Linkage of microbiota and osteoporosis: A mini literature review
2019-03-21DavidYatsonskyIIKarenPanVithalShendgeJiayongLiuNabilEbraheim
David Yatsonsky II, Karen Pan, Vithal B Shendge, Jiayong Liu, Nabil A Ebraheim
Abstract The gut microbiota (GM) has become a recent topic of interest in the role of many disease states. Assessing patients with osteoporosis (OP), there is a strong correlation between gut microbe dysregulation and decreased bone density. Gut dysbiosis may lead to inflammation, dysregulation of nutrient and calcium transport across the intestine into circulation and systemic inflammation.Investigation of microbial profile relative to normal gut microbiomes, assessment of inflammatory markers such as interleukin-1 (IL-1), IL-6, and tumor necrosis factor-alpha. Therapies to normalize GM in patients with OP or prevent occurrence of OP to be investigated include: High fiber prebiotic diets to promote growth of normal gut bacteria and short chain fatty acid production, Probiotics to encourage growth of normal gut microbes, and antibiotic treatment followed by fecal matter transplant.
Key words: Osteoporosis; Microbiota; Linkage; Bone density; Gut microbiota
GUT MICROBIOTA DYSREGULATION AND DECREASED BONE DENSITY
There exists a correlation between gut microbiota (GM) dysregulation and decreased bone density. Osteoporosis (OP) is a disease state: The loss of bone density and increased risk of bone fracture[1-3]. The most current United States data shows that 10.3% of adults in United States over 50 years of age had OP based on data collected from 2005-2010, affecting over 10 million adults with 43.9% of adults over 50 experiencing low bone mass[4]. There are two forms of OP: Primary, due to estrogen deficiency and natural aging and secondary, due to disease pathology[3,5-9]. Both forms of OP result from an imbalance of bone remodeling slated toward bone loss[1-3,5-9].
The flora of the human gastrointestinal tract has nearly one hundred trillion microbes[10,11]. For millions of years these organisms have symbiotically evolved to habituate the human gut: Converting much of what mankind consumes into nutrients that can cross the GI tract into circulation[11]. The contents of each person’s gut varies;however, there are four main classes that are consistent in most normal GM:Firmicutes, Bacteroides, Proteobacteria, and Actinobacteria, with Bacteroidetes and Firmicutes comprising over 90% of the phylogenic categories[11-13]. The ratio of Firmicutes to Bacteroidetes GM is indicative of various dysregulation of biological processes[11-13].
Ratio of Firmicutes/Bacteriodetes negatively correlated with bone volume and levels of clostridia and lachnospiraceae. Abundance of actinobacteria phylum members:Bifidobacteriaceae were positively correlated with bone volume[11-13].
Osteomicrobiology: The role of microbiota in bone health may bridge the gap between bone physiology, gastroenterology, immunology, and microbiology. GM may lead to novel targets for OP and as biomarkers for future susceptibilities[7,10].
POSSIBLE MECHANISM OF GUT DYSBIOSIS LEADS TO OP
Microbiota: Normal function, dysbiosis and its role in calcium transport and inflammatory response
GM dysregulation is correlated with increased inflammatory response and bone resorption. Proposed mechanisms include impaired calcium transport, T cell response, systemic inflammation via cytokine activation: Osteoclast activation and bone resorption. Short-term colonization of germ-free mice with GM results in activation of CD4+ T cells, increased pro-inflammatory cytokines in bone and subsequent activation of Osteoclastic bone resorption[14]. Mice with normal GF demonstrated decreased frequency of CD4+ T cells and CD11b+/GR1 osteoclast precursors than those mice with dysbiosis[14]. GM is also involved in nutritional uptake and may thereby regulate overall body growth and bone sizes mediated by altered insulin-like growth factors-1 levels. Mechanism of bone degradation, in murine models, is inflammatory in nature and normalization of GM may lead to prevention or limitation of OP[14].
A diversity analysis of the GM in OP and osteopenia (ON) patients was conducted using 16s ribosomal RNA sequencing. There was an inverse correlation between diversity estimators and bone density. In OP, ON, and control groups the four dominant phyla included Firmicutes, Bacteroidetes, Proteobacteria, and Actinobacteria[11-13].In OP samples Firmicutes were significantly increased and Bacteroidetes significantly decreased but were largest proportion in all groups. In the control group Bacteroides,Faecalibacterium, and Prevotella contributed more than half of the bacterial community,while in ON and OP groups five and 11 genera accounted for 50% of the bacterial community[15]. Blautia, Parabacteroides, and Ruminococcaceae genera differed significantly between OP and control group[15]. Lachnoclostridium and Klebsiella were more abundant in OP and ON than control[15]. The immune-inflammatory axis is hypothesized to be the mediator between GM and Bone metabolism.
Microbiota play an integral role in the transport and uptake of nutrients needed for bone growth and remodeling: Vitamin D[11,16,17]. Vitamin D stimulates intestinal calcium absorption, while 1,25-dihydroxyvitamin D3can regulate calcium homeostasis including calcium channel: TRPV6 and calbindin-D9K, which mediates intracellular calcium diffusion[11,16]. Intestinal resistance of 1,25(OH)2D3and decreased calcium absorption increase in old age and are strongly correlated to gut dysbiosis[11,16,17]. Subsequently, dysbiosis can impact absorption of calcium and vitamin D contributing to OP.
Colonization of Firmicutes and increased biodiversity is associated with increased inflammatory response in gut. Increased inflammation is correlated with osteoclast activation at the site of the bones. Osteoclasts originate from monocytic precursors in the bone marrow (CD4+/Gr1-)[18]. Osteoclasts carry out bone resorption and are regulated by several pathways including calcium and vitamin D, estrogen, and Inflammation[18]. Therefore gut-mediated inflammation, specifically markers: Tumor necrosis factor-α, interleukin-1 (IL-1), and IL-6 can play a role in osteoclast activation and if sustained OP[18].
How normalization of GM can decrease/prevent occurrence of OP and related disorders
Probiotics modify the microbiota composition, intestinal barrier function and immune system resulting in systemic benefits to the host: Bone health (growth, density, and structure) under conditions of dysbiosis, intestinal permeability, and inflammation[5,19,20]. Probiotics may be useful in postmenopausal OP[19]. Probiotic supplementation may prevent OP in healthy individuals and slow its progression in those with the disease based on testing in rodents[5]. In a rat study the probiotic(Bacillus subtilis) supplementation the number of osteoclasts decreased versus vehicle,while number of osteoblasts increased[21]. Additionally, Probiotic Lactobacillus reuteri supplementation mice studies, blocked type-1 diabetes-induced OP and postmenopausal OP: Mimicked by ovariectomized mice[22,23]. Lactobacillis reuteri supplementation suppressed increase in CD4+ T-Lymphocytes, and directly suppressed Osteoclastogenesis in vitro[23]. Probiotics supplemented with the isoflavone contributing red clover extract RCE, which has estrogen receptor affinity, have shown to decrease bone loss in postmenopausal osteopenic women supplemented with calcium, magnesium, and calcitriol[24]. This trial demonstrated potential benefit over hormone replacement therapy, which has increased risk of cancer[24]. Further benefits of Probiotics are related to intestinal permeability and inflammation, which mediates bone loss and OP)[18].
Prebiotics, non-digestible carbohydrate compounds, have also shown to increase calcium absorption in the lower gut of animals and humans and improve bone mineral density and strength in rodent models[24,25]. Non-digestible oligosaccharides have much potential to improve bone health and merit more investigation[25]. Most commonly accepted mechanism is microbial fermentation of prebiotics. This leads to increase in short-chain fatty acids and decrease in pH increasing bioavailability of calcium in the colon[24,26].
Antibiotics can vastly alter the composition of the GM. In a normal GM antibiotics used for other purposes may clear out normal flora and promote growth of resistant strains that may induce inflammation and associated symptoms[26]. Alternatively,antibiotic treatment and subsequent fecal microbiota transplants can be used to correct dysbiosis: Overgrowth of Clostridioides difficile and to promote growth of normal microbiota[27](Table 1).
Short chain fatty acids such as acetate mediate anti-inflammatory conditions systemically[28,29]. These have demonstrated positive effects in preventing bone density loss and preventing OP progression in a rat model by directly feeding them to rats,which not only bolstered anti-inflammatory effects but also enabled normal GM to proliferate[25]. Exercise alters ratio of Firmicutes/Bacteriodetes and may be another venue of investigation into its effects on the GM and potentially as a preventative or therapeutic solution to OP[30,31].
In conclusion, the current literature shows that there may be a linkage between the GM and OP; subsequently, understanding the microbiota and how changes in diet and exercise can modify microbiota can prevent or improve OP disease pathology.
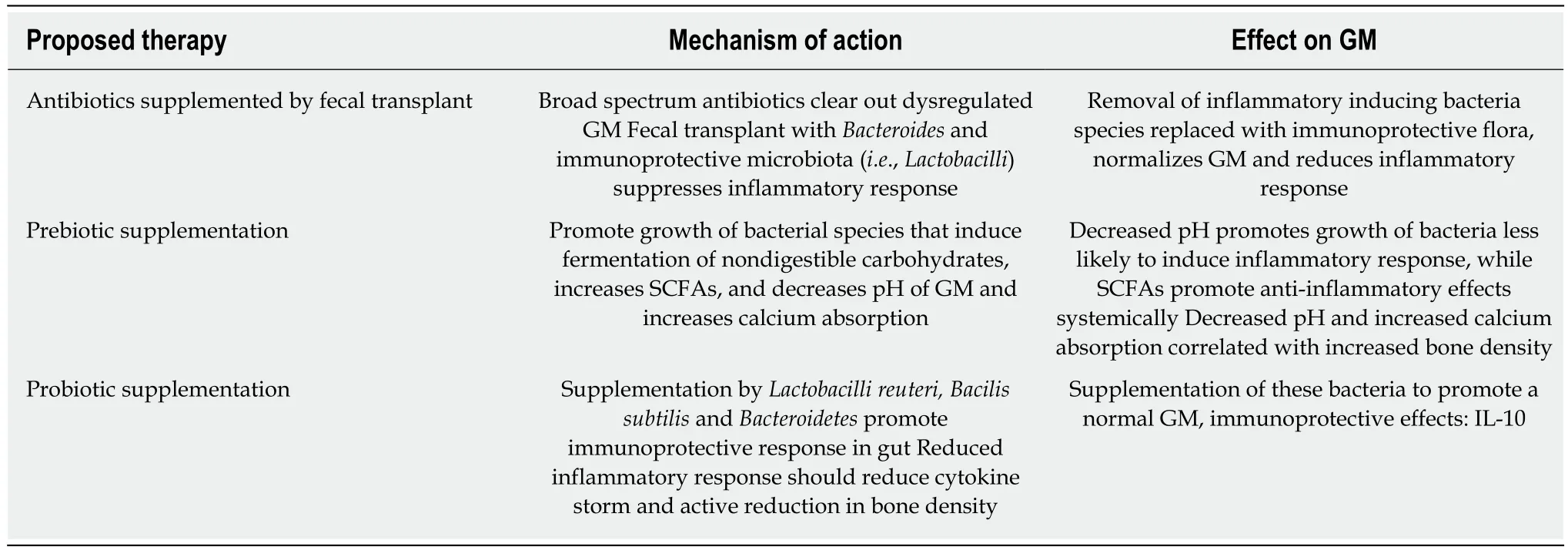
Table 1 Potential therapies of gut microbiota, proposed mechanisms of action, and subsequent immunological and metabolic effects
杂志排行
World Journal of Orthopedics的其它文章
- Changing trends in the mortality rate at 1-year post hip fracture - a systematic review
- Aetiology of Legg-Calvé-Perthes disease: A systematic review
- Comparison of implant related complications amongst patients with opioid use disorder and non-users following total knee arthroplasty
- Short-term differences in anterior knee pain and clinical outcomes between rotating and fixed platform posterior stabilized total knee arthroplasty with a new femoral component design
