酸水解酶;[3]-肌醇激酶;[4]-磷酸甘油酸激酶;[5]-多磷酸-肌醇5,6-激酶;[6]-1,3,4,5,6-5-肌醇-2-磷酸激酶;[7]-磷脂酰肌醇合成酶;[8]-磷酸磷脂酶C;[9]-肌醇1,4,5-三磷酸激酶;[10]-ABC转运蛋白;MRP转运蛋白;[11]-六磷酸肌醇激酶;[12]-植酸酶或磷酸酶。
[1], MIPS,-inositol-3-phosphate synthase; [2], Ins(3)P1-monophosphatase IMP,-inositol-phosphate monophosphatase; [3], MIK,-inositol- kinase; [4], PGK,phosphoglycerate kinase; [5], ITP5/6K,inositol 1,3,4-triphosphate 5/6-kinase; [6], IPK1,inositol 1,3,4,5,6-pentakisphosphate 2-kinase; [7], PtdIns Synthase,phosphatidy linositol synthase; [8], Phospholipase C; [9], Inositol 1,4,5-tris-phosphate kinase; [10], ABC transporter; MRP transporter; [11], InsP6 Kinase; [12], Phytases or phosphatase.
图1 植酸的生物合成
Fig. 1. Biosynthetic pathways of phytic acid.
2 植酸合成过程的重要功能基因以及低植酸水稻种质筛选
培育低植酸新品种是提高作物籽粒微营养及生物有效性的一条有效途径。近年来随着分子生物技术的发展,在传统理化诱变的基础上,利用基因操作已成为作物低植酸突变材料创建的常用手段之一。目前,国内外已获得大麦、玉米、水稻和大豆等作物的低植酸突变材料,其籽粒植酸下降30%~95%[1, 4, 9, 14, 16-18]。人体和动物试验结果均表明,低植酸作物铁、钙的利用率增加了30%~50%,锌的有效性增幅高达76%[1, 19-22]。其中,通过基因工程技术对植酸合成的限速酶基因进行干扰或敲除,籽粒植酸含量降低了14.9%~75%[23-26]。Ali等[27]和Kuwano等[23]在前人利用组成型表达启动子进行低植酸作物选育的基础上,进一步利用籽粒特异性启动子对()进行RNA干扰,转基因后代中籽粒的表达量为原来的21.8%,籽粒植酸含量降低了68%~75%,特异性启动子的选择有效避免了相关基因突变对营养器官中磷代谢的干扰。除外,近年来对参与植酸代谢过程其他基因进行突变,也正不断丰富低植酸的突变类型。据Kim等[28]报道,通过EMS筛选出的(肌醇激酶基因)型低植酸突变水稻,籽粒植酸含量降低了34%~75%,这与通过基因工程筛选出的型低植酸突变体的植酸降幅相近(37.0%~50.7%)[25, 28]。对以及()等基因进行突变或干扰,也筛选出相应的低植酸突变材料[29],籽粒植酸降幅分别可达39%~71%和46%~68%[29-31]。虽然目前对PGK的功能研究相对较少,但研究者在细菌中观察到PGK在ATP参与下会催化生成2,3-二磷酸甘油酸盐,此物质会抑制肌醇多磷酸的产生,这可能是基因缺失引起植物细胞中肌醇单磷酸盐增加以及植酸含量降低的一个原因。IPK1催化植酸合成的最后一步。Ali等[32]利用特异性启动子()对基因进行RNA干扰,转基因后代中籽粒基因表达量降至原来的26.0%,植酸含量也同步降低了69%。基因的突变一般并不会影响低价肌醇磷酸的合成,考虑到肌醇、磷脂酰肌醇以及低价磷酸肌醇(IP1-3)在作物生长发育过程中参与了多个重要生理代谢过程,而高价磷酸肌醇,如4-磷酸肌醇或5-磷酸肌醇具有与植酸相似的生理功能,因此对该基因进行突变后产生的低植酸突变材料,其农艺性状的劣变趋势比突变小。同时,虽然IMP在植酸合成中的功能现已基本明确,主要参与催化肌醇的合成,但迄今尚未获得此基因突变的低植酸水稻材料。
此外,对参与植酸合成后从胞质向液泡转运/分储的转运蛋白(如MRP)的相关基因进行突变,也已在不同主栽粮食作物中筛选出相应的低植酸突变材料,如拟南芥[33]、水稻[34, 35]、豆类[36, 37]、玉米[38-40]和小麦[41, 42]等。由于植酸合成过程中存在肌醇回补路径[43, 44],因此,MRP蛋白可能充当了植酸在不同器官和组织中相互交流的媒介。Mitsuhashi等[43]在拟南芥中发现,IMP催化产生的肌醇在肌醇转运蛋白的作用下从胚乳转移到胚中参与肌醇顺序磷酸化,这一过程也受到MRP蛋白的调控。利用T-DNA插入技术对水稻MRP基因进行突变,籽粒植酸降幅可达90%[45]。由于MRP同时还参与作物颖花发育、激素调控、信号代谢、氧化胁迫响应等多个生理过程[46, 47],该基因的突变还会影响除籽粒植酸合成外的其他生理过程,如突变后拟南芥对激素调控的响应变得更为敏感[33]。MRP蛋白还可以通过与谷胱甘肽偶联参与重金属的抗性表达,对该基因进行突变后,小麦根系对镉胁迫的敏感度增加,同时还改变了籽粒中矿质元素的空间分布以及铁离子的再运转[41]。基于MRP酶的多功能性,此基因的突变可能是导致水稻、小麦等作物的生长发育(如籽粒灌浆、营养器官的形态建成)及农艺性状劣变(粒重降低,萌发延迟,胚芽鞘生长缓慢,感病性增强)的原因之一。同时,由于不同作物中MRP同源基因组成/功能有所差异,型低植酸突变体的农艺性状在不同作物中的表现有所不同。水稻中多表现为农艺性状劣变的趋势,而豆科作物由于不同基因的同工型之间表现为一定程度的功能互补(如同工型在一定程度上可补偿缺失对营养器官中磷代谢造成的影响,而不参与籽粒中植酸合成),因此在一定程度上补偿了突变后造成的农艺性状劣变的多效性[21, 37, 48]。前人研究表明型低植酸大豆籽粒中肌醇含量表现为增加[49],而()三基因突变后,肌醇含量变化不显著,推测可能与位点突变补偿了突变后肌醇的降低有关,对型低植酸大豆的转录谱分析也表明,基因突变促进植酸水解生成肌醇/磷酸肌醇[50],说明型低植酸突变体中肌醇和磷酸肌醇中间产物含量的增加和植酸的降解有关。但也有观点认为,沉默后肌醇含量增加,可能与植酸(或肌醇、磷酸肌醇)减少后通过反馈调节激活肌醇转运子相关基因的表达有关[51]。因此,关于基因突变后降低植酸积累的生理原因还有待进一步研究。除MRP转运蛋白外,研究者通过对已筛选出的低植酸突变体-Z9B-1和-MH86-1的突变位点进行定位分析,结果表明硫酸盐转运蛋白(sulfate transporter,sultr3;3)酶基因突变也会显著影响水稻籽粒植酸的合成。研究表明在大麦、水稻和拟南芥中对;基因进行突变,籽粒植酸降幅可达45%,同时籽粒总磷的含量也会显著降低[52-56],这种植酸磷和总磷同步降低的低植酸类型是未来低植酸作物选育的一个新目标。此外,Zhang等[57]对玉米转录谱分析表明,除上述功能基因直接参与植酸合成外,还有三个候选基因可能也参与了植酸的合成,包括(编码磷酸肌醇合成相关酶基因)、(参与磷酸肌醇在不同器官中的转运)和(参与磷酸肌醇向液泡转运),但其同源基因在水稻中尚未见相关报道,这可能是未来水稻植酸合成相关基因克隆以及相应低植酸突变筛选的一个新方向。
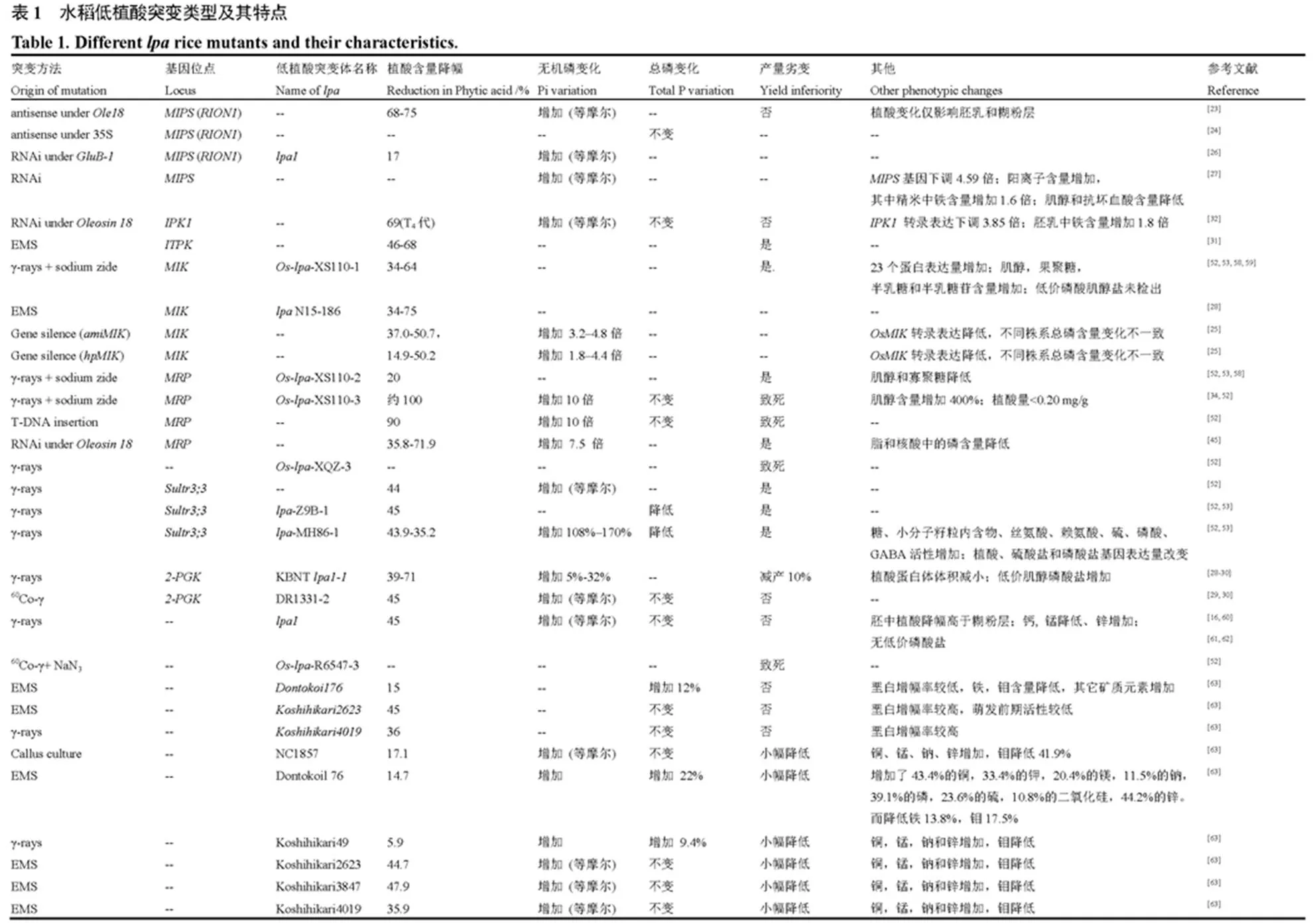
根据水稻籽粒中植酸磷、无机磷和总磷含量的变化特点,可将不同低植酸突变体分为4种类型(表1):1)类低植酸突变。该类低植酸突变体的籽粒植酸磷变化特点表现为籽粒植酸含量明显降低的同时,无机磷含量等摩尔增加,而不同价位的磷酸肌醇和籽粒总磷含量保持不变。类低植酸突变体通常是由于植酸合成代谢路径中作用于肌醇供应阶段(从葡萄糖-6-磷酸到三磷酸肌醇合成)的相关功能基因发生突变所引起的(如和等);2)类低植酸突变。该类低植酸突变体籽粒植酸含量明显下降,而籽粒中无机磷和其他形态的磷酸肌醇(如IP1~IP5)均有所增加,总磷含量保持不变。类低植酸突变体多是由多磷酸肌醇磷酸化环节的重要功能基因发生突变所引起的(如、、和等),籽粒植酸含量的降幅一般小于类低植酸突变;3)类低植酸突变。主要是肌醇激酶(MIK)基因发生突变所引起,其籽粒植酸磷和肌醇含量的变化特点表现为籽粒植酸含量明显下降,肌醇含量也显著降低。与和型低植酸突变有所不同,类低植酸突变体的籽粒总磷含量也会随籽粒植酸含量的降低而发生明显改变;4)其他类低植酸突变。此类突变相关基因通常与植酸在植物不同组织中的转运分配有关。如ABC转运子家族中MRP蛋白以及硫酸盐转运蛋白(sultr3;3)基因(表1)。其中,基因突变所导致的籽粒植酸磷、总磷含量变化特征与类低植酸突变体相似,因而也有研究将其划归为类低植酸突变体。而sultr3;3酶基因突变可引起籽粒植酸磷和总磷含量的同步下降,其籽粒磷组分变化与突变类型不一致。
3 植酸生理代谢的调控网络
明确植酸合成代谢的调控网络,并建立起植酸代谢与其他生理过程之间的联系,对于籽粒植酸积累的调控、低植酸“优质种性”的发挥具有重要的意义。研究表明植酸合成与糖代谢、信号转导[Ca2+和Ins(1,4,5)-P3]、激素调控、肌醇/磷酸肌醇代谢、ROS响应密切相关。例如,型低植酸作物降低籽粒植酸含量的同时,还影响了糖代谢[64]。Edwards等[65]通过QTL定位分析表明,水稻籽粒植酸和垩白形成相关基因高度连锁,推测植酸含量降低是水稻垩白率增加的原因之一。Zhou等[66]分析低植酸水稻(9311-)及其野生对照(9311)的萌发生理差异发现,低植酸水稻籽粒中Ins(1,4,5)-P3升高抑制了ROS活性(SOD、CAT、POD、NOX和APX)。Redekar等[51]对低植酸大豆发育籽粒的转录谱分析表明,在发育籽粒的不同阶段,(低植酸基因型,单基因突变)和1MWT(野生对照基因型)相比共有250个差异表达基因。而(,三基因突变)与3MWT(野生对照)相比,差异表达的基因增加到4000个。同时,低植酸突变体与其野生对照在肌醇代谢、磷酸肌醇代谢、激素信号代谢(如auxin-ABA信号,肌醇-生长素信号)均表现出显著差异。对大豆和玉米的植酸代谢研究表明,植酸代谢相关基因的表达同时受到转录因子的调控,如(调控逆境胁迫和激素响应)、(参与ABA信号转导、寡聚糖合成以及钙调蛋白结合转录激活因子表达)、、和等[50, 51, 57]。Zhang等[57]用系统生物学方法,利用转录组测序(RNA-Seq)以及小RNA测序(microRNA-Seq)对不同植酸遗传背景玉米(Qi319,低植酸基因型;B73,高植酸基因型)进行转录表达分析,发现低植酸突变体和野生对照基因型之间差异最大的基因为和,这与IPs含量的变化一致。对参与植酸合成的代谢网络进行分析表明,在植酸和激素关系上,磷酸肌醇合成路径和赤霉素(GA)合成路径之间通过泛素化途径相关联。而在植酸与信号代谢上,Ca2+信号路径是植酸和其他代谢路径之间的桥梁。在植酸和碳水化合物代谢上,植酸(磷酸肌醇)转运相关基因与碳水化合物转运代谢密切相关。此外,、乙烯响应相关转录因子(参与初级和次级代谢调控)、以及(参与调控作物生长发育,逆境响应等),是突变体及其野生对照比较中表达差异最显著的转录因子。Redekar等[50]在低植酸大豆中的转录谱分析表明,和相比,肌醇转运子相关基因都表现为显著上调表达,推测两种突变类型均诱导了相同的信号代谢。明确植酸代谢和调控的网络联系,为今后利用栽培调优或外源调控效应降低籽粒植酸积累具有重要的借鉴意义,相关转录因子的发现也为未来低植酸品种选育提供了新的研究思路。
4 低植酸水稻的农艺性状和环境生态适应性表现
低植酸作物在增加籽粒锌、铁生物有效性的同时,农艺性状却表现出不同程度的劣变特征[60, 67, 68],如籽粒活性、发育、萌发率、出苗率或花粉育性降低,灌浆充实不畅,籽粒充实度下降,易早衰,营养器官生长缓慢,花期及成熟延迟等[43, 67, 69, 70],并最终导致产量降低[1, 46, 71, 72]。且籽粒植酸的降幅与农艺性状劣变程度还表现出正相关趋势,即籽粒植酸降幅越大,其灌浆充实度和产量水平的劣变趋势就越明显[9, 30, 67, 68, 73]。低植酸水稻籽粒植酸降幅从35%增加到63.6%时,对应产量降幅从12.5%上升至25.6%。籽粒植酸降幅超过70%时,农艺性状劣变的多效性开始加剧。对植酸降幅90%的低植酸大麦(-M955)进行转录分析表明,在发育籽粒的信号代谢、激素代谢(细胞分裂素、乙烯)、碳水化合物转运和合成代谢路径中,相关酶基因的表达量均表现出显著降低的趋势[74]。当籽粒植酸含量降幅达90%以上时,作物的正常生长发育都会受到严重影响[1, 9, 73];植酸降幅超过95%时甚至无法完成正常的发育进程(表现为胚发育缺陷或致死)[1, 46, 75, 76]。因此,低植酸作物的优质种性,往往因其农艺性状劣变表现,难以进一步在育种中得以推广和应用。探明低植酸突变作物籽粒灌浆特点,以及低植酸作物籽粒灌浆不良和产量下降的生理机制及其与植酸合成积累间的代谢生理联系,对于通过育种、栽培等途径协调作物籽粒植酸含量降低与产量性状劣变之间的矛盾具有重要的理论和生产指导意义。
籽粒植酸含量的降低也会伴随作物生态适应性的改变。据Meis等[69]报道,型大豆和玉米低植酸突变体在适宜的温度区域种植,其子代的田间出苗率为63%,同一低植酸材料种植于高温区域,其子代的出苗率仅为8%,而野生型对照品种在不同生态区域的出苗率差异却不明显。相似地,在非胁迫环境中具有优良田间农艺表现的低植酸大麦(胚乳特异性),在逆境条件下产量也表现出显著下降的趋势[77]。Bregitzer等[68]研究表明,低植酸作物农艺性状的劣变程度在逆境条件下尤为明显,并把这一现象称为“种源效应”。前人对“种源效应”的分析认为,低植酸作物早衰以及籽粒成熟过程中化学结构/组分的变化可能是后代籽粒萌发率降低的主要原因[1, 46, 69]。然而,环境生态因子或外源物质调控对作物籽粒植酸积累的影响是否还与品种本身的植酸遗传特性有关?即关于低植酸作物的农艺表现的争议是否忽略了其环境生态稳定性变化?Naidoo等[78]研究表明,相比野生对照,型低植酸玉米()对干旱胁迫表现更敏感。Su等[71]利用多对低植酸水稻进行灌浆期高温胁迫处理,结果证实水稻籽粒植酸的种性与其在逆境条件下的生态稳定性之间也存在密切联系。与野生型对照相比,低植酸水稻对逆境表现更“敏感”,其籽粒植酸含量和结实特性(结实率、花粉育性和千粒重)的生态稳定性也明显变差。
虽然近年来利用基因工程选用器官特异性启动子(如胚、糊粉层等特异性启动子和)对植酸代谢相关基因(如、、和等)进行沉默,筛选出的低植酸突变体(玉米、大麦、水稻)表现出了与野生型对照相似的农艺性状特征[1, 23, 26, 39, 46, 79]。如Kuwano等[23]以糊粉层特异表达的启动子对水稻()基因进行沉默,籽粒植酸含量显著降低的同时,产量性状并未受到显著影响。对植酸代谢路径中下游基因的沉默和敲除(如等),由于未影响到低价磷酸肌醇及1,4,5-3-磷酸肌醇等信号物质的合成,低植酸突变体的产量优势也得以保留[32]。然而上述低植酸突变体及其野生型的产量对照多在初代遗传材料之间或单一生态环境条件下进行,低植酸性状的同源性还有待进一步提高。因此,低植酸突变体是否能表现出与野生型对照相似的农艺表现还有待进一步观察。事实上,Raboy等[77]经多代筛选,并进行了多年、多点的大田试验,发现初代具有产量优势的低植酸突变体,纯合后的农艺性状再次表现出一定的劣变特征,如花期延迟、萌发率降低等。因此,低植酸作物品质和产量的同步提升在未来品质育种与改良中依然是个挑战。
5 低植酸水稻的农艺性状劣变和生态适应性降低的生理原因
明确低植酸作物产量降低的生理机制及其与植酸合成积累的生理联系,是协调与优化低植酸作物品质与产量潜力的基础。低植酸作物农艺性状变劣、结实/灌浆障碍、产量水平下降可能与以下四方面有关:
1)肌醇及磷酸肌醇是植酸代谢的主要产物,在胞间信号转导以及磷酸肌醇信号转导[如1,4,5-3-磷酸肌醇作为胞内第二信使通过诱导Ca2+离子释放激活信号级联、肌醇1-3,4-5-6-5磷酸(IP5)是COI1-JAZ信号代谢路径的配体][80]、激素调控/代谢与平衡(肌醇与生长素受体结合参与逆境响应、葡萄糖醛酸代谢)[50]、钙和糖信号生理[81, 82]、糖转运、碳水化合物代谢(寡聚糖、蔗糖和淀粉合成)、生长发育调节、生物和非生物胁迫[83, 84]、渗透调节和保护、磷贮存和平衡[85, 86]、萌发、光合形态建成[87]、质膜和细胞壁合成、膜/囊泡转运、能量调控、基因调控、染色体修饰和重组[88]、DNA 修复、mRNA输送/转运、细胞程序性死亡、环醇合成、多元化合物贮存以及病原体防御中均发挥着重要的生理调控作用[50, 74, 89-92]。以肌醇为例,肌醇会通过抗坏血酸、磷脂酰肌醇等途径参与植物抗逆生理。研究表明肌醇甲基化后形成的甲酯、D-芒柄醇或D-松醇等代谢物质,可通过保护细胞结构和维持膨压提高植物对干旱胁迫的耐受能力[93, 94]。过表达基因会引起肌醇含量同步增加,作物耐盐性也同步增强[94, 95]。这些代谢过程涉及籽粒从萌发、出苗、营养生长以及灌浆等发育过程的各个方面,植酸代谢路径中相关基因的突变,不仅会直接降低籽粒植酸含量,还会影响作物生长发育的其他生理过程,降低作物的逆境适应性,并最终体现为农艺性状的变化[50]。除植酸代谢的中间产物外,调控植酸代谢相关的酶基因(如等)也参与了多个生理代谢过程,如除是植酸合成的限速酶外,对逆境响应也较为敏感,高温、冷害、干旱、脱水和强光等逆境胁迫均会诱导基因的显著上调表达[8, 96-100]。Das-Chatterjee等[101]将基因转入烟草,转基因后代的耐盐性得以显著提升。此外,植酸合成代谢途径的中间产物还可能通过调控磷酸肌醇信号传导和ATP能量转换等生理代谢过程对低植酸作物的生长发育和结实产生影响。
2)低植酸作物在籽粒植酸含量降低的同时,无机磷含量显著增加。由于常规水稻品种的籽粒植酸主要隔离于液泡中,其相对独立的细胞区位保证了胞内的离子平衡,可能并不会影响籽粒中包括淀粉、蛋白以及脂类在内的其他内含物的合成和积累。但当植酸含量显著降低后,游离出的过量无机磷不仅会打破细胞内的离子平衡,同时还会通过抑制ADPG焦磷酸化酶和淀粉磷酸化酶的活性影响淀粉的合成及积累,从而影响发育籽粒的正常灌浆和充实[102]。也有研究认为过量无机磷的胞外泄漏可能是低植酸作物易感病和籽粒活力降低的原因之一[1];
3)植酸代谢路径较为复杂,涉及多条代谢路径、多种酶/基因、多底物、多器官共同协调参与调控。除籽粒高表达的植酸代谢相关基因(如、、)外,营养器官(、、、、)及花器()中植酸(或磷)代谢受阻也会影响光合同化物的合成、运输与分配[10]。
6 籽粒植酸合成的环境调控效应
作物籽粒植酸积累除与作物类型和品种基因型的高遗传力有关外,还显著受生态因素和栽培环境的影响。土壤类型/结构、种植区域、肥水管理(磷肥、氮肥和锌肥运筹)、气象因素、种植年份或播期、温室效应等,均会显著影响作物籽粒的植酸积累[103-115]。Liu等[116]通过多点生态区域种植,对24个常规粳稻品种的籽粒植酸进行分析,结果表明品种基因型、环境条件以及基因型和环境的互作效应(G×E)均会显著影响水稻籽粒的植酸含量,其中以环境(种植地点)效应最为显著。Magallane-Lopez等[117]对小麦的研究结果表明,环境效应对籽粒中植酸和铁的生物有效性的影响最为显著,且对铁的生物有效性的影响(57.8%)高于对植酸(46%)的影响。Su等[71]和Hummel等[118]在水稻和大豆的研究结果表明,灌浆期高温或全球气候变化所导致的干旱等逆境胁迫会显著增加作物籽粒中的植酸含量。由于植酸是磷的最主要贮存形式,多数研究结果均表明土壤磷水平会对作物籽粒中的植酸积累产生显著正调控的影响。这可能是不同种植区域、土壤类型条件下作物籽粒植酸含量显著变化的原因之一。Gibson等[119]和Thavarajah等[110]以豆科作物为研究对象,发现高温胁迫下籽粒植酸含量和总磷积累量均呈显著增加的趋势。同一大田中同一作物品种在不同播期和年际效应所引起的籽粒植酸含量变异,可能就与作物灌浆结实期间的温度变化有关。此外,肥水管理也会显著影响作物籽粒的植酸积累,合理氮肥运筹可同步实现小麦籽粒植酸降低以及蛋白质含量增加[120],且不同氮源形态(氯化铵、硫酸铵和尿素)对籽粒植酸含量的影响一致[121]。灌浆初期叶片喷施锌肥可抑制植酸积累、提高籽粒锌的生物有效性[121, 122]。适宜的水肥调控也会使水稻籽粒中植酸含量有所降低[123]。相反,增施磷肥可引起谷物籽粒中植酸含量显著上升[124-128]。最近的研究进一步表明,外源无机磷供应虽然能在一定程度上提高籽粒植酸含量和积累量,但过量磷水平下,水稻籽粒的植酸积累量却表现出显著降低的趋势[124]。明确低植酸突变水稻的环境调控效应,对进一步提高低植酸作物产量的同时,保持低植酸的优质种性发挥具有重要的参考价值。
7 研究展望
关于植物籽粒植酸合成的大致路径现已基本清楚,相关关键酶及其编码基因的功能、克隆和定位也已在多种作物中得到验证,如、、、、、、、和等。然而在脂独立途径中,低价磷酸肌醇之间(如IP1和IP2等)、低价磷酸肌醇向高价磷酸肌醇转化的过程,以及脂依赖途径中磷脂酰肌醇向1,3,4,5,6-5磷酸肌醇转化的过程,究竟还受到哪些其他关键调控位点或酶(基因)催化的影响,目前的研究还较少。未来利用数量性状基因座(quantitative trait locus, QTL)、精细定位、基因组测序、关联分析、关键调控基因克隆和定位以及CRISPR-Cas9基因编辑等方法,并结合ICS-HPLC对不同价位磷酸肌醇衍生物及其同分异构体(InsP1-InsP5)变化进行分析,明确低价肌醇磷酸的磷酸化过程,有利于进一步明确植酸的生物合成过程、遗传机理以及两条代谢路径的功能特点。同时,明确相关转录因子在植酸合成以及调控中的作用,可继续丰富低植酸的突变类型,为筛选具有产量优势的低植酸基因型提供依据。此外,筛选低植酸和低总磷双性状水稻基因型,在改善作物品质的同时,还能进一步节约磷肥资源。考虑到水稻籽粒植酸积累的环境调控效应以及低植酸水稻突变体在逆境生态系统中的不稳定性,如何利用栽培调优(如肥料运筹、环境调控、株型改造)或外源调控等措施,确保低植酸的优质种性发挥的同时,进一步提升其产量潜力,也是栽培学研究的重要方向。这些研究将会对以锌、铁生物强化为目标的水稻品质育种提供借鉴和补充。
[1] Raboy V. Seeds for a better future: 'Low phytate', grains help to overcome malnutrition and reduce pollution., 2001, 6(10): 458-462.
[2] Borg S, Brinch-Pedersen H, Tauris B, Holm P B. Iron transport, deposition and bioavailability in the wheat and barley grain., 2009, 325(1-2): 15-24.
[3] Rosa-Sibakov N, Kaisa P, Valérie M. How does wheat grain, bran and aleurone structure impact their nutritional and technological properties?, 2015, 41(2): 118-134.
[4] 张倩雯, 丁广大, 王效华, Liu L, King J G, 徐芳森, 石磊. 植物种子植酸研究进展. 植物科学学报, 2016, 34(5): 814-820.
Zhang Q W, Ding G D, Wang X H, Liu L, King J G, Xu F S, Shi L. Research progress on plant seed phytate., 2016, 34(5): 814-820. (in Chinese with English abstract)
[5] Brinch-Pedersen H, Sorensen L D, Holm P B. Engineering crop plants: Getting a handle on phosphate., 2002, 7(3): 118-125.
[6] Welch R M, Graham R D. Breeding for micronutrients in staple food crops from a human nutrition perspective., 2004, 55(396): 353-364.
[7] Boncompagni E, Gregorio O, Eleonora C, Prakash I G, Stefania G, Theophilus T K Z, Maria G D, Erik N, Francesca S. Antinutritional factors in pearl millet grains: Phytate and goitrogens content variability and molecular characterization of genes involved in their pathways., 2018, 13(6): e0198394.
[8] Loewus F A, Murthy P.-inositol metabolism in plants., 2000, 150(1): 1-19.
[9] Raboy V. Low-phytic-acid grains., 2000, 21(4): 423-427.
[10] Suzuki M, Tanaka K, Kuwano M, Yoshida K T. Expression pattern of inositol phosphate-related enzymes in rice (L.): Implications for the phytic acid biosynthetic pathway., 2007, 405(1-2): 55-64.
[11] Coelho C M, Tsai S M, Vitorello V A. Dynamics of inositol phosphate pools (tris-, tetrakis- and pentakisphosphate) in relation to the rate of phytate synthesis during seed development in common bean (L.)., 2005, 162(1): 1-9.
[12] Shi J, Wang H, Wu Y, Hazebroek J, Meeley R B, Ertl D S. The maize low-phytic acid mutantis caused by mutation in an inositol phosphate kinase gene., 2003, 131(2): 507-515.
[13] Cui M, Liang D, Ma F W. Molecular cloning and characterization of a cDNA encoding kiwifruit L--inositol-1-phosphate synthase, a key gene of inositol formation., 2013, 40(1): 697-705.
[14] Perera I, Saman S, Naoki H. Manipulating the phytic acid content of rice grain toward improving micronutrient bioavailability., 2018, 11(1): 4.
[15] Coelho C M, Benedito V A, Figueira A, Vitorello V A, Azevedo R A. Variation in the enzyme activity and gene expression of-inositol-3-phosphate synthase and phytate accumulation during seed development in common bean (L.)., 2007, 190(3): 24-39.
[16] Larson S R, Rutger J N, Young K A, Raboy V. Isolation and genetic mapping of a non-lethal rice (L.) low phytic acid 1 mutation., 2000, 40(5): 1397-1405.
[17] Wilcox J R, Premachandra G S, Young K A, Raboy V. Isolation of high seed inorganic P, low-phytate soybean mutants., 2000, 40(6): 1601-1605.
[18] Yuan F J, Zhao H J, Ren X L, Zhu S L, Fu X J, Shu Q Y. Generation and characterization of two novel low phytate mutations in soybean (L. Merr.)., 2007, 115(7): 945-957.
[19] Hambidge K M, Krebs N F, Westcott J L, Sian L, Miller L V, Peterson K L, Raboy V. Absorption of calcium from tortilla meals prepared from low-phytate maize., 2005, 82(1): 84-87.
[20] Poulsen H D, Johansen K S, Hatzack F, Boisen S, Rasmussen S R K. Nutritional value of low-phytate barley evaluated in rats., 2001, 51(1): 53-58.
[21] Petry N, Egli I, Campion B, Nielsen E, Hurrell R. Genetic reduction of phytate in common bean (L.) seeds increases iron absorption in young women., 2013, 143(8): 1219-1224.
[22] Hambidge K M, Huffer J W, Raboy V, Grunwald G K, Westcott J L, Sian L, Miller L V, Dorsch J A, Krebs N F. Zinc absorption from low-phytate hybrids of maize and their wild-type isohybrids., 2004, 79(6): 1053-1059.
[23] Kuwano M, Mimura T, Takaiwa F, Yoshida K T. Generation of stable 'low phytic acid' transgenic rice through antisense repression of the 1D--inositol 3-phosphate synthase gene () using the 18-kDa oleosin promoter., 2009, 7(1): 96-105.
[24] Feng X, Yoshida K T. Molecular approaches for producing low-phytic-acid grains in rice., 2004, 21(3): 183-189.
[25] Li W X, Huang J Z, Zhao H J, Tan Y Y, Cui H R, Poirier Y, Shu Q Y. Production of low phytic acid rice by hairpin RNA- and artificial microRNA-mediated silencing ofin seeds., 2014, 119(1): 15-25.
[26] Kuwano M, Ohyama A, Tanaka Y, Mimura T, Takaiwa F, Yoshida K T. Molecular breeding for transgenic rice with low-phytic-acid phenotype through manipulating-inositol 3-phosphate synthase gene., 2006, 18(3): 263-272.
[27] Ali N, Paul S, Gayen D, Sarkar S N, Datta S K, Datta K. RNAi mediated down regulation of-inositol-3- phosphate synthase to generate low phytate rice., 2013, 6(1): 1-12.
[28] Kim S I, Andaya C B, Newman J W, Goyal S S, Tai T H. Isolation and characterization of a low phytic acid rice mutant reveals a mutation in the rice orthologue of maize., 2008, 117(8): 1291-1301.
[29] Kim S I, Andaya C B, Goyal S S, Tai T H. The ricegene encodes a novel protein involved in phytic acid metabolism., 2008, 117(5): 769-779.
[30] Zhao H J, Liu Q L, Ren X L, Wu D X, Shu Q Y. Gene identification and allele-specific marker development for two allelic low phytic acid mutations in rice (L.)., 2008, 22(4): 603-612.
[31] Kim S, Tai T H. Identification of novel rice low phytic acid mutations via TILLING by sequencing., 2014, 34(4): 1717-1729.
[32] Ali N, Paul S, Gayen D, Sarkar S N, Datta K, Datta S K. Development of low phytate rice by RNAi mediated seed-specific silencing of inositol 1,3,4,5,6-pentakis phosphate 2-kinase gene ()., 2013, 8(7): e68161.
[33] Nagy R, Grob H, Weder B, Green P, Klein M, Frelet-Barrand A, Schjoerring J K, Brearley C, Martinoia E. The Arabidopsis ATP-binding cassette protein ATMRP5/ATABCC5 is a high-affinity inositol hexakisphosphate transporter involved in guard cell signaling and phytate storage., 2009: jbc. M109. 030247.
[34] Xu X H, Zhao H J, Liu Q L, Frank T, Engel K H, An G H, Shu Q Y. Mutations of the multi-drug resistance-associated protein ABC transporter gene 5 result in reduction of phytic acid in rice seeds., 2009, 119(1): 75-83.
[35] Wanke D, Üner Kolukisaoglu H. An update on the ABCC transporter family in plants: Many genes, many proteins, but how Many functions?, 2010, 12: 15-25.
[36] Maroof M A, Glover N M, Biyashev R M, Buss G R, Grabau E A. Genetic basis of the low-phytate trait in the soybean line CX1834., 2009, 49(1): 69-76.
[37] Panzeri D, Cassani E, Doria E, Tagliabue G, Fort L, Campion B, Bollini R, Brearley C A, Pilu R, Nielsen E, Sparvoli F. A defective ABC transporter of the MRP family, responsible for the beanmutation, affects the regulation of the phytic acid pathway, reduces seed-inositol and alters ABA sensitivity., 2011, 191(1): 70-83.
[38] Cerino B F, Amelotti M, Cassani E, Pilu R. Study of low phytic acid1-7 (), a new ZmMRP4 mutation in maize., 2012, 103(4): 598-605.
[39] Shi J, Wang H, Hazebroek J, Harp T. The maize low-phytic acid 3 encodes a-inositol kinase that plays a role in phytic acid biosynthesis in developing seeds., 2005, 42(5): 708-719.
[40] Pilu R, Panzeri D, Cassani E, Cerino B F, Landoni M, Nielsen E. A paramutation phenomenon is involved in the genetics of maize low phytic acid 1-241 () trait., 2009, 102(3): 236-245.
[41] Bhati K K, Alok A, Kumar A, Kaur J, Tiwari S, Pandey A K, Notes A. Silencing of ABCC13 transporter in wheat reveals its involvement in grain development, phytic acid accumulation and lateral root formation., 2016, 67(14): 4379-4389.
[42] Bhati K K, Aggarwal S, Sharma S, Mantri S, Singh S P, Bhalla S, Kaur J, Tiwari S, Roy J K, Tuli R, Pandey A K. Differential expression of structural genes for the late phase of phytic acid biosynthesis in developing seeds of wheat (L.)., 2014, 224: 74-85.
[43] Mitsuhashi N, Kondo M, Nakaune S, Ohnishi M, Hayashi M, Hara-Nishimura I, Richardson A, Fukaki H, Nishimura M, Mimura T. Localization of-inositol-1- phosphate synthase to the endosperm in developing seeds of., 2008, 59(11): 3069-3076.
[44] Chiera J M, Grabau E A. Localization of-inositol phosphate synthase (-) during the early stages of soybean seed development., 2007, 58: 2261-2268.
[45] Li W X, Zhao H J, Pang W Q, Cui H R, Poirier Y, Shu Q Y. Seed-specific silencing ofreduces seed phytic acid and weight in rice., 2014, 23(4): 585–599.
[46] Sparvoli F, Cominelli E. Seed biofortification and phytic acid reduction: a conflict of interest for the plant?, 2015, 4(4): 728-755.
[47] Borghi L, Kang J, Ko D, Lee Y, Marinoia E. The role of ABCG-type ABC transporters in phytohormone transport., 2015, 43(5): 924-930.
[48] Cominelli E, Confalonieri M, Carlessi M, Cortinovisa G, Daminati M G, Porch T G, Losa A, Sparvoli F. Phytic acid transport in Phaseolus vulgaris: A new low phytic acid mutant in the PvMRP1 gene and study of the PvMRPs promoters in two different plant systems., 2018, 270: 1-12.
[49] Israel D W, Taliercio E, Kwanyuen P, Burton J W, Dean L. Inositol metabolism in developing seed of low and normal phytic acid soybean lines., 2011, 51: 282-289.
[50] Redekar N, Pilot G, Raboy V, Song L, Saghai-Maroof M A. Inference of transcription regulatory network in low phytic acid soybean seeds., 2017, 8: 2029.
[51] Redekar N R, Biyashev R M, Jensen R V, Helm R, Grabau E A, Maroof M A. Genome-wide transcriptome analyses of developing seeds from low and normal phytic acid soybean lines., 2015, 16(1): 1074.
[52] Liu K S, Xu X H, Ren X L, Fu H W, Wu D X, Shu Q Y. Generation and characterization of low phytic acid germplasm in rice (L.)., 2007, 114(5): 803-814.
[53] Zhao H J, Liu Q L, Fu H W, Xu X H, Wu D X, Shu Q Y. Effect of non-lethal low phytic acid mutations on grain yield and seed viability in rice., 2008, 108(3): 206-211.
[54] Zhao H, Frank T, Tan Y. Disruption ofreduces phytate and phosphorus concentrations and alters the metabolite profile in rice grains., 2016, 211(3): 926-939.
[55] Ye H, Zhang X Q, Broughton S, Westcott S, Wu D, Lance R, Li C. A nonsense mutation in a putative sulphate transporter gene results in low phytic acid in barley., 2011, 11(1): 103-110.
[56] Takahashi H, Buchner P, Yoshimoto N, Hawkesford M J, Shiu S H. Evolutionary relationships and functional diversity of plant sulfate transporters., 2012, 2: 119.
[57] Zhang S, Yang W, Zhao Q, Zhou X, Jiang L, Ma S, Liu X, Li Y, Zhang C, Fan Y, Chen R. Analysis of weighted co-regulatory networks in maize provides insights into new genes and regulatory mechanisms related to inositol phosphate metabolism., 2016, 17(1): 129.
[58] Frank T, Norenberg S, Engel K H. Metabolite profiling of two novel low phytic acid () soybean mutants., 2009, 57(14): 6408-6416.
[59] Emami K, Morris N J, Cockell S J, Golebiowska G, Shu Q Y, Gatehouse A M R. Changes in protein expression profiles between a low phytic acid rice (L. ssp.) line and its parental line: a proteomic and bioinformatic approach., 2010, 58(11): 6912-6922.
[60] Rutger J N, Raboy V, Moldenhauer K A K, Bryant R J, Lee F N, Gibbons J W. Registration of KBNT1-1 low phytic acid germplasm of rice., 2004, 44(1): 363-364.
[61] Lott J N A, Liu J C, Ockenden I, Truax M. Phytic acid-phosphorus and other nutritionally important mineral nutrient elements in grains of wild-type and low phytic acid () rice., 2004, 14(2): 109-116.
[62] Andaya C B, Tai T H. Fine mapping of the rice low phytic acid (Lpa1) locus., 2005, 111(3): 489-495.
[63] Yatou O, Aoki H, Aii J, Tanaka H. Selection of novel non-lethal, low phytic acid mutants and evaluation of their agronomic traits/mineral compositions in rice ()., 2018, 52(1): 39-47.
[64] Hitz W D, Carlson T J, Kerr P S, Sebastian S A. Biochemical and molecular characterization of a mutation that confers a decreased raffinosaccharide and phytic acid phenotype on soybean seeds., 2002, 128(2): 650-660.
[65] Edwards J D, Jackson A K, McClung A M. Genetic architecture of grain chalk in rice and interactions with a low phytic acid locus., 2017, 205: 116-123.
[66] Zhou L, Ye Y, Zhao Q, Du X, Zakari S A, Su D, Pan G, Cheng F M. Suppression of ROS generation mediated by higher InsP3 level is critical for the delay of seed germination inrice., 2018, 85(3): 411-424.
[67] Oltmans S E, Fehr W R, Welke G A, Raboy V, Peterson K L. Agronomic and seed traits of soybean lines with low-phytate phosphorus., 2005, 45(2): 593-598.
[68] Bregitzer P, Raboy V. Effects of four independent low-phytate mutations in barley on (L.) seed phosphorus characteristics and malting quality., 2006, 83: 460-464.
[69] Meis S J, Fehr W R, Schnebly S R. Seed source effect on field emergence of soybean lines with reduced phytate and raffinose saccharides., 2003, 43(4): 1336-1339.
[70] Hulke B S, Fehr W R, Welke G A. Agronomic and seed characteristics of soybean with reduced phytate and palmitate., 2004, 44: 2027-2031.
[71] Su D, Lei B T, Li Z W, Cao Z Z, Huang F D, Pan G, Ding Y, Cheng F M. Influence of high temperature during filling period on grain phytic acid and its relation to spikelet sterility and grain weight in non-lethal low phytic acid mutations in rice., 2014, 60(2): 331-338.
[72] Pilu R, Landoni M, Cassani E, Doria E, Nielsen E. The maizemutation causes a remarkable variability of expression and some pleiotropic effects., 2005, 45: 2096-2105.
[73] Raboy V, Gerbasi P F, Young K A, Stoneberg S D, Pickett S G, Bauman A T, Murthy P, Sheridan W F, Ertl D S. Origin and seed phenotype of maize low phytic acid 1-1 and low phytic acid 2-1., 2000, 124(1): 355-368.
[74] David E B, Edward J S, Mary J G, Victor R, Jianming F. A low phytic acid barley mutation alters seed gene expression., 2007, 47: S149.
[75] Ertl D S, Young K A, Raboy V. Plant genetic approaches to phosphorus management in agricultural production.1998, 27(2): 299-304.
[76] Nunes A C, Vianna G R, Cuneo F, Amayafarf A, De-Capdeville G, Rech L, Arag A. RNAi-mediated silencing of the-inositol-1-phosphate synthase gene () in transgenic soybean inhibited seed development and reduced phytate content., 2006, 224(1): 125-132.
[77] Raboy V, Peterson K. A substantial fraction of barley (L.) low phytic acid mutations have little or no effect on yield across diverse production environments., 2015, 4 (2): 225-239.
[78] Naidoo R, Tongoona P, Derera J, Laing M D, Watson G M F. Combining ability of low phytic acid (1-1) and quality protein maize (QPM) lines for seed germination and vigour under stress and non-stress conditions., 2012, 185(3): 529-541.
[79] Ockenden I, Dorsch J A, Reid M M, Lin L, Grant L K, Raboy V, Lott J N A. Characterization of the storage of phosphorus, inositol phosphate and cations in grain tissues of four barley (L.) low phytic acid genotypes., 2004, 167(5): 1131-1142.
[80] Sheard L B, Tan X, Mao H, Withers J, Ben-Nissan G, Hinds T R, Kobayashi Y, Hsu F F, Sharon M. Jasmonate perception by inositol-phosphate-potentiated COI1-JAZ co-receptor., 2010, 468(7322): 400-405.
[81] Ananieva E, Gillaspy G. Switches in nutrient and inositol signaling., 2009, 4 (4): 304-306.
[82] Lemtiri-Chlieh F, MacRobbie E, Webb A, Manison N F, Brownlee C, Skeppe J N, Chen J, Prestwich G D, Brearley C A. Inositol hexakisphosphate mobilizes an endomembrane store of calcium in guard cells., 2003, 100(17): 10091-10095.
[83] Murphy A M, Otto B, Brearley C A, Carr J P, Hanke D E. A role for inositol hexakisphosphate in the maintenance of basal resistance to plant pathogens., 2008, 56(4): 638-652.
[84] Munnik T, Vermeer J E. Osmotic stress-induced phosphoinositide and inositol phosphate signalling in plants., 2010, 33(4): 655-669.
[85] Stevenson-Paulik J, Bastidas R J, Chiou S T, Frye R A, York J D. Generation of phytate-free seeds inthrough disruption of inositol polyphosphate kinases., 2005, 102(35): 12612-12617.
[86] Kuo H, Chang T, Chiang S.inositol pentakisphosphate 2-kinase, AtIPK1, is required for growth and modulates phosphate homeostasis at the transcriptional level., 2014, 80(3): 503-515.
[87] Qin Z, Chen Q, Tong Z. Theinositol 1,3,4-trisphosphate 5/6 kinase, AtItpk-1, is involved in plant photomorphogenesis under red light conditions, possibly via interaction with COP9 signalosome., 2005, 43(10-11): 947-954.
[88] Latrasse D, Jegu T, Meng P H, Mazubert C, Hudik E, Delarue M, Charon C, Crespi M, Hirt H, Raynaud C, Bergounioux C, Benhamed M. Dual function of MIPS1 as a metabolic enzyme and transcriptional regulator., 2013, 41(5): 2907-2917.
[89] Loewus F A, Loewus M W. Myo-inositol: its biosynthesis and metabolism., 1983, 34(1): 137-161.
[90] Gumber S C, Loewus M W, Loewus F A. Further studies on-Inositol-1-phosphatase from the pollen of Lilium longiflorum Thunb., 1984, 76(1): 40-44.
[91] Irvine R F, Schell M J. Back in the water: The return of the inositol phosphates., 2001, 2(5): 327-338.
[92] Hui Q, Yang R, Shen C, Zhou Y, Gu Z. Mechanism of calcium lactate facilitating phytic acid degradation in soybean during germination., 2016, 64(27): 5564-5573.
[93] Nelson D E, Rammesmayer G, Bohnert H J. Regulation of cell-specific inositol metabolism and transport in plant salinity tolerance., 1998, 10(5): 753-764.
[94] Patra B, Ray S, Richter A, Majumder A L. Enhanced salt tolerance of transgenic tobacco plants by co-expression ofandis accompanied by increased level of-inositol and methylated inositol., 2010, 245(1-4): 143-152.
[95] Majee M, Maitra S, Dastidar K G, Pattnaik S, Chatterjee A, Hait N C, Das K P, Majumder A L. A novel salt-tolerant L-myo-inositol-1-phosphate synthase from Porteresia coarctata (Roxb.) Tateoka, a halophytic wild rice molecular cloning, bacterial overexpression, characterization, and functional introgression into tobacco-conferring salt tolerance phenotype., 2004, 279(27): 28539-28552.
[96] Ishitani M, Majumder A L, Bornhouser A, Michalowski C B, Jensen R G, Bohnert H J. Coordinate transcriptional induction of-inositol metabolism during environ mental stress., 1996, 9(4): 537-548.
[97] Boominathan P, Shukla R, Kumar A, Manna D, Negi D, Verma P K, Chattopadhyay D. Long term transcript accumulation during the development of dehydration adaptation in Cicer arietinum., 2004, 135(3): 1608-1620.
[98] Abreu E, Aragao F. Isolation and characterization of a-inositol-1-phosphate synthase gene from yellow passion fruit (Passiflora edulis f. flavicarpa) expressed during seed development and environmental stress., 2007, 99(2): 285-292.
[99] Iwai T, Takahashi M, Oda K, Terada Y, Yoshida K T. Dynamic changes in the distribution of minerals in relation to phytic acid accumulation during rice seed development., 2012, 160(4): 2007-2014.
[100]Kaur H, Shukla R K, Yadav G, Chattopadhyay D, Majee M. Two divergent genes encoding L-inositol 1-phosphate synthase1 () and 2 () are differentially expressed in chickpea., 2008, 31(11): 1701-1716.
[101]Das-Chatterjee A, Goswami L, Maitra S, Dastidar K G, Ray S, Majumder A L. Introgression of a novel salt-tolerant L--inositol 1-phosphate synthase from Porteresia coarctata (Roxb.) Tateoka () confers salt tolerance to evolutionary diverse organisms., 2006, 580(16): 3980-3988.
[102]Plaxton W C, Preiss J. Purification and properties of nonproteolytic degraded ADPglucose pyrophosphorylase from maize endosperm., 1987, 83(1): 105-112.
[103]Bilgrami S S, Houshmand S, Kadivar M, Fakheri B, Zandi P, Shariati V, Razavi K, Tavakol E, Mahdinezhad N, Sabouri S J, Kumar B S, Możdżeń K. Phytic acid, iron and zinc content in wheat ploidy levels and amphiploids: The impact of genotype and planting seasons., 2018, 64(3): 331-346.
[104]Miller G A, Youngs V L. Environmental and cultivar effects on oat phytic acid concentration., 1980, 57: 189-191.
[105]Batten G D, Lott J. The influence of phosphorus nutrition on the appearance and composition of globoid crystals in wheat aleurone cells., 1986, 63(1): 14-18.
[106]Feil B, Fossati D. Phytic acid in triticale grains as affected by cultivar and environment., 1997, 37(3): 916-921.
[107]Jaksomsak P, Tuiwong P, Rerkasem B, Guild G, Palmer L, Stangoulis J, Prom-u-thai C T. The impact of foliar applied zinc fertilizer on zinc and phytate accumulation in dorsal and ventral grain sections of four Thai rice varieties with different grain zinc.,, 2018 79: 6-12.
[108]Dost K, Tokul O. Determination of phytic acid in wheat and wheat products by reverse phase high performance liquid chromatography., 2006, 558(1-2): 22-27.
[109]Dai F, Wang J, Zhang S, Xu Z, Zhang G. Genotypic and environmental variation in phytic acid content and its relation to protein content and malt quality in barley., 2007, 105(2): 606-611.
[110]Thavarajah D, Thavarajah P, See C T, Vandenberg A. Phytic acid and Fe and Zn concentration in lentil (L.) seeds is influenced by temperature during seed filling period., 2010, 122(1): 254-259.
[111]Fernando N, Panozzo J, Tausz M, Norton R M, Fitzgerald G J, Myers S, Nicolas M E, Seneweera S. Intra-specific variation of wheat grain quality in response to elevated [CO2] at two sowing times under rain-fed and irrigation treatments., 2014, 59(2): 137-144.
[112]Dhole V J, Reddy K S. Genetic variation for phytic acid content in mungbean (L. Wilczek)., 2015, 3(2): 157-162.
[113]赵宁春, 张小明, 叶胜海, 程方民. 不同栽培方式和施氮量对稻米营养品质及植酸积累的影响. 浙江农业学报, 2009, 21(3): 259-263.
Zhao N C, Zhang X M, Ye S H, Cheng F M. Effects of different cultivation methods and nitrogen application on grain phytic acid contents and nutritional quality for japonica rice., 2009, 21(3): 259-263. (in Chinese with English abstract)
[114]赵宁春, 张其芳, 程方民, 周伟军. 氮、磷、锌营养对水稻籽粒植酸含量的影响及与几种矿质元素间的相关性. 中国水稻科学, 2007, 21(2): 185-190.
Zhao N C, Zhang Q F, Cheng F M. Phosphorus and zinc supply levels on grain phytic acid content and its correlation with several mineral nutrients in rice grain., 2007, 21(2): 185-190. (in Chinese with English abstract)
[115]Steiner T, Mosenthin R, Zimmermann B, Greiner R, Roth S. Distribution of phytase activity, total phosphorus and phytate phosphorus in legume seeds, cereals and cereal by-products as influenced by harvest year and cultivar., 2007, 133(3-4): 320-334.
[116]Liu Z H, Cheng F M, Zhang G P. Grain phytic acid content in japonica rice as affected by cultivar and environment and its relation to protein content., 2005, 89(1): 49-52.
[117]Magallanes-López A M, Hernandez-Espinosa N, Velu G, Posadas-Romano G, Ordoñez-Villegas V M G, Crossa J, Ammar K, Guzmán C. Variability in iron, zinc and phytic acid content in a worldwide collection of commercial durum wheat cultivars and the effect of reduced irrigation on these traits., 2017, 237: 499-505.
[118]Hummel M, Hallahan B F, Brychkova G, Ramirez-Villegas J, Guwela V, Chataika B, Curley E, McKeown P C, Morrison L, Talsma E F, Beebe S, Jarvis A, Chirwa R, Spillane C. Reduction in nutritional quality and growing area suitability of common bean under climate change induced drought stress in Africa., 2018, 8: 16187.
[119]Gibson L R, Mullen R E. Mineral concentrations in soybean seed produced under high day and night temperature., 2001, 81(4): 595-600.
[120]Ning H, Liu Z, Wang Q, Lin Z, Chen S, Li G, Wang S, Ding Y. Effect of nitrogen fertilizer application on grain phytic acid and protein concentrations in japonica rice and its variations with genotypes., 2009, 50(1): 49-55.
[121]Khan A M, Hussain S, Rengel Z, Shah M A A. Zinc bioavailability and nitrogen concentration in grains of wheat crop sprayed with zinc sulfate, ammonium sulfate, ammonium chloride, and urea., 2018, 41(15): 1926-1936.
[122]Wang Z M, Liu Q, Pan F, Yuan L X, Yin X B. Effects of increasing rates of zinc fertilization on phytic acid and phytic acid/zinc molar ratio in zinc bio-fortified wheat., 2015, 184: 58-64.
[123]张其方, 刘奎刚, 苏达, 王复标, 程方民. 氮素和水分处理对稻米植酸含量和蛋白组分的影响. 植物营养与肥料学报, 2012, 18(3): 542-550.
Zhang Q F, Liu K G, Su D, Wang F B, Cheng F M. Effects of different nitrogen and water treatments on phytic acid contents and protein components in rice grain., 2012, 18(3): 542-550. (in Chinese with English abstract)
[124]Su D, Zhou L J, Zhao Q, Pan G, Cheng F M. Different phosphorus supplies altered the accumulations and quantitative distributions of phytic acid, zinc, and iron in rice (L.) Grains., 2018, 66(7): 1601-1611.
[125]Raboy V, Dickinson D B. Phytic acid levels in seeds of Glycine max and G. soja as influenced by phosphorus status., 1993, 33(6): 1300-1305.
[126]Buerkert A, Haake C, Ruckwied M, Marschner H. Phosphorus application affects the nutritional quality of millet grain in the Sahel., 1998, 57(2): 223-235.
[127]Zhang W, Liu D Y, Liu Y M, Chen X P, Zou C Q. Overuse of phosphorus fertilizer reduces the grain and flour protein contents and zinc bioavailability of winter wheat (L.)., 2017, 65(8):1473-1482.
[128]Lickfett T, Matthaus B, Velasco L, Mollers C. Seed yield, oil and phytate concentration in the seeds of two oilseed rape cultivars as affected by different phosphorus supply., 1999, 11(3-4): 293-299.
Research Advances on the Low Phytic Acid Rice Breeding and Their Genetic Physiological Regulation and Environmental Adaptability
SU Da1,2, WU Liangquan2, Søren K Rasmussen3, ZHOU Lujian4, CHENG Fangmin4,*
(,,,,,;International Magnesium Institute,,;Department of Plant and Environmental Sciences,,,,;,,,;,:)
Breeding variety with improved quality while maintaining or improving yields is one of the primary objectives in rice breeding. Among which, reducing the anti-nutritional factors, such as grain phytic acid content, is an effective strategy to cope with hidden hunger and increase grain bioavailabilities of zinc and iron. In this paper, we reviewed the biosynthesis of phytic acid and the genetic characteristics of related functional genes, the co-regulatory networks of phytic acid synthesis and other physiological metabolism, breeding of low phytic acid () germplasm resource and their genetic characteristics, agronomic performance and environmental ecological adaptability ofmutants, the possible reasons for their agronomic deterioration and ecological adaptation change, and the environmental regulation of grain phytic acid accumulation. Those contents could provide reference for production ofrice with suitable agronomic cultivation practices.
phytic acid; rice (L.); grain nutrition; genetic regulation; ecological effect
10.16819/j.1001-7216.2019.8083
S482.8; S511.02
A
1001-7216(2019)02-0095-13
2018-07-16;
2018-12-31。
国家自然科学基金资助项目(31571602和31271655); 福建省中青年教师教育科研项目(JAT170156); 国家留学基金委资助项目; 国家重点研发计划资助项目(2017YFD0200200)。
