Targeted puncture of left branch of intrahepatic portal vein in transjugular intrahepatic portosystemic shunt to reduce hepatic encephalopathy
2019-03-11c
c
Abstract BACKGROUND Transjugular intrahepatic portosystemic shunt (TIPS) is currently used for the treatment of complications of portal hypertension. The incidence of hepatic encephalopathy (HE) remains a problem in TIPS placement. It has been reported that the right branch mainly receives superior mesenteric venous blood while the left branch mainly receives blood from the splenic vein. We hypothesized that targeted puncture of the left portal vein would divert the non-nutritive blood from the splenic vein into the TIPS shunt; therefore, targeted puncture of the left branch of the intrahepatic portal vein during TIPS may reduce the risk of HE.AIM To evaluate the influence of targeted puncture of left branch of portal vein in TIPS on HE.METHODS A retrospective analysis of 1244 patients with portal-hypertension-related complications of refractory ascites or variceal bleeding who underwent TIPS from January 2000 to January 2013 was performed. Patients were divided into group A(targeting left branch of portal vein, n = 937) and group B (targeting right branch of portal vein, n = 307). TIPS-related HE and clinical outcomes were analyzed.RESULTS The symptoms of ascites and variceal bleeding disappeared within a short time.By the endpoint of follow-up, recurrent bleeding and ascites did not differ significantly between groups A and B (P = 0.278, P = 0.561, respectively).Incidence of HE differed significantly between groups A and B at 1 mo (14.94% vs 36.80%, χ2 = 4.839, P = 0.028), 3 mo (12.48% vs 34.20%, χ2 = 5.054, P = 0.025), 6 mo(10.03% vs 32.24%, χ2 = 6.560, P = 0.010), 9 mo (9.17% vs 31.27%, χ2 = 5.357, P =0.021), and 12 mo (8.21% vs 28.01, χ2 = 3.848, P = 0.051). There were no significant differences between groups A and B at 3 years (6.61% vs 7.16%, χ2 = 1.204, P =0.272) and 5 years (5.01% vs 6.18%, χ2 = 0.072, P = 0.562). The total survival rate did not differ between groups A and B (χ2 = 0.226, P = 0.634, log-rank test).CONCLUSION Targeted puncture of the left branch of the intrahepatic portal vein during TIPS may reduce the risk of HE but has no direct influence on prognosis of portalhypertension-related complications.
Key words: Portal hypertension; Transjugular intrahepatic portosystemic shunt; Portal vein branch; Hepatic encephalopathy
INTRODUCTION
Transjugular intrahepatic portosystemic shunt (TIPS) is currently used for the treatment of complications of portal hypertension[1]. The establishment of TIPS is widely accepted as an alternative to surgery in the management of complications from portal hypertension such as variceal bleeding, refractory ascites, Budd-Chiari syndrome, hepatorenal syndrome, hepatic hydrothorax, and even hepatopulmonary syndrome[2]. After TIPS was introduced as an alternative treatment for complications related to portal hypertension, it was progressively recognized as an effective therapeutic option in a growing number of clinical situations[3,4].
With the advances in materials, many experimental and clinical studies[5]have been conducted using covered stent grafts, especially stent grafts covered with polytetrafluoroethylene, to improve the long-term patency of TIPS[6]. The incidence of hepatic encephalopathy (HE) remains a problem in TIPS placement and affects the quality of life and long-term outcomes of patients.
It has been reported[7]that the reflux blood from the splenic and superior mesenteric veins is distributed hydrodynamically in the main portal vein, that is,alongside the trunk on both sides of the wall of the portal vein. However, it is not fully mixed and enters the left and right branches of the portal vein. The right branch mainly receives superior mesenteric venous blood while the left branch mainly receives blood from the splenic vein[8].
We hypothesized (Figure 1) that targeted puncture of the left portal vein would divert the non-nutritive blood from the splenic vein into the TIPS shunt and reduce the incidence of HE. The purpose of this study was to evaluate the influence of targeted puncture of the left or right branches of the portal vein on the incidence of HE in patients who required TIPS placement for portal-hypertension-related complications of ascites or variceal bleeding.
MATERIALS AND METHODS
Patient information
Between January 2000 and January 2013, 1244 patients were referred to us on an intention-to-treat basis and underwent a TIPS procedure. Indications for stent graft shunt were variceal hemorrhage, refractory ascites, or both. The outcomes of recurrent variceal bleeding and/or ascites, mortality, and HE were compared between two groups. The Institutional Review Board approved the study protocol. The patients’medical records and images were reviewed to gather information regarding the underlying etiology, clinical presentation, age, sex, and severity of cirrhosis (Table 1).
Study design
This study was a single-center and retrospective study that compared the influence of targeted puncture of the left and right branches of the portal vein on the incidence of HE in patients who required TIPS placement for portal-hypertension-related complications of ascites or variceal bleeding. The patients were divided into two groups: A (targeting of left branch of portal vein,n= 937) and B (targeting of right branch of portal vein,n= 307) (Figure 2). The outcomes of HE, recurrent variceal bleeding and/or ascites, and mortality were compared and analyzed between the groups. The inclusion criteria were: Recurrent variceal bleeding after a session of variceal sclerotherapy, refractory ascites, or both that required TIPS placement with portal-hypertension-related complications. The exclusion criteria were: Variceal bleeding as an emergency indication, portal vein thrombosis, history of HE, severe right-sided heart failure, severe liver failure (bilirubin > 4 mg/dL), polycystic liver disease, dilated biliary ducts, age > 75 years, Child-Pugh score > 11, Model of End-Stage Liver Disease score > 18, hepatic carcinoma, sepsis, spontaneous bacterial peritonitis, and patients who underwent liver transplantation.
TIPS procedure
TIPS was performed under standard local anesthesia as described previously[9]. The entire length of the intrahepatic tract was covered by the stent graft (BARD, Fluency,Voisins le Bretonneux, France; or Viatorr, W.L. Gore & Associates, Flagstaff, AZ,United States). Hepatic venous pressure gradient and portal vein pressure were measured during the procedure, and the shunts were dilated to their full nominal diameter to reach a target portosystemic gradient (PSG) of < 12 mmHg. Obvious gastroesophageal collateral vessels observed during the TIPS procedure were embolized with coils (Cook Inc., Bloomington, IL, United States; or Interlock Coil,Boston Scientific Corporation, Natikeshi, MA, United States). Subsequent direct portography was performed to evaluate whether the portal venous system was completely patent. After the TIPS procedure, intravenous heparin (4000 U/d; Chase Sun Pharma Co. Ltd., Tianjin, China) was administered for 3 d and oral warfarin was given at 2.5 mg/d (Orion Pharma Co. Ltd., Orionintie, Finland) to achieve an international normalized ratio ≤ 2.0, if prolonged international normalized ratio, oral warfarin was not given.
Follow-up
After TIPS deployment, baseline duplex sonography was performed on the day.Shunt velocities were compared with this baseline result during follow-up. Patients were placed into a routine follow-up protocol identical for each group. They were seen as outpatients 1 mo after the procedure and then 3, 6 and 9 mo and 1, 3 and 5 years, or whenever needed. Each consultation included a clinical examination, blood chemistry, upper abdominal ultrasonography, and assessment of HE. TIPS angiography was performed in patients with recurrent symptoms or suspected shunt dysfunction. TIPS revision was performed when a hemodynamically significant shunt stenosis (> 50%) was present with recurrent variceal bleeding, recurrent or gradually worsening ascites, and PSG ≥ 15 mmHg unless grade III/IV encephalopathy was present (Practice Guideline of the European Association for the Study of the Liver and the American Association for the Study of Liver Diseases). Patients lost to follow-up were censored at the time of the last known imaging of the shunt (duplex ultrasonography or shunt venography).
Statistical analysis
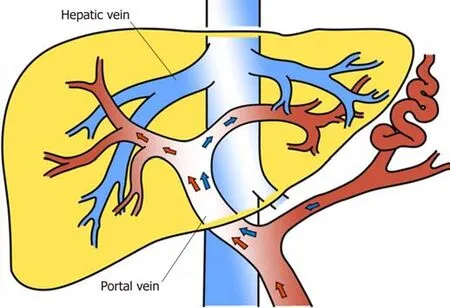
Figure 1 Blood distributed hydrodynamically in the main portal vein. It is reported that the reflux blood from the splenic and superior mesenteric veins is distributed hydrodynamically in the main portal vein, that is, alongside the trunk on both sides of the wall of the portal vein. However, it is not fully mixed and enters the left and right branches of the portal vein. The right branch mainly receives superior mesenteric venous blood, while the left branch mainly receives blood from the splenic vein.
Results were expressed as mean ± standard deviation and compared using the independent samplettest or one-way analysis of variance, and categorical variables were expressed as frequencies and compared usingχ2tests. The differences between the groups were compared using one-way analysis of variance followed by least significant differencettests. Differences were considered significant atP< 0.05. The statistical analyses were performed with SPSS version 20.0 (SPSS, Armonk, NY,United States).
RESULTS
None of the 1244 patients died within 30 d after TIPS, with an early survival rate of 100%. Both TIPS procedures demonstrated similar efficacy in decreasing PSG before and after TIPS placement from 27.08 ± 5.47 to 10.75 ± 3.67 mmHg in group A (P=0.003) and from 26.42 ± 3.53 to 10.96 ± 2.41 mmHg in group B (P= 0.001) (Table 2).
HE occurrence in group A was lower than in group B at 1, 3, 6, 9, and 12 mo, and showed a downward trend (Figure 3). At 3 and 5 years, there was no significant difference in HE occurrence between the two groups. After drug treatment, the symptoms disappeared in patients with covert and grade II HE. In patients with grade III or IV HE, the symptoms disappeared after shunt reduction, but three patients who underwent shunt reduction still had hepatic myelopathy (Table 3).
At 1 mo after TIPS placement, in group A, 140 patients manifested HE; among them, 44 cases were covert, 78 were grade II, 10 were grade III, and eight were grade IV. In group B, 113 patients manifested HE; among them, 27 cases were covert, 56 were grade II, 18 were grade III, and 12 were grade IV. The incidence of HE in group A was lower than in group B (14.94%vs36.80%,χ2= 4.839,P= 0.028). The symptoms in the 48 patients with grade III and IV HE disappeared after shunt reduction in both groups.
At 3 mo after TIPS placement, in group A, 117 patients manifested HE; among them, 34 cases were covert, 59 were grade II, 17 were grade III, and seven were grade IV. In group B, 105 patients manifested HE; among them, 26 cases were covert, 58 were grade II, 11 were grade III, and 10 were grade IV. The incidence of HE in group A was lower than that in group B (12.48%vs34.20%,χ2= 5.054,P= 0.025). The symptoms in the 42 patients with grade III and IV HE disappeared after shunt reduction in both groups.
At 6 mo after TIPS placement, in group A, 94 patients manifested HE; among them,53 cases were covert, 32 were grade II, six were grade III, and three were grade IV. In group B, 99 patients manifested HE; among them, 36 cases were covert, 46 were grade II, nine were grade III, and eight were grade IV. The incidence of HE in group A was lower than that in group B (10.03%vs32.24%,χ2= 6.560,P= 0.010). The symptoms in the 26 patients with grade III and IV HE disappeared after shunt reduction.
At 9 mo after TIPS placement, in group A, 86 patients manifested HE; among them,42 cases were covert, 31 were grade II, seven were grade III, and six were grade IV. In group B, 96 patients manifested HE; among them, 46 cases were covert, 37 were grade II, nine were grade III, and four were grade IV. The incidence of HE in group A was lower than that in group B (9.17%vs31.27%,χ2= 5.357,P= 0.021). The symptoms in the 26 patients with grade III and IV HE disappeared after shunt reduction.
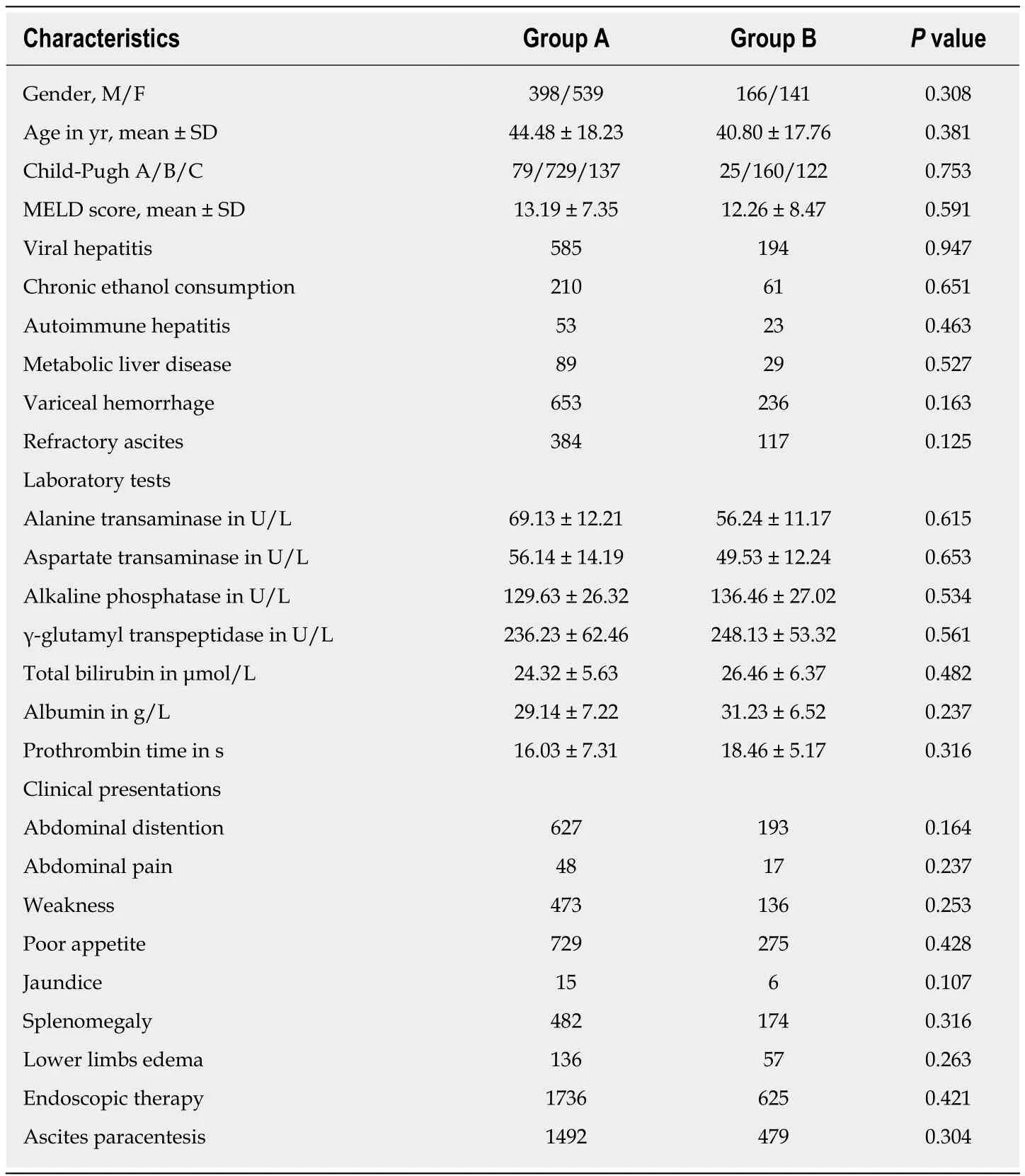
Table 1 Baseline characteristics in the two groups
At 12 mo after TIPS placement, in group A, 77 patients manifested HE; among them, 34 cases were covert, 35 were grade II, six were grade III, and three were grade IV. In group B, 86 patients manifested HE; among them, 28 cases were covert, 39 were grade II, 11 were grade III, and eight were grade IV. The incidence of HE in group A was lower than that in group B (8.21%vs28.01%,χ2= 3.848,P= 0.051). The symptoms in the 25 patients with grade III and IV HE disappeared after shunt reduction,although three patients who underwent shunt reduction still had hepatic myelopathy.
At 3 years after TIPS placement, in group A, 62 patients manifested HE; among them, 25 cases were covert, 31 were grade II, six were grade III, and none were grade IV. In group B, 22 patients manifested HE; among them, 12 cases were covert, six were grade II, four were grade III, and none were IV. There was no significant difference in the incidence of HE between group A and group B (6.61%vs7.16%,χ2= 1.204,P=0.272).
At 5 years after TIPS placement, in group A, 47 patients manifested HE; among them, 23 cases were covert, 24 were grade II, and none were grade III or IV. In group B, 19 patients manifested HE; among them, 12 cases were covert, five were grade II,and two were grade III. There was no significant difference in the incidence of HE between group A and group B (5.01%vs6.18%,χ2= 0.072,P= 0.562).
The symptom of ascites in 357 cases in group A and 119 cases in group B disappeared within the first week without paracentesis, and there was no significant difference between the groups (P= 0.364). No patient experienced re-bleeding within a week. By the endpoint of follow-up, 112 cases had recurrent bleeding in group A compared with 49 cases in group B, and there was no significant difference between the groups (P= 0.278). There were 185 patients with recurrent ascites in group A and 64 patients in group B, and there was no significant difference between the groups (P= 0.561). After stent revision, the symptoms disappeared (Table 4).
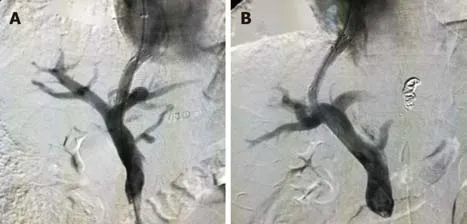
Figure 2 Shunt in the left or right branch of portal vein. A: Shunt in the left branch of portal vein; B: Shunt in the right branch of portal vein.
During follow-up, at 1 year, 141/937 patients (84.95%) were lost to follow-up in group A and 62/307 patients (79.80%) in group B. At 3 years, 211/937 patients(77.48%) were lost to follow-up in group A and 88/307 patients (71.33%) in group B.The endpoint of this study was at 5 years, 305/937 patients (67.44%) were lost to follow-up in group A and 114/307 patients (62.86%) in group B. The 1-, 3-, and 5-year survival rates did not differ between groups A and B (χ2= 0.326,P= 0.568;χ2= 0.364,P= 0.564 andχ2= 0.178,P= 0.673, respectively), and the total survival rates did not differ between groups A and B (χ2= 0.226,P= 0.634, log-rank test) (Figure 4). Among them, 221 patients died from hepatic tumor, 151 from multiorgan failure, and 47 from other causes (Table 5).
DISCUSSION
The use of TIPS in the treatment of portal-hypertension-related complications has progressively increased and has achieved good results[9,10]. However, the clinical benefit of this intervention has been hampered due to a high rate of HE, up to 20%-40% at 12 mo follow-up[3]. HE has become an important issue to be taken into consideration in TIPS candidates and a problem to be addressed after the procedure,which influence its widespread use in clinical practice.
Numerous studies[11,12]have evaluated the risk factors associated with post-TIPS HE in patients with portal hypertension due to cirrhosis, such as the selection of candidates for TIPS placement, the patient’s age, and liver function, as measured by Child-Pugh or Model of End-Stage Liver Disease scores. However, few studies focus on whether targeted puncture of right or left intrahepatic branch of portal vein in TIPS may reduce the incidence of post-TIPS HE[13].
HE is mainly due to absorption of toxic substances from the intestinal portal vein system, through the shunt without the liver first pass effect into the systemic circulation, caused by dysfunction of the central nervous system; a syndrome with mental and nervous symptoms[14]. One of the toxic substances that causes HE is blood ammonia[15]. There are two aspects of the source of blood ammonia in the body,endogenous ammonia is produced by catabolismin vivo, and exogenous ammonia is produced by catabolism of nitrogenous substances in the intestine[16]. In the latter, 90%is found in blood urea and is diffused into the intestinal cavity through the gastrointestinal mucosal blood vessels and decomposed by bacterial urease[17].
The superior mesenteric vein and splenic vein are composed of two branches of main portal vein; the former mainly collects blood reflux in the small intestine, colon,and pancreatic head; the latter mainly collects blood from the spleen, pancreatic body and tail; and other parts or inferior mesenteric vein collect blood reflux in the left colon[18]. Thus, exogenous ammonia is absorbed into the body mainly through the superior mesenteric vein. It is reported that the reflux blood from the splenic and superior mesenteric veins is distributed hydrodynamically in the main portal vein,that is, alongside the trunk on both sides of the wall of the portal vein. However, it is not fully mixed and enters the left and right branches of the portal vein. The right branch mainly receives superior mesenteric venous blood, while the left branch mainly receives blood from the splenic vein[7,8]. We hypothesized that targeted puncture of the left portal vein would divert the non-nutritive blood from the splenic vein into the TIPS shunt and reduce the incidence of HE.
Normally, urea is produced by the circulation of ornithine through the liver[19]. However, almost no exogenous ammonia was produced in the blood collected from the splenic vein. The concentration of ammonia in the superior mesenteric vein was higher than that in the splenic vein and the left and right branches of the portal vein,and the latter was higher than in the vena cava[20]. The anatomy of the portal vein and its mechanism of ammonia production are consistent with those of the portal vein.Similar results[21]have been found in humans: blood ammonia concentration, superior mesenteric vein > portal vein > splenic vein > peripheral vein, and the differences are significant. Later, the isotope hypothesis[22,23]was used to confirm the hypothesis. The results showed that the concentration of ammonia in the superior mesenteric vein was higher than that in the splenic vein and vena cava, suggesting that exogenous ammonia removal was not timely and would cause systemic circulation of ammonia to increase rapidly.
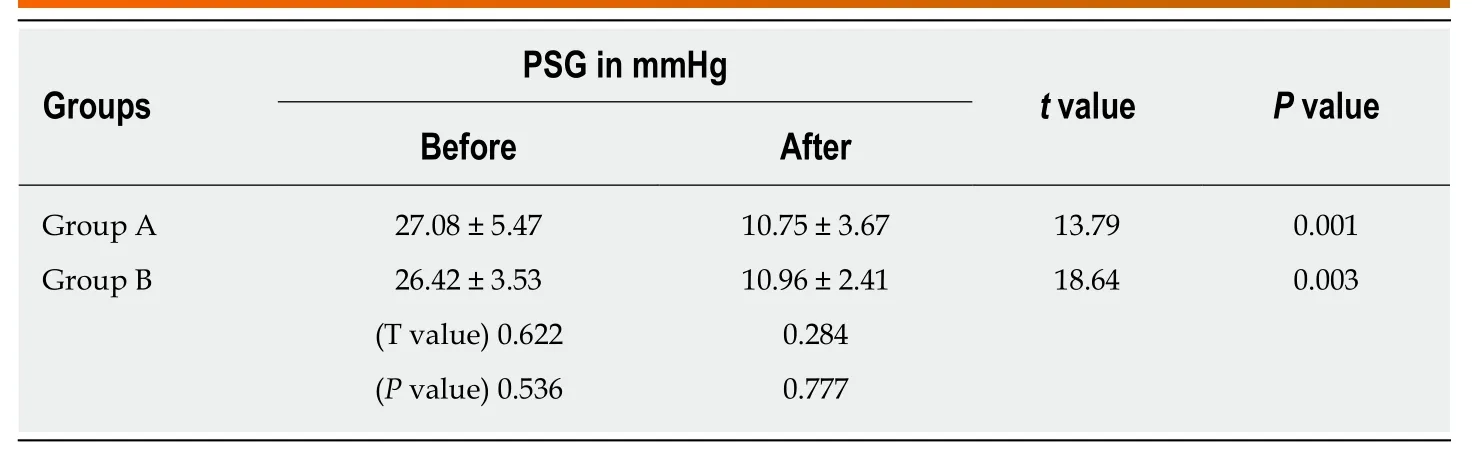
Table 2 Portosystemic gradient changes in the two groups
In early TIPS technology, it was easier to use the right than left branch of the portal vein for puncture target[24]. Currently, experienced interventional radiologists can choose the left or right branch as a puncture target, with no technical difficulty and a near 100% success rate[25]. Further consideration is how to improve the clinical success rate and reduce the incidence of HE and liver failure. It was hypothesized[26]that the blood components of the left and right branches of the intrahepatic portal vein are different. We believe that the choice of TIPS method plays a decisive role in treatment outcome.
In addition, from the anatomical point of view[27], the right branch of the portal vein supplies more of the right liver, and if it is partially or completely diverted, liver function impairment is aggravated. Moreover, the mesenteric vein blood, which contains a large number of toxins (including ammonia) and the liver factor[28], enters the right branch of the portal vein, and then is diverted, which increases the concentration of circulating blood ammonia. Liver failure and the high concentration of ammonia in the systemic circulation are the main causes of portal body shunt encephalopathy[29,30]. The pathogenesis of HE is complex, especially in TIPS treatment.In addition to being shunted around the liver, blood ammonia is not cleared, but because the liver blood supply is reduced after shunting, liver dysfunction and decreased removal of ammonia lead to the occurrence of HE. The hepatic dysfunction after shunting is related to the shunt flow and the quality of the shunt. As with insulin, high concentrations of glucagon and other liver factors in the blood likely lead to the occurrence of liver failure[31].
We can only correctly select the TIPS procedure after fully understanding the important differences between the delivery and concentration of substances in the left and right branches of the portal vein and the complications of shunting. The results of the present study provide evidence that after TIPS treatment, targeted puncture of the left branch of the intrahepatic portal vein may reduce the risk of HE. In group A, the incidence of HE was lower than that in group B and the occurrence of HE showed a downward trend. Our results confirmed in the TIPS process that targeted puncture of the left portal vein diverted the non-nutritive blood that came from the splenic vein into the TIPS shunt and minimized the incidence of HE.
The previous literature[15]only compared the overall incidence of HE in 3 years, and showed that HE occurred one year after TIPS[32]. Therefore, we compared the occurrence of HE after 1, 3, 6, 9 and 12 mo, and the total incidence of HE after 5 years.We showed that targeted puncture of the left branch of the intrahepatic portal vein reduced the risk of HE, but it had no direct influence on prognosis of portalhypertension-related complications of refractory ascites or variceal bleeding.
In our study, the survival rate and recurrence rate for ascites and bleeding did not differ between the two groups. These results indicate the prognostic importance of TIPS placement for portal-hypertension-related complications. We believe that, as long as intrahepatic vein angiography shows that the anatomical structure meets the requirements, we should puncture the left portal vein, which significantly decreases the incidence of HE.
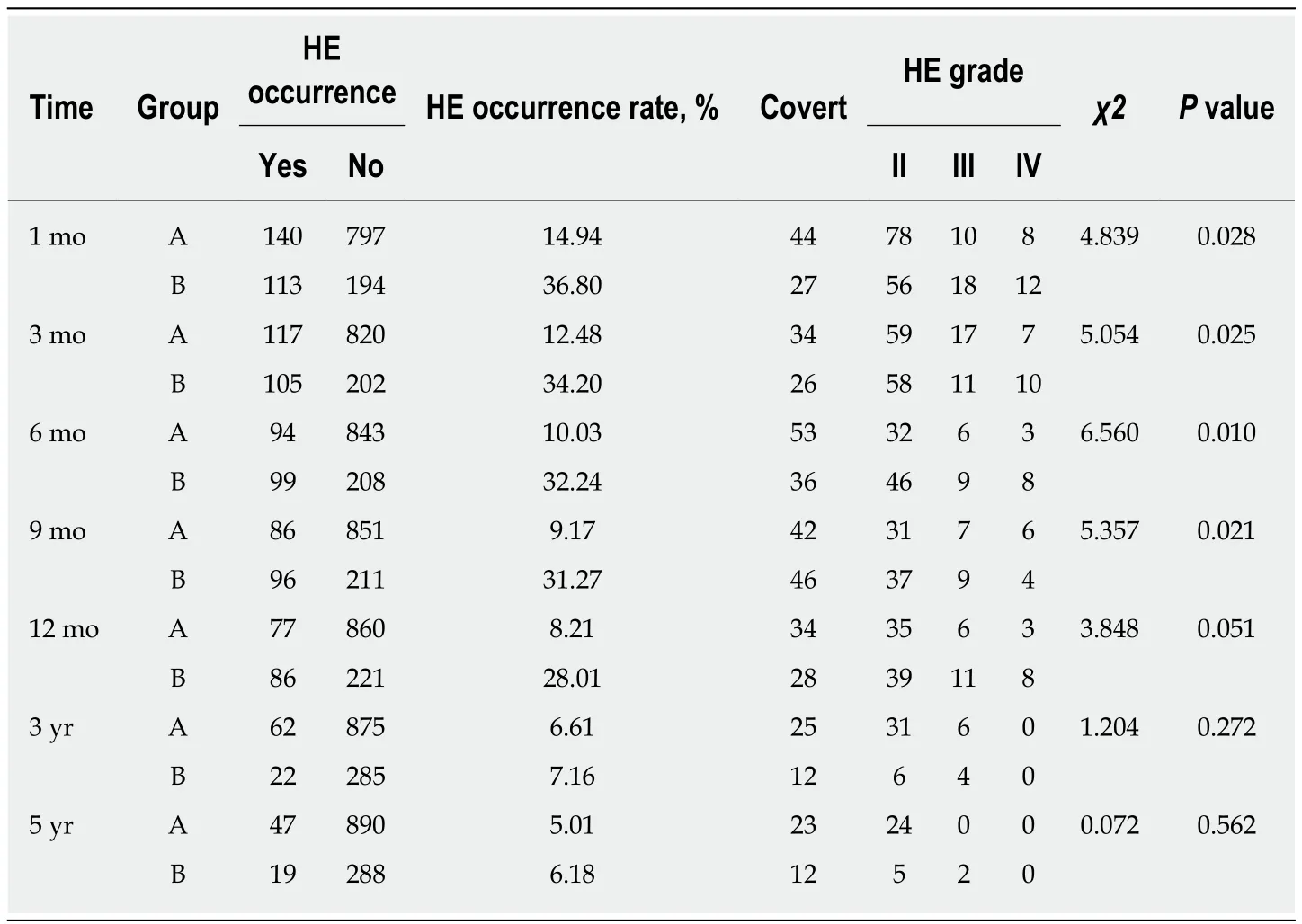
Table 3 HE occurrence in the two groups
This study had several limitations. First, randomized controlled trials are needed to verify our results. Second, targeted puncture of the left intrahepatic portal vein during TIPS procedure is difficult for those who are used to targeted puncture of the right intrahepatic portal vein, which will take some time for such change. Finally, our hypothesis need to be validated by animal experiments and further study.
In conclusion, targeted puncture of the left branch of the intrahepatic portal vein during TIPS does not directly influence the prognosis of portal-hypertension-related complications of refractory ascites or variceal bleeding but may reduce the risk of HE.

Table 4 Outcomes of symptoms in the two groups
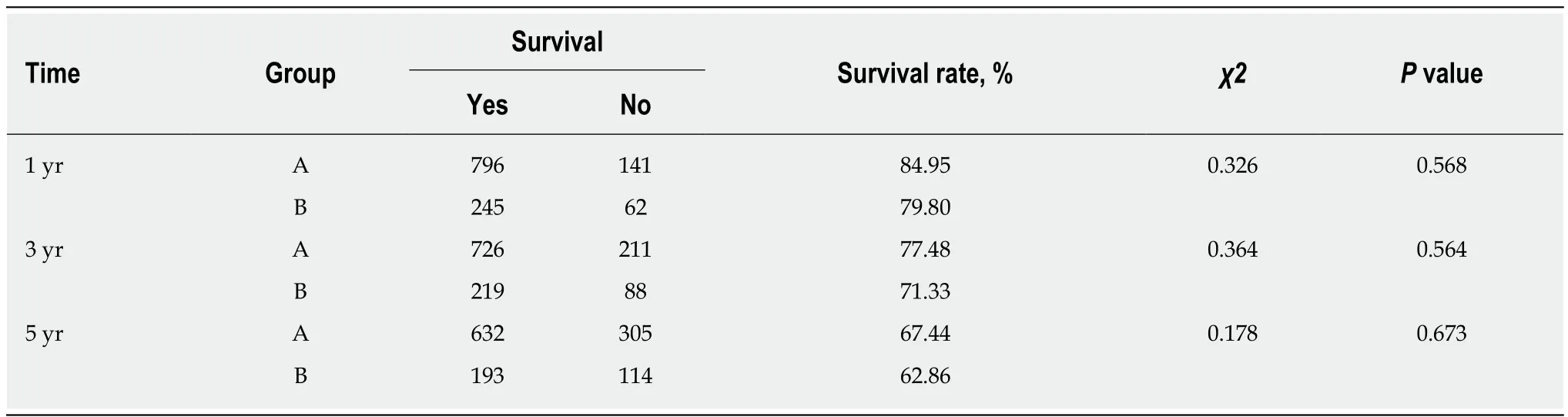
Table 5 One-, three- and five-year survival rates in the two groups
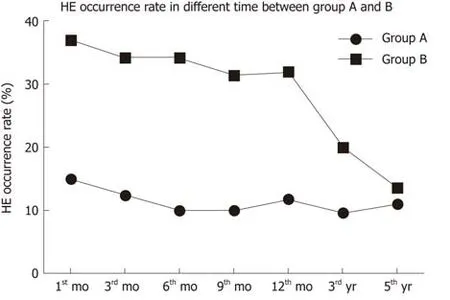
Figure 3 HE occurrence in the two groups. HE occurrence rate in group A was lower than that in group B at 1, 3, 6, 9, and 12 mo, and the occurrence of HE showed a downward trend. HE: Hepatic encephalopathy.
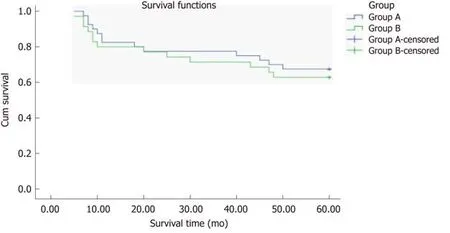
Figure 4 Total survival rate in the two groups. The total survival rate did not differ between groups A and B (χ2 = 0.226, P = 0.634, log-rank test).
ARTICLE HIGHLIGHTS
Research background
Transjugular intrahepatic portosystemic shunt (TIPS) is currently used for the treatment of complications of portal hypertension. The incidence of hepatic encephalopathy (HE) remains a problem in TIPS placement. It has been reported that the right branch mainly receives superior mesenteric venous blood, while the left branch mainly receives blood from the splenic vein. We hypothesized that targeted puncture of the left portal vein would divert the non-nutritive blood from the splenic vein into the TIPS shunt; therefore, targeted puncture of the left branch of the intrahepatic portal vein during TIPS may reduce the risk of HE.
Research motivation
TIPS is currently used for the treatment of complications of portal hypertension. With advances in materials, many experimental and clinical studies have been conducted using covered stent grafts, especially stent grafts covered with polytetrafluoroethylene, to improve the long-term patency of TIPS. However, the incidence of HE remains a problem in TIPS placement and affects the quality of life and long-term outcomes of patients.
It has been reported that the reflux blood from the splenic and superior mesenteric veins is distributed hydrodynamically in the main portal vein, that is, alongside the trunk on both sides of the wall of the portal vein. However, it is not fully mixed and enters the left and right branches of the portal vein. The right branch mainly receives superior mesenteric venous blood,while the left branch mainly receives blood from the splenic vein. We hypothesized that targeted puncture of the left portal vein would divert the non-nutritive blood from the splenic vein into the TIPS shunt and reduce the incidence of HE. The purpose of this study was to compare the effect of the left and right branches of the portal vein as TIPS shunt on the incidence of HE in patients who required TIPS placement for portal-hypertension-related complications of ascites or variceal bleeding. In the future, randomized controlled trials are needed to verify our results,and our hypothesis needs to be validated by animal experiments and further study.
Research objectives
The main objective was to establish whether the left branch of the intrahepatic portal vein as TIPS shunt reduced the risk of HE. If we realized this objective for future clinical practice in TIPS, we should target puncturing the left branch of the intrahepatic portal vein during TIPS as far as possible, because we hypothesized that targeted puncture of the left portal vein would divert the non-nutritive blood from the splenic vein into the TIPS shunt and reduce the incidence of HE.
Research methods
We hypothesized that targeted puncture of the left portal vein would divert the non-nutritive blood from the splenic vein into the TIPS shunt and reduce the incidence of HE. To achieve this objective, we conducted a single-center retrospective study that compared the influence of targeted puncture of the left and right branches of the portal vein on the incidence of HE in patients who required TIPS placement for portal-hypertension-related complications of ascites or variceal bleeding. The patients were divided into two groups: A (targeting of left branch of portal vein,n= 937) and B (targeting of right branch of portal vein,n= 307) (Figure 2). The outcomes of HE, recurrent variceal bleeding and/or ascites, and mortality were compared and analyzed between the groups. This study was not reported previously. Results were expressed as mean ± standard deviation and compared using the independent samplettest or one-way analysis of variance, and categorical variables were expressed as frequencies and compared usingχ2tests. The differences between the groups were compared using one-way analysis of variance followed by least significant differencettests. Differences were considered significant atP< 0.05. The statistical analyses were performed with SPSS version 20.0.
Research results
This study showed that targeted puncture of the left branch of the intrahepatic portal vein during TIPS reduced the risk of HE, although it did not directly influence the prognosis of portal-hypertension-related complications of refractory ascites or variceal bleeding. It verified the hypothesis that the reflux blood from the splenic and superior mesenteric veins is distributed hydrodynamically in the main portal vein, that is, alongside the trunk on both sides of the wall of the portal vein. However, it is not fully mixed and enters the left and right branches of the portal vein. The right branch mainly receives superior mesenteric venous blood, while the left branch mainly receives blood from the splenic vein indirectly.
Research conclusions
We found that the left branch of the intrahepatic portal vein during TIPS reduced the risk of HE.One of the toxic substances that causes HE is blood ammonia. HE is mainly caused by absorption of toxic substances from the intestinal portal vein system, through the shunt without the liver first pass effect into the systemic circulation, caused by dysfunction of the central nervous system. It has been reported that the reflux blood from the splenic and superior mesenteric veins is distributed hydrodynamically in the main portal vein, that is, alongside the trunk on both sides of the wall of the portal vein. However, it is not fully mixed and enters the left and right branches of the portal vein. The right branch mainly receives superior mesenteric venous blood,while the left branch mainly receives blood from the splenic vein. We hypothesized that targeted puncture of the left portal vein would divert the non-nutritive blood from the splenic vein into the TIPS shunt and reduce the incidence of HE. As far as possible, we should target puncturing the left branch of the intrahepatic portal vein during TIPS procedure for clinical practice in the future.
Research perspectives
We can learn from this study that, to reduce the risk of HE, we should target puncturing the left branch of the intrahepatic portal vein during TIPS for clinical practice in the future. We believe that, as long as intrahepatic vein angiography shows that the anatomical structure meets the requirements, we should puncture the left portal vein, which significantly decreases the incidence of HE. In future research, randomized controlled trials are needed to verify our results,and it will take some time to switch to the targeted puncture of the left intrahepatic portal vein during TIPS procedure. Finally, our hypothesis needs to be validated by animal experiments to find direct evidence for hydrodynamics of blood.
ACKNOWLEDGEMENTS
The authors thank all the patients who were involved in this study and colleagues of the Department of Radiology of Air Force Medical Center of PLA for their contributions to the data collection.
杂志排行
World Journal of Gastroenterology的其它文章
- Liver stem cells: Plasticity of the liver epithelium
- Reaction of antibodies to Campylobacter jejuni and cytolethal distending toxin B with tissues and food antigens
- Integrated network analysis of transcriptomic and protein-protein interaction data in taurine-treated hepatic stellate cells
- Computed tomography scan imaging in diagnosing acute uncomplicated pancreatitis: Usefulness vs cost
- Optimized protocol of multiple post-processing techniques improves diagnostic accuracy of multidetector computed tomography in assessment of small bowel obstruction compared with conventional axial and coronal reformations
- Comprehensive lifestyle intervention vs soy protein-based meal regimen in non-alcoholic steatohepatitis
