Reaction of antibodies to Campylobacter jejuni and cytolethal distending toxin B with tissues and food antigens
2019-03-11AristoVojdaniElroyVojdani
Aristo Vojdani, Elroy Vojdani
Abstract BACKGROUND The bacteria Campylobacter jejuni (C. jejuni) is commonly associated with Guillane-Barré syndrome (GBS) and irritable bowel syndrome (IBS), but studies have also linked it with Miller Fisher syndrome, reactive arthritis and other disorders, some of which are autoimmune. It is possible that C. jejuni and its toxins may be crossreactive with some human tissues and food antigens, potentially leading to autoimmune responses.AIM To measure the immune reactivity of C. jejuni and C. jejuni cytolethal distending toxin (Cdt) antibodies with tissue and food antigens to examine their role in autoimmunities.METHODS Using enzyme-linked immunosorbent assay (ELISA) methodology, specific antibodies made against C. jejuni and C. jejuni Cdt were applied to a variety of microwell plates coated with 45 tissues and 180 food antigens. The resulting immunoreactivities were compared to reactions with control wells coated with human serum albumin (HSA) which were used as negative controls and with wells coated with C. jejuni lysate or C. jejuni Cdt which served as positive controls.RESULTS At 3 SD above the mean of control wells coated with HSA or 0.41 OD, the mouse monoclonal antibody made against C. jejuni showed moderate to high reactions with zonulin, somatotropin, acetylcholine receptor, β-amyloid and presenilin.This immune reaction was low with an additional 25 tissue antigens including asialoganglioside, and the same antibody did not react at all with another 15 tissue antigens. Examining the reaction between C. jejuni antibody and 180 food antigens, we found insignificant reactions with 163 foods but low to high immune reactions with 17 food antigens. Similarly, we examined the reaction of C. jejuni Cdt with the same tissues and food antigens. The strongest reactions were observed with zonulin, intrinsic factor and somatotropin. The reaction was moderate with 9 different tissue antigens including thyroid peroxidase, and reaction was low with another 10 different antigens, including neuronal antigens.The reaction of C. jejuni Cdt antibody with an additional 23 tissue antigens was insignificant. Regarding the reaction of C. jejuni Cdt antibody with different food antigens, 160 out of 180 foods showed insignificant reactions, while 20 foods showed reactions ranging from low to high.CONCLUSION Our findings indicate that C. jejuni and its Cdt may play a role in inflammation and autoimmunities beyond the gut.
Key words:Campylobacter jejuni; Cytolethal distending toxin; Tissue antigens; Food antigens; Autoimmune reactivities; Cross-reactivity
INTRODUCTION
Campylobacter jejuni(C. jejuni) is a Gram-negative spiral-shaped bacteria that belongs to the group of Epsilonproteobacteria, which can inhabit mucosal tissues of the gastrointestinal (GI) tract[1].C. jejunicurrently is the most common bacteria associated with food contamination and bacterial gastroenteritis worldwide. This bacterium is found in environmental niches such as agricultural land and riverine water sources that have been contaminated by the fecal materials of wild birds or agricultural animals. The major sources for disease induction are meat products from farm animals, mainly poultry, pigs and cattle. In many cases these bacteria live quietly within the GI tract of poultry, causing no obvious systems of disease due to their evolutionary relationship with chickens and other birds. Initial colonization of a chicken flock occurs when birds are about 2 wk old, later on spreading throughout the chicken population[2]. This bacterium makes its way into the human gut when an individual consumes insufficiently-cooked contaminated chicken meat or drinks contaminated water. Depending on the genetic makeup of the individual host, the bacteria may then induce diseases both within and beyond the gut[3]. In humans,C.jejunihas been associated with different GI conditions, including irritable bowel syndrome (IBS), inflammatory bowel disease (IBD), Barrett’s esophagus and colorectal cancer. Moreover,C. jejunihas also been reported to be involved in extraintestinal manifestations, including bacteremia, reactive arthritis, lung infections, brain abscesses, meningitis, and the autoimmune conditions known as Guillain-Barré and Miller Fisher syndromes in a very small percentage of infected individuals[2,4-7].Guillain-Barré syndrome (GBS) is an acute post-infection autoimmune disease in which the immune system attacks the gangliosides of the peripheral nervous system,resulting in peripheral neuropathy and complete loss of movement[8].
GBS stems from the mimicry between theC. jejunisurface structural molecule called lipooligosaccharide and the ganglioside found in the human nervous system.Exposure of the immune system to theC. jejunicellular antigens can induce crossreactive antibodies that target not only the bacteria but also specific antigens of the nerve cells such as ganglioside GM1, GM1b, GD1a and GQ1b[9-11]. This continuous immune attack against gangliosides seems to be responsible for the symptoms of paralysis in a small percentage of patients with a specific genetic makeup[12,13].
Cytolethal distending toxin (Cdt) is another virulence factor produced by C.jejuniand other enterobacters; secretedviaouter membrane vesicles, Cdts have the capacity to disrupt epithelial tight junction proteins (e.g., occludin, claudin) and induce the epithelial barrier dysfunction observed in patients with IBD and IBS[1,14-18].
Animal models have shown that IBS may be linked to changes in the microbiome and the production of bacterial cytotoxins. Introduction ofC. jejunito rats in one study resulted in changes in stool form, small intestine bacterial overgrowth (SIBO),and the increased rectal intra-epithelial lymphocytes characteristic of humans with IBS[19]. The effects in this study appeared to be due to changes in gut neuroanatomy,with a notable reduction in interstitial cells of Cajal (ICC). Furthermore, rats infected with a mutantC. jejunistrain lacking Cdt exhibited significant mitigation in the IBS-like phenotype compared to those infected with wild-typeC. jejuni; this suggests that Cdt had an important role in the development of IBS in this animal model[19-21].
Moreover, rabbit anti-bacterial cytotoxin antibody has been found to exhibit affinity to enteric ganglia and ICC through molecular mimicry and the production of crossreactive antibodies.
Based on the affinity of bacterial cytotoxin antibodies to ICC and ganglia, it has been concluded that these cross-reactive antibodies play a role in the pathophysiology of IBS by affecting gut motor function, leading to SIBO[22,23].
Bacteria use these cytotoxins or enterotoxins to penetrate the gut epithelial cells and change the dynamic of cytoskeletal proteins (vinculin, talin, actinin and others).
Through elegant experiments, it was determined that enterotoxins utilize a mechanism of cross-reactivity with host proteins such as vinculin and talin, allowing them to bind to the vinculin and talin, causing the loss of cytoskeletal proteins in the intestinal wall[22-24]. Additionally, levels of these circulating antibodies to bacterial cytotoxins and cytoskeletal proteins correlated with the development and levels of SIBO in these animals[23]. These circulating antibodies against bacterial cytotoxins and their cross-reaction with cytoskeletal proteins are linked to autoimmunity against cytoskeletal proteins and reduction in their functionality. In addition to the animal model of bacterial cytotoxin-induced autoimmunity, these measurements were performed on humans with diarrhea-predominant IBS (D-IBS), Crohn’s disease,ulcerative colitis, celiac disease and controls. It was shown that anti-bacterial cytotoxins and anti-vinculin antibodies were significantly higher (P< 0.001) in D-IBS individuals compared to celiac disease, IBD and healthy subjects[25]. The detection of these antibodies in blood with a sensitivity of 92% as shown in this study suggests that IBS may have an organic basis.
The three-dimensional structure of Cdt is highly compatible with the structure of the deoxyribonuclease I (DNase I) from different mammalian species[26,27].
Furthermore, there is a significant amino acid similarity between Cdt and DNAse I.Due to this DNAse I reactivity, exposure to Cdt would produce epithelial cell damage altering the functional capacity of the tissues and facilitating bacterial invasion into the underlying tissue, resulting in severe diarrhea and the loss of absorbed nutrients.When the epithelium is damaged, bacteria collectively gain entry into the underlying connective tissue where microbial products can affect processes and pathways in infiltrating inflammatory cells, culminating in the destruction of the epithelial barrier and the underlying tissue[28,29]. This results in increased intestinal permeability to large macromolecules, including food antigens that escape enzymatic digestion[30,31].
The integrity and functionality of barrier proteins of epithelial layers are regulated by intercellular adherent junctions such as actinin, talin and vinculin, which are generally called cytoskeletal proteins. It is generally believed that Cdts and other inflammatory stimuli increase transepithelial permeability to large molecules by inducing junctional disassembly, and reorganization of F-actin cytoskeleton[32,33].
Due to its unique structure, vinculin not only binds to talin and α-actinin, but also,due to molecular mimicry, to a bacterial cytotoxin called invasin protein, which bacteria use to bind to the N-terminal domain of vinculin in order to invade the epithelial cells. This is the best example of how a bacterial toxin subverts vinculin’s functions, causing F-actin depolymerization through a remarkable level of molecular mimicry of talin-vinculin interaction to activate vinculin. Therefore, mimicry of vinculin and talin by bacterial toxin could be a general mechanism for bacterial invasion of the host cells[34-36].
This mucosal inflammation commonly leads to epithelial barrier compromise and increases in body exposure to the external environmental triggers, thereby further aggravating the consequences of enhanced gut permeability. Thus, understanding the mechanisms involved in the disruption of tight junctions and the reorganization of adherens junction proteins such as vinculin, talin and α-actinin is important in developing strategies to remove the triggers and repair the barriers which play such an important role in health and diseases[37-39].
It has been demonstrated thatC. jejunicross-reacts with basal ganglia, and its bacterial cytotoxins cross-react with DNase, talin and vinculin, and that through its binding to these structural proteins it can breach the epithelial cell barrier. Therefore,the goal of this study was to examine possible cross-reactivity betweenC. jejuniand its toxins with other tissue antigens as well as food proteins to which the human body is exposed on a daily basis.
MATERIALS AND METHODS
Antibody and antigens
Mouse immunoglobulin G (IgG) monoclonal anti-C. jejuniwhole cell suspension and affinity-purified rabbit anti-C. jejuniCdt IgG isotype was purchased from Creative Diagnostics (Shirley, NY, United States).C. jejunilysate was prepared fromC. jejuniNCTC11168 strain after its growth on Mueller-Hinton agar under microaerophillic conditions at 42 °C.C. jejuniCdt peptide was purchased from BioSynthesis,Lewisville, TX, United States.
Proteins, parietal cell antigen, intrinsic factor, fibrinogen, laminin, thyroid peroxidase (TPO), thyroglobulin, myeloperoxidase, collagen type V, and neuraminidase were purchased from MP Biologicals (Solon, OH, United States).
Cardiolipin, actin, myelin basic protein (MBP), tropomyosin, ganglioside GM1,insulin, liver microsomes, transglutaminases, enolase, β-amyloid protein, tau protein,somatotropin, human serum albumin (HSA), dipeptidylpeptidase,Saccharomycescerevisiae, and lipopolysaccharides were purchased from Sigma-Aldrich (St Louis,MO, United States).
Glial fibrillary acidic protein (GFAP), brain-derived neurotrophic factor (BDNF),myoglobin platelet glycoprotein, a-synuclein, acetylcholine receptor, lysosome, and elastase were purchased from Calbiochem (San Diego, CA, United States).
Different peptides of occludin, zonulin, claudin-5, aquaporin-4 (AQP4), presenilin,fibulin, protein disulfide isomerase (PDI), cerebellar, rabaptin-5 (rab-5), enteric nerve neuronal nuclear antigen (NNA), glutamate-R, dopamine-R, and glutamic acid decarboxylase 65 (GAD-65), all with purity greater than 90%, were synthesized by Biosynthesis (Lewisville, TX, United States).
One hundred and eighty different food extracts were prepared according to the procedure described in our earlier study[40]. Briefly, food products including grains,seeds, beans, nuts, fruits, vegetables, meats, seafood, spices and gums were purchased in either raw, modified or cooked form. For the preparation of antigens, 10 g of food products were put in a food grinder using 75% ethanol, and another 10 g were put in the grinder with 0.1 mol/L phosphate buffer saline (PBS) pH 7.4. After mixing for 1 h each preparation was kept on the stirrer for 12 h at 4 °C, then centrifuged at 20000 g for 15 min. The liquid layers were then removed and dialysed. Finally, the solutions were combined, protein concentration was measured, and the combined solution was kept at -20 °C until it was used in the enzyme-linked immunosorbent assay (ELISA).
Reaction of anti-C. jejuni and anti-C. jejuni Cdt with different tissue and food antigens
Proteins and peptides at a concentration of 1 mg/mL were diluted 1:100 in 0.1 mol/L carbonate buffer; 100 mL or 1 mg of each antigen was added to a series of Costar microtiter ELISA plate wells. After incubation for 8 h at room temperature (RT) and 18 h at 4 °C, plates were washed 3 times using ELISA washer, and 200 mL of 2% BSA was added to each well and incubated for 24 h at 4°C in order to block the nonspecific binding of the antibody to the antigen-coated wells. For this immunoreactivity study,100 mL of monoclonal mouse anti-C. jejuniat a dilution of 1:200 was added to one series of ELISA coated plates and 100 mL of affinity-purified rabbit anti-C. jejuniCdt dilution of 1:100 was added to another series of ELISA microwell plates. Both primary antibodies were diluted with 2% BSA in 0.01 mol/L PBS containing 0.05% Tween 20.Each antibody was added to quadruplicate wells that were coated with a variety of tissues and food antigens. The ELISA plates were incubated for 60 min at 25 °C. After washing 5 times with 0.01 mol/L PBS 0.05% Tween 20, 100 mL of alkaline phosphatase- labeled anti-rabbit or anti-mouse IgG at a dilution of 1:300 were added to appropriate wells and incubated again for 1 h at RT. The enzyme reaction was started by adding 100 mL of paranitrophenyl phosphate at a concentration of 1 mg/mL in diethanolamine buffer containing 1 mmol/L MgCl2and sodium azide at a pH of 9.8. The reaction was stopped 45 min later with 50 mL of 1 mol/L NaOH, and the color intensity was read by an ELISA reader at 405 nmol/L.
To determine the specificity of mouse monoclonal and rabbit polyclonal antibodies,these antibodies were replaced with the same dilution of non-immunized serum and added to quadruplicate wells. Furthermore, the antibodies and other reagents were added to 4 wells coated with HSA and 4 wells coated with 2% BSA alone; these were then used as negative controls. After the addition of other reagents to these control wells, the ODs were measured.
Binding of serially diluted anti-C. jejuni and anti-C. jejuni Cdt to tissue and food antigens
For the demonstration of the specificity of anti-C. jejunilysate and anti-C. jejuniCdt antibodies binding to different tissue and food antigens, 2 sets of 5 different strips of ELISA plate, each containing 8 wells, were coated respectively withC. jejunilysate,zonulin, somatotropin, presenilin and ω-gliadin. In the second set of microplate wells,the first strip was coated withC. jejuniCdt followed by the other antigens on each microwell strip. In addition toC. jejuniand Cdt antigens the additional 4 antigens were chosen as being representative of the antigens that showed from moderate to strong immune reactivity with the anti-C. jejuniandC. jejuniCdt.C. jejuni or Cdt antibody that had been serially diluted from 1:200-1:25, 600 was then added to appropriate sets of microtiter plate wells. After incubation, washing and addition of secondary antibodies, and completion of other required ELISA steps, the ODs were recorded at 405 nmol/L.
Inhibition of anti-C. jejuni antigens and Cdt antibodies binding to C. jejuni, tissue and food antigens with the same antigens in liquid phase
For this inhibition study, 100 µL of diluents was first added to all wells of 5 different rows of microtiter plates coated withC. jejuniantigens, zonulin, somatotropin,presenilin, or ω-gliadin. Twenty microliter of 0.1 mol/L PBS was added to the first well of each row; to the additional rows of the antigen-coated wells, 20 µL of PBS containing 1.5-96 µg ofC. jejuni, zonulin, somatotropin, presenilin, or ω-gliadin was added respectively and incubated for 1 h at RT. After the addition of 100 µL of rabbit anti-C. jejuniandC. jejuniCdt, incubation, washing, and further addition of the secondary antibody or anti-rabbit IgG, and completion of all ELISA steps, the ODs were recorded at 405 nmol/L, and the percentage of inhibition of this antigenantibody reaction was calculated in proportion to the antigen concentration in the liquid phase.
Statistical analysis
Statistical analysis using Microsoft Excel’sttest function was performed.
Comparison of the ODs of all wells used as controls to the ODs of theC. jejuniantibody reactivity against all tested tissue and food antigens was performed. A Bonferroni adjustment account for type 1 errors with multiple comparisons was set to adjust the alpha (P> 0.001). TheP-values are listed in Figures 1-4.
RESULTS
Based on earlier studies about the structure ofC. jejunilipooligosaccharide, its mimicry with ganglioside, and the detection of antibodies against them in patients suffering from Guillain-Barré and Miller Fisher syndromes[9-13], we measured the degree of immune reactivity of mouse monoclonal antibody made againstC. jejuniwith many tissue antigens and a variety of food proteins and peptides. Using ELISA methodology, we first found that unimmunized mouse serum did not react withC.jejunilysate and all 45 different tissue antigens including HSA used in the examination. The ELISA ODs for all these reactions were within 3 SD above the mean of control values or 0.41. To interpret the results of the antibody level measurements,we used the following key: 0-0.41 OD = insignificant; 0.42-1.0 = low; 1.1-1.6 =moderate; 1.61-2.2 = high; > 2.21 = very high.
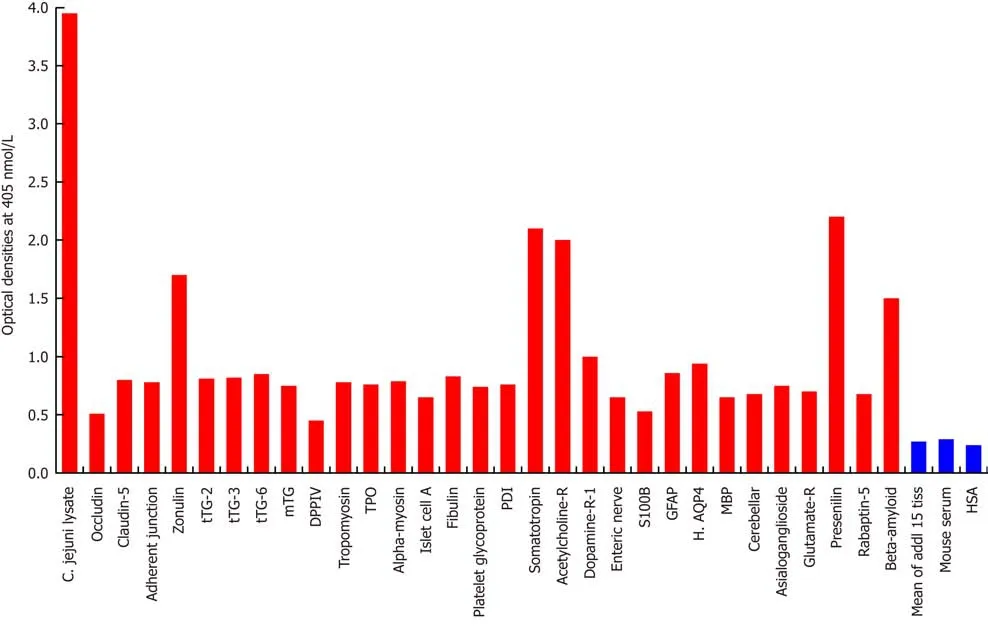
Figure 1 Reaction of mouse monoclonal anti-Campylobacter jejuni with tissue antigens. Compared to the monoclonal antibody’s reaction with Campylobacter jejuni (C. jejuni) lysate as positive control and human serum albumin (HSA) or unimmunized mouse serum as negative control, the reaction of this antibody with 15 tissue antigens is insignificant, with 25 additional antigens is low, with β-amyloid, zonulin, acetylcholine receptor, somatotropin and presenilin ranges from moderate to high, and with C. jejuni lysate is very highly positive. The variation of quadruplicate ODs was less than 10% for all determinations. Comparison of the means of the antibody reactivity to various tissue antigens with the means of controls resulted in insignificant values (P > 0.05) for 15 different tissue antigens and highly significant P values (P < 0.00001) for the others. 0-0.4 OD = insignificant; 0.42-1.0 = low; 1.1-1.6 = moderate; 1.61-2.2 = high; > 2.21 = very high. tTG: Transglutaminase; mTG:Microbial transglutaminase; DPPIV: Dipeptidyl peptidase-4; TPO: Thyroid peroxidase; PDI: Protein disulfide isomerase; GFAP: Glial fibrillary acidic protein; H. AQP4:Human aquaporin-4; MBP: Myelin basic protein; HSA: Human serum albumin.
As was expected, the strongest reaction was observed between anti-C. jejuniantibody andC. jejunilysate with an OD of 3.9, which is very close to the maximum detection limits of the assay (OD of 4.0) (Figure 1). In relation to reactivity with different tissue antigens, theC. jejuniantibody reacted from moderate to high with βamyloid (OD of 1.6), zonulin (1.7), acetylcholine receptor (2.0), somatotropin (2.1) and presenilin (2.2). With 25 additional tissue antigens, this immune reaction was low,with ODs ranging from 0.5-1.0. The same mouse monoclonal antibody did not react at all with another 15 antigens, including HSA. The ODs for these 15 antigen-antibody reactions were below 0.27, which is considered as ELISA background (Figure 1).
Looking at the reaction between this monoclonal antibody and 180 different food antigens, our study found no reaction with 163 food antigens, but a low reaction (OD:0.5-0.94) with 17 different food antigens (Figure 2).
Since the structure ofC. jejuniCdt highly mimics the structure of DNase I, which controls the progression of the epithelial cell cycle[27], and immunity against it links vinculin and other cytoskeletal proteins to the pathophysiology of IBS[21-25], we examined the reaction of anti-C. jejuniCdt with the same tissue proteins and food antigens. With tissue proteins, as expected, we found the strongest reaction betweenC. jejuniCdt antibody andC. jejuniCdt with an OD of 3.3, followed by the reaction ofEscherichia coli(E. coli) Cdt with an OD of 2.8; both of these are considered very high reactions. The next strongest reactions of Cdt antibody were observed with somatotropin, intrinsic factor and zonulin with ODs of 2.0 or slightly greater, which are considered high. With 9 tissue antigens [adherent junction, transglutaminase(tTG)-2, microbial transglutaminase (mTG), TPO, islet cell, a-enolase, GFAP,dopamine-R1, and platelet glycoprotein], reaction with this antibody resulted in ODs of 1.1-1.6 (moderate). The reaction of this Cdt antibody withE. colilipopolysaccharide(LPS), a-myosin, claudin-5 and 8 different neuronal or associated antigens (enteric nerve, S100B, AQP4, MBP, asialoganglioside GM1, presenelin and GAD-65) resulted in ODs of 0.6-1.09 (low). The reactions of this antibody with an additional 3 different tissue antigens were insignificant (Figure 3). In relation to the immunoreactivity of this antibody with a variety of food antigens, 160 out of the 180 antigens showed practically no reaction (mean OD of 0.27 ± 0.18), but the remaining 20 tested food antigens had reactions ranging from low to very high. The strongest reaction was observed with watermelon, followed by pumpkin+squash, carrot, peanut, peanut agglutinin, banana, peanut butter, potato, garlic, yam, kiwi, seaweed, gums, wheat and milk proteins (Figure 4).
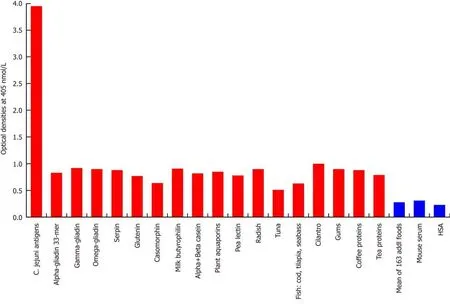
Figure 2 Reaction of mouse monoclonal anti-Campylobacter jejuni with different food antigens. Compared to the monoclonal antibody’s reaction with Campylobacter jejuni (C. jejuni) antigens as positive control and human serum albumin or unimmunized mouse serum as negative control, the reaction of this antibody with 163 food antigens is insignificant, and with 17 additional antigens is low. The variation of quadruplicate ODs was less than 10% for all determinations.Comparison of the means of the antibody reactivity to various food antigens with the means of controls resulted in insignificant values (P > 0.05) for 163 different food antigens and highly significant P values (P < 0.00001) for the other 17 antigens. HSA: Human serum albumin.
Demonstration of analytical specificity of anti-C. jejuni and anti-Cdt antibodies binding to representative antigens
The analytical specificity of mouse monoclonal anti-C. jejuniand rabbit anti-Cdt binding to different tissue and food antigens was confirmed by serial dilution and inhibition studies. As shown by the ODs summarized in Figure 5A and B, the binding of these antibodies to the specific and cross-reactive antigens declined significantly in proportion to the dilutions. For example, anti-C. jejuniantibody at a dilution of 1:400 gave an OD of 3.6, a dilution of 1:3200 resulted in an OD of 2.2, and a dilution of 1:25600 gave an OD of 0.5. The reaction of this same anti-C. jejuniantibody with crossreactive antigens such as zonulin, somatotropin, presenilin and ω-gliadin also declined in proportion to dilutions (Figure 5A). Similar results were obtained when serially diluted anti-C. jejuniCdt was applied to fixed concentrations of Cdt, zonulin,somatotropin, presenilin and ω-gliadin (Figure 5B).
To further demonstrate the specificity of these antibody reactions between eitherC.jejuniorC. jejuniCdt and zonulin, somatotropin, presenilin and ω-gliadin, different amounts of antigen concentrations ranging from 0-96 µg were added to the liquid phase of the ELISA plates that were coated with optimal concentrations of the same antigens. Compared to the controls (buffer with no antigens) shown in Figure 6A and B, the addition of higher concentrations of specific tissue and food antigens to the liquid phase, followed by the addition of primary antibodies, resulted in significant inhibition of anti-C. jejuniand anti-C. jejuniCdt antibodies binding to the specific as well as cross-reactive antigens. This decline in antibody-antigen reaction due to the addition of antigens in liquid phase was more obvious when higher concentrations of the antigens were used as inhibitors (Figure 6A and B).
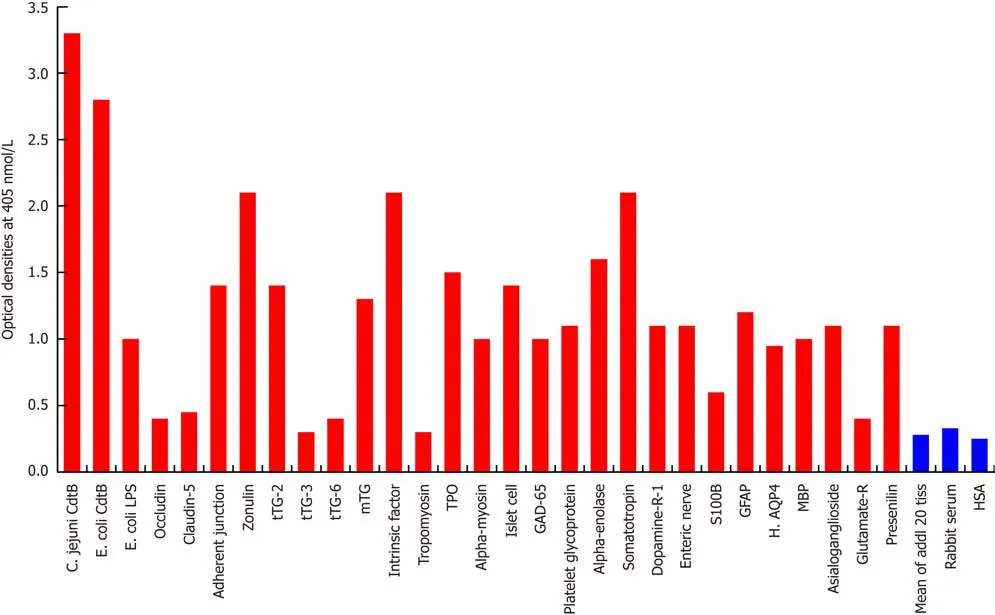
Figure 3 Reaction of affinity-purified rabbit anti-Campylobacter jejuni cytolethal distending toxin with different tissue antigens. Compared to the reaction with Campylobacter jejuni (C. jejuni) cytolethal distending toxin (Cdt) as positive control and human serum albumin (HSA) or unimmunized rabbit serum as negative control, the reaction of this antibody with 20 tissue antigens is insignificant, with an additional 5 tissue antigens slightly higher than the mean of the 20 but still insignificant; with Escherichia coli (E. coli) lipopolysaccharide (LPS), α-myosin, claudin-5 and 8 different neuronal or associated antigens (enteric nerve, S100B, AQP4,myelin basic protein, asialoganglioside GM1, presenelin and glutamic acid decarboxylase-65) is low, with adherent junction, transglutaminase-2, microbial transglutaminase, thyroid peroxidase, islet cell, a-enolase, glial fibrillary acidic protein, dopamine-R1, and platelet glycoprotein is moderate, with somatotropin, intrinsic factor and zonulin is high, and with E. coli Cdt is very highly positive. The variation of quadruplicate ODs was less than 10% for all determinations. Comparison of the means of the antibody reactivity to various food antigens with the means of controls resulted in insignificant values (P > 0.05) for 25 different tissue antigens and highly significant P values (P < 0.00001) for the other antigens. E. coli: Escherichia coli; Cdt: Cytolethal distending toxin; LPS: Lipopolysaccharide; tTG: Transglutaminase;mTG: Microbial transglutaminase; DPPIV: Dipeptidyl peptidase-4; TPO: Thyroid peroxidase; GAD-65: Glutamic acid decarboxylase-65; GFAP: Glial fibrillary acidic protein; H. AQP4: Human aquaporin-4; MBP: Myelin basic protein; Glutamate-R: Glutamate receptor; HSA: Human serum albumin.
DISCUSSION
The aim of this study was to evaluate the immunoreactivity of specific antibodies made againstC. jejuniandC. jejuniCdt with different tissue and food antigens in order to demonstrate the possible role that this bacteria plays beyond food poisoning,GBS, or IBS and its overlap with SIBO[22-25,41]. Many investigators have clearly establishedC. jejunias the most prevalent cause of food-related enteritis worldwide[1,3-5,42-47]. This bacterium releases a very strong toxin called Cdt that contributes to microbial virulence and possible disease pathogenesis by acting as a triperditious toxin that impairs host diseases[47]. The precise mechanisms by whichC.jejuniand its toxins contribute to the development of the aforementioned clinical conditions is largely unknown. Consequently, the focus of our research was to identify tissue and food proteins that have the potential to cross-react withC. jejuniantigens. We therefore appliedC. jejuni-specific antibodies to tissue antigens that are major components of intestinal barriers, tissue enzymes, enteric nerve, thyroid, heart,joints, pancreas, growth hormone, blood-brain barriers, and brain tissues with their receptors. Overall the reaction of these antibodies with proteins such as claudin-5,adherent junction and zonulin were from low to high. More specifically, the reactions of bothC. jejuniandC. jejuniCdt with zonulin were high, but with adherent junction was from low to moderate (Figures 1 and 3). We were not surprised by these findings,since it has been shown thatC. jejuniinteracts directly with intestinal epithelial cells and induce the production of inflammatory cytokines such as TNF-α and IFN-g that have synergistic effects on barrier integrity[48]. Moreover,C. jejuniinfection has been linked to the pathophysiology of chronic functional bowel changes through the structural mimicry between vinculin and bacterial virulence factors such as Cdt, and the autoimmune reactivity against them[23,24]. Interestingly, although the antibodies used in our study reacted strongly withC. jejunilysate,C. jejuniandE. coliCdt, the same antibodies did not react with vinculin, as shown in Figures 1 and 3. The ODs generated from the reaction of these antibodies with vinculin were less than 0.4. This lack of reaction betweenC. jejuniantibodies and vinculin in contrast with their strong reaction with zonulin and adherent junction could be related to the immunogens used for the preparation of these antibodies. At this point the nature of these Cdt crossreactive antibodies is not clear, and more research is needed in order to examine their roles in IBS, SIBO and autoimmune disorders.

Figure 4 Reaction of affinity-purified rabbit anti-Campylobacter jejuni cytolethal distending toxin with different food antigens. Compared to the reaction with Campylobacter jejuni (C. jejuni) antigens as positive control and HSA or unimmunized rabbit serum as negative control, the reaction of this antibody with 160 out of 180 food antigens is insignificant, and with 20 additional antigens ranges from low to very high. The variation of quadruplicate ODs was less than 10% for all determinations. Comparison of the means of the antibody reactivity to various food antigens with the means of controls resulted in insignificant values (P > 0.05) for 160 different food antigens and highly significant P values (P < 0.00001) for the other 20 antigens. E. coli: Escherichia coli; Cdt: Cytolethal distending toxin; LPS:Lipopolysaccharide; HSA: Human serum albumin.
The enteric nerve system (ENS) consists of a mesh-like network of neurons that governs the function of the GI tract and is capable of autonomous functions such as coordination of the reflexes[49]. The main antigen of the ENS is the enteric nerve neuronal nuclear antigen (enteric nerve NNA); antibodies against this antigen are detected in patients with irritable bowel syndrome[50]. Rabbit anti-bacterial cytotoxin antibody has been found to exhibit affinity to enteric ganglia and ICC through molecular mimicry and the production of cross-reactive antibodies. In previous studies, a single exposure of rats toC. jejuniresulted in SIBO in 26% of the rats. In double-exposed rats SIBO was detected in 46% (P< 0.05). Anti- bacterial cytotoxins and cytoskeletal proteins were detected in all rats exposed toC. jejuni[22,23].
Based on the affinity of bacterial cytotoxin antibodies to ICC and ganglia, it was concluded that these antibodies play a role in the pathophysiology of IBS by affecting gut motor function, leading to SIBO[23]. As shown in Figure 3, the anti-C. jejuniand anti-Cdt reacted moderately with enteric nerve antigens. This may indicate that mimicry between Cdt and enteric nerve NNA plays a role in the pathophysiology of IBS. We also found thatC. jejuniantibodies reacted from moderately to strongly with tissue and microbial transglutaminases.
tTGs are a group of enzymes that catalyze various posttranslational modifications of glutamine residues in proteins and peptides[51]. Tissue transglutaminases such as tTG-2 and tTG-3 are known as endogenous transglutaminases. Antibodies against tTG-2, tTG-3 and tTG-6 are detected in patients with celiac disease (CD), dermatitis herpetiformis, and gluten ataxia[52,53-55]. The exogenous mTG is a universal protein cross-linker and translational modifier of peptides made fromStreptoverticillium mobaraensethat imitates the function of endogenous tTGs[56]. It is used industrially as meat glue to bind lesser cuts of meat and other kinds of food together. Studies indicate that the widespread use of mTG in different industries has contributed to the surge of CD and nonceliac gluten sensitivity (NCGS)[56,57].
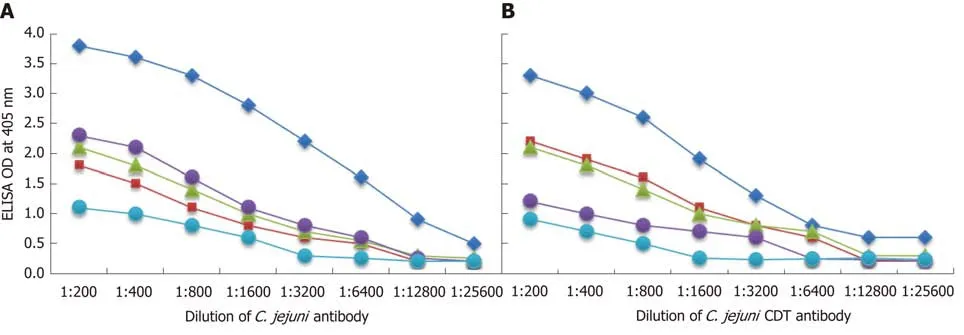
Figure 5 Demonstration of analytical specificity by dilution study. A: Reaction of various dilutions of mouse monoclonal anti-Campylobacter jejuni (C. jejuni)bacteria antibody with the same concentrations of C. jejuni = blue rhombus, zonulin = red square, somatotropin = green triangle, presenilin = purple circle, and ωgliadin = blue circle. B: Reaction of various dilutions of mouse monoclonal anti-C. jejuni bacterial cytolethal distending toxin (Cdt) antibody with the same concentrations of C. jejuni Cdt = blue rhombus, zonulin = red square, somatotropin = green triangle, presenilin = purple circle, and ω-gliadin = blue circle. CDT:Cytolethal distending toxin; C. jejuni: Campylobacter jejuni.
Cross-reaction betweenC. jejuniantigens and different transglutaminases may indicate the involvement ofC. jejuninot only in the induction of SIBO/IBS but of CD and NCGS as well. Guillain-Barré syndrome-relatedC. jejuniis very often used for the demonstration of the bi-directional signaling that exists between the GI and the brain[13]. This concept of a “gut-brain axis” was covered extensively in the March 2018 issue of Nature Collections by Katrina Ray[58]. In this collection of articles, it was shown that the disruption of the gut-brain axis has been implicated in the etiopathogenesis or manifestation of a diverse range of neurodevelopmental,psychiatric, and neurodegenerative diseases, including autism spectrum disorder,depression, Alzheimer’s disease, and Parkinson’s disease[58]. In turn, common pathophysiological mechanisms have been associated with GI comorbidity[58].Everything is interconnected: the gut can influence the brain, the brain can influence the gut, and they both can influence and be influenced by the immune system.
In view of this, we set out to study the immune reactivity of anti-C. jejuniantibodies with a variety of neuronal antigens that have been associated with various neuroimmune and neurodegenerative disorders[59,60]. The antibodies used in our study reacted from moderate to high with these neural antigens: MBP, asialoganglioside GM1, S100B, GFAP, AQP4, glutamate receptor, acetylcholine receptor, β-amyloid and presenilin (Figures 1 and 3). Antibodies against some of these antigens and peptides are detected in the sera and cerebrospinal fluids (CSF) of patients with neuroimmune and neurodegenerative disorders[60,61]. These antibodies are also found but at much lower levels in the blood of many healthy individuals[62]. Overall, high levels of Aβ protein, presenilin, GFAP and MBP have been linked with neurodegeneration and diseases such as AD, Parkinson’s disease (PD), and multiple sclerosis (MS)[61-67]. Since we have shown thatC. jejuniantibodies react with these neural antigens,C. jejuniand its toxin may therefore play a role in neurodegenerative disorders including AD through molecular mimicry.
Aquaporin 4 (AQP4) and S100B have been linked to increased permeability of the blood-brain barrier (BBB), neuromyelitis optica (NMO), and dementia, among others[66,67]. Glutamate receptor (glutamate-R), dopamine receptors and glutamic acid decarboxylase 65 (GAD-65) are associated with neuroautoimmunity, including Sydenham’s chorea and gluten ataxia[68,69]. Finally, application of the anti-C. jejunito TPO, a-enolase and somatotropin resulted in moderate to strong reactions (Figures 1 and 3).
Although thyroid autoantibodies are used for confirming the diagnosis of thyroid autoimmunities[70-72], many infectious agents such asYersinia enterocolitica,Helicobacter pylori(H. pylori),Candida albicans, B. burgdorferi, and, as our own study now demonstrates,C. jejuni, may contribute to the presence of these antibodies in blood[73-75]. a-enolase or non- neuronal enolase, in addition to its enzymatic activity,performs many physiological functions in eukaryotes and prokaryotes[76]. Antibodies against this enzyme have been detected in a variety of infections and autoimmune diseases. In infections, it seems that anti-a-enolase could play a role in limiting microbial tissue invasion by binding to bacterial surface enolase[77-78], while in autoimmunities the a-enolase antibody could induce tissue injury through the initiation of immune complex formation and the activation of complement cascade[79-82].
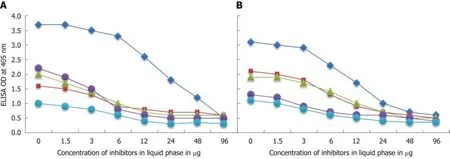
Figure 6 Demonstration of analytical specificity by inhibition study. A: Inhibition of the reaction of mouse monoclonal anti-Campylobacter jejuni (C. jejuni)antibody binding to C. jejuni antigens = blue rhombus, zonulin = red square, somatotropin = green triangle, presenilin = purple circle, and ω-gliadin = blue circle with different concentrations of the same antigen in liquid phase. B: Inhibition of the reaction of affinity-purified rabbit anti-C. jejuni cytolethal distending toxin antibody binding to C. jejuni cytolethal distending toxin = blue rhombus, zonulin = red square, somatotropin = green triangle, presenilin = purple circle, and ω-gliadin = blue circle with different concentrations of the same antigen in liquid phase.
Finally, as shown in Figures 1 and 3, bothC. jejuniandC. jejuniCdt antibodies reacted strongly with somatotropin or human growth hormone (HGH). In addition to growth promotion, other significant effects of HGH are stimulation of protein synthesis, enhancement of immune function, and activation of lipolysis. In a recent review article, it was shown that the gut microbiota communicates with the host’s tissue and cells, including adipocytes[83]. Thus, alteration in the composition of the gut microbiota and their metabolites can affect not only the serum level of HGH but also different organs of the human body[84-87]. Although the underlying molecular mechanisms are unknown, the production of antibodies againstC. jejuniand their strong reaction with somatotropin may result in the binding of these antibodies to HGH, thus preventing its binding to the HGH receptor and inhibiting its potent pleiotropic biological effects. In our earlier studies[40,88], we showed that in addition to infections, many food antigens or peptides contribute to the increased levels of antibodies that are detected in patients with type 1 diabetes, thyroid autoimmunity and Alzheimer’s disease[40,59,88,89]. Therefore, similar to these other studies, we measured the immunoreactivities ofC. jejuniantibodies with 180 different food antigens. Results depicted in Figures 3 and 4 show that although theC. jejunibacterial antibody had low reactions with 17 out of 180 foods, theC. jejuniCdt antibody reactions were from moderate to high with 19 out of 180 food antigens or peptides(Figure 4). Detection in the blood of theseC. jejuniantibodies that cross-react with different food antigens such as watermelon, pumpkin, squash, carrots, peanut agglutinin, wheat, milk, and gums is clinically significant if macromolecules from food escape enzymatic digestion due toC. jejuniand its toxins. In the state of altered intestinal permeability, a heightened rate of food antigens is detected in the systemic circulation. The reaction ofC. jejunicross-reactive antibodies in the presence of C1q complement results in the formation of food immune complexes. It has been suggested that if these circulating soluble immune complexes are not removed by the liver, they may then play a significant role in allergies and a variety of inflammatory and autoimmune disorders[31,90,91]. Furthermore, our findings may provide new insight into the diversity ofC. jejunitoxins that mimic mammalian tissue antigens, as well as the ways by whichC. jejunican bind with cell surface glycans on different foods and then use them as vehicles forC. jejunitoxic invasion into the blood. This could initiate not only autoimmunities but also neurodegenerative disorders such as Alzheimer’s disease and Parkinson’s disease.
While the presence of antibodies in the blood is not by itself an indication of specific autoimmune disease, the binding of anti-C. jejuniand its Cdt to a variety of tissue antigens may contribute to the molecular mechanism that is involved in the induction of not only IBS, SIBO, and GBS but many other autoimmune and neurodegenerative disorders with which these tissue antibodies are associated. The reaction ofC. jejuniandC. jejuniCdt antibody with neural antigens and growth factors such as α-amyloid, presenilin, S100B, GFAP, AQP4, somatotropin, enteric nerve and more justifies the addition ofC. jejuniand its toxins to the list of microbes that are implicated in the etiology of Alzheimer’s disease. This suggestion is further supported by our recent study, where we show that anti-Aβ-42 antibody not only reacted moderately withE. coli,Salmonella, andC. jejuni, but also reacted very strongly withC. jejuniCdt, and, in decreasing degrees, withE. coliandSalmonellaCdts[59].Therefore, by identifying the triggers such asC. jejuniand its toxin that induce the production of cross-reactive tissue antibodies, it may be possible to develop therapeutic protocols to treat patients with autoimmune disease. In this particular case, the treatment for toxin- producingC. jejunicould result first in the reduction of the antigenic load, followed by the reduction of tissue antibodies in the blood. This offers some hope for alleviating the symptoms of patients with autoimmune disorders. Otherwise, the release of bacterial antigens and toxins into the blood and their binding to the formed antibodies can result in the generation of immune complexes and activation of the C1q complement that may induce tissue damage and cell death through an apoptotic process.
Given that the intestinal microbiome has been shown to signal extraintestinal organs[83], it is not surprising that alterations in the composition of the gut microbiota due to food-borneC. jejunihas been linked to different diseases. Thus, modulation of the composition of the gut microbiota with medication, prebiotics, probiotics and dietary intervention represents a promising therapeutic avenue for the management of autoimmune disorders that affect about 10% of the world population[92].
ARTICLE HIGHLIGHTS
Research background
The bacteriaCampylobacter jejuni(C. jejuni) is commonly associated with GBS and IBS, but studies have also linked it with Miller Fisher syndrome, reactive arthritis and other disorders,some of which are autoimmune. It is possible thatC. jejuniand its toxins may be cross-reactive with some human tissues and food antigens, potentially leading to autoimmune responses.Cross-reaction betweenC. jejuniantigens and different transglutaminases may indicate the involvement ofC. jejuninot only in the induction of small intestine bacterial overgrowth(SIBO)/irritable bowel syndrome (IBS) but of celiac disease (CD) and nonceliac gluten sensitivity(NCGS) as well.C. jejuniis very often used for the demonstration of the bi-directional signaling that exists between the gastrointestinal tract and the brain. The disruption of the gut-brain axis has been implicated in the etiopathogenesis or manifestation of a diverse range of neurodevelopmental, psychiatric, and neurodegenerative diseases. In turn, common pathophysiological mechanisms have been associated with gastrointestinal comorbidity.Everything is interconnected.
Research motivation
Our earlier research showed that antibodies against amyloid-beta peptide reacted strongly with different tissue and food antigens. Infections have been shown to be involved in the pathogenesis of various disorders. Our earlier study showed that anti-amyloid-beta-42 antibody reacted very strongly withC. jejunicytolethal distending toxin (Cdt).C. jejuniand its toxins have been shown to be involved not only in IBS, Guillain-Barré syndrome (GBS), SIBO, Miller Fisher syndrome and the like, but in various extragastrointestinal conditions as well, including some that involve the brain. This inspired us to investigate whether perhapsC. jejuniand its toxins were reacting to certain tissues or foods, resulting in food reactivity, molecular mimicry,or even autoimmunity and neurodegenerative disorders. One of the most crucial problems was finding reagents of sufficient quality and purity in order to achieve accurate and reliable results in our study’s testing, and we tried different sources before finally finding and using mouse monoclonal anti-C. jejuniand rabbit affinity-purified anti-C. jejuniCdt isotypes. This enabled our study to accurately detect and measure antibody levels to a degree that had not previously been possible, and the employment of high quality highly-purified reagents should greatly increase the accuracy of similar lab testing or even lab testing in general in future research. The findings of our study regarding immunoreactivity ofC. jejuniand its Cdt antibodies with many human tissues and food antigens may indicate a more widespread role of this bacteria in many autoimmune and neurodegenerative disorders. This deserves the initiation of further studies.
Research objectives
To measure the immune reactivity ofC. jejuniandC. jejuniCdt antibodies with tissue and food antigens to examine their role in autoimmunities.
Research methods
Using enzyme-linked immunosorbent assay (ELISA) methodology, specific antibodies made againstC. jejuniandC. jejuniCdt were applied to a variety of microwell plates coated with 45 tissues and 180 food antigens. The resulting immunoreactivities were compared to reactions with control wells coated with human serum albumin (HSA) which were used as negative controls and with wells coated withC. jejunilysate orC. jejuniCdt which served as positive controls.
Research results
Monoclonal antibody made againstC. jejunishowed moderate to high reactions with zonulin,somatotropin, acetylcholine receptor, β-amyloid and presenilin. This immune reaction was low with an additional 25 tissue antigens, and the same antibody did not react at all with another 15 tissue antigens. Examining the reaction betweenC. jejuniantibody and 180 food antigens, we found insignificant reactions with 163 foods but low to high immune reactions with 17 food antigens. Similarly, withC. jejuniCdt antibody, the reactions with tissues were strongest with zonulin, intrinsic factor and somatotropin, moderate with 9 different tissue antigens including thyroid peroxidase, low with another 10 different antigens, including neuronal antigens, and insignificant with an additional 23 tissue antigens. BetweenC. jejuniCdt antibody and different food antigens, 160 out of 180 foods showed insignificant reactions, while 20 foods showed reactions ranging from low to high. Further research with the proper reagents and samples from patients with autoimmune and neurodegenerative disorders can lead to the clinical application of the methods and specific antibody tests used in this study.
Research conclusions
C. jejuniand its toxins are involved in different autoimmune and neurodegenerative disorders beyond the gut.C. jejuniand its toxins play a major role in a variety of autoimmune and neurodegenerative disorders through molecular mimicry. Many investigators have clearly establishedC. jejunias the most prevalent cause of food-related enteritis worldwide. It has been associated with a range of gastrointestinal conditions, including inflammatory bowel disease(IBD), Barrett’s esophagus, and colorectal cancer, and has also been reported to be involved in extragastrointestinal manifestations, including bacteremia, lung infections, brain abscesses,meningitis, and reactive arthritis. Monoclonal and affinity-purified antibodies againstC. jejuniand its toxins have moderate to strong reactions with a variety of tissue and food antigens.Studies have shown that chronic exposure toC. jejuniand its toxin Cdt could induce epithelial cell damage, facilitating not only bacterial invasion but also the penetration of undigested food antigens into the submucosa and into the circulation. This body exposure to these external triggers may initiate an immune response in which antibody production against the bacterial food toxins and food antigens may result in cross-immunoreactivity with a variety of tissue antigens, thereby setting the stage for multiple autoimmune reactivities beyond the gut. Reaction of antibodies againstC. jejuniand its toxins with tissue and food antigens, which has not previously been studied. The reaction ofC. jejuniandC. jejuniCdt with different tissue antigens, including neural tissues, and different food antigens were made possible by finding the proper reagents of high quality and purity. The results of our study confirmed that there is crossreactivity betweenC. jejuniand its toxins with other tissue antigens as well as food proteins to which the human body is exposed on a daily basis. This reaction may be due to molecular mimicry and may contribute to the pathogenesis of autoimmune and neurodegenerative disorders. Treatment forC.jejunishould therefore be a part of the strategy for prevention and treatment of autoimmune and neurodegenerative disorders.
Research perspectives
The availability and use of excellent and highly purified reagents such as monoclonal mouse and affinity-purified rabbit antibodies can help us to accurately detect and measure in the blood levels of antibodies that had previously been overlooked or ignored but which may actually have important roles in devastating diseases. The reaction of antibodies made againstCampylobacter jejuniwith so many human tissues and food antigens encourages future research in the role that this organism may play in multiple autoimmune diseases. Future research should seek to further confirm the involvement ofC. jejuniand its toxins in autoimmune and neurodegenerative disorders, and then expand the search to examine the possible involvement of other pathogens. The best method for ensuring the most accurate and reliable results in testing forC. jejuniand other pathogens is to use only specific antibodies that are of high quality and high purity.
ACKNOWLEDGEMENTS
The authors wish to thank Joel Bautista for the preparation of this manuscript for publication.
杂志排行
World Journal of Gastroenterology的其它文章
- Liver stem cells: Plasticity of the liver epithelium
- Integrated network analysis of transcriptomic and protein-protein interaction data in taurine-treated hepatic stellate cells
- Computed tomography scan imaging in diagnosing acute uncomplicated pancreatitis: Usefulness vs cost
- Targeted puncture of left branch of intrahepatic portal vein in transjugular intrahepatic portosystemic shunt to reduce hepatic encephalopathy
- Optimized protocol of multiple post-processing techniques improves diagnostic accuracy of multidetector computed tomography in assessment of small bowel obstruction compared with conventional axial and coronal reformations
- Comprehensive lifestyle intervention vs soy protein-based meal regimen in non-alcoholic steatohepatitis
